- *Corresponding Author:
- M. A. Rode
Department of Chemistry, Radhabai Kale Mahila Mahavidyalaya, India
E-mail: milindrode@yahoo.com
| Date of Submission | 05 December 2013 |
| Date of Revision | 08 November 2014 |
| Date of Acceptance | 24 March 2015 |
| Indian J Pharm Sci, 2015;77(2):230-236 |
Abstract
The reaction of 5-nitrobenzimidazolone with phenoxyethyl bromide in presence of potassium carbonate in dimethyl formamide obtained 6-nitro-1,3-bis(2-phenoxyethyl)-1,3-dihydro-2H-benzimidazol-2-one. It was reduced using stannous chloride to get 6-amino -1,3-bis(2-phenoxyethyl)-1, 3-dihydro-2H-benzimidazol -2-one, which was further treated with aromatic sulphonyl chloride to obtain benzimidazolone derivatives, 6a-k. These compounds were tested for antibacterial, antituberculosis and antifungal activity. Most of them have shown very good activity against some gram positive and gram negative microorganisms and fungal strains. Some of them have shown moderate activity against Mycobacterium tuberculosis.
Keywords
Benzimidazolone, antifungal, antibacterial and antituberculosis activity
The incidence of bacterial and fungal infections has increased dramatically in the past 20 years partly because of the increase in the number of people whose immune systems are compromised by with AIDS, aging, organ transplantation or cancer therapy. Accordingly, the increase in rates of morbidity and mortality because of bacterial and fungal infections has been now recognized as a major problem. In response to the increased incidence of bacterial and fungal infections, researchers are working on the development of newer less toxic antiinfective agents for clinical use.
Sulfonamide drugs were the first antimicrobial drugs, and paved the way for the antibiotic revolution in medicine. There are several sulfonamide-based groups of antiinfective drugs e.g. sulfamethoxazole, which are known as the reversible inhibitors of folic acid synthesis [1]. Sulfa drugs are still widely used for conditions such as acne and urinary tract infections, and are receiving renewed interest for the treatment of infections caused by bacteria resistant to other antibiotics.
In the last few years, benzimidazole and benzimidazolone have been studied extensively for their antitumor [2], antiviral [3] and antibiotic activities such as the antiprotozoal and antibacterial [4]. Recently, Monforte et al. identified some 1,3-dihydrobenzimidazol-2-one derivative and their sulfones as a potent and novel class of non-nucleoside reverse transcriptase inhibitor [5]. Aryloxyalkyl benzimidazole derivatives have been explored for antimicrobial activity by Khalafi-Nezhad et al. and have suggested that negative electrostatic potentials around oxygen of the phenoxy and nitrogen of the imidazole moieties have direct effect on the antibacterial activity towards Staphylococcus aureus [6].
Very few attempts have been made so far to explore antimicrobial activity of sulfonamide linked benzimidazolone. In order to explore the potential role of sulfonamide linked benzimidazolone in antiinfective treatment, we have synthesized some aryloxyalkyl benzimidazolone linked to various heterocyclic ring systems through sulfonamide linkage and tested them for antimicrobial activity.
All the recorded melting points were determined in open capillary and are uncorrected. IR spectra were recorded on Perkin-Elmer FTIR spectrophotometer in KBr disc. 1H-NMR and 13C-NMR spectra were recorded on 400 MHz spectrophotometer in DMSO-d6 as a solvent and TMS as an internal standard. Peak positions are shown in ppm values. Mass spectra were obtained by Waters mass spectrometer. Thin layer chromatography (TLC) was performed on precoated aluminum sheets of Silica Gel 60 F254 (Merck, Art. 5554), visualization of products being accomplished by UV absorption.
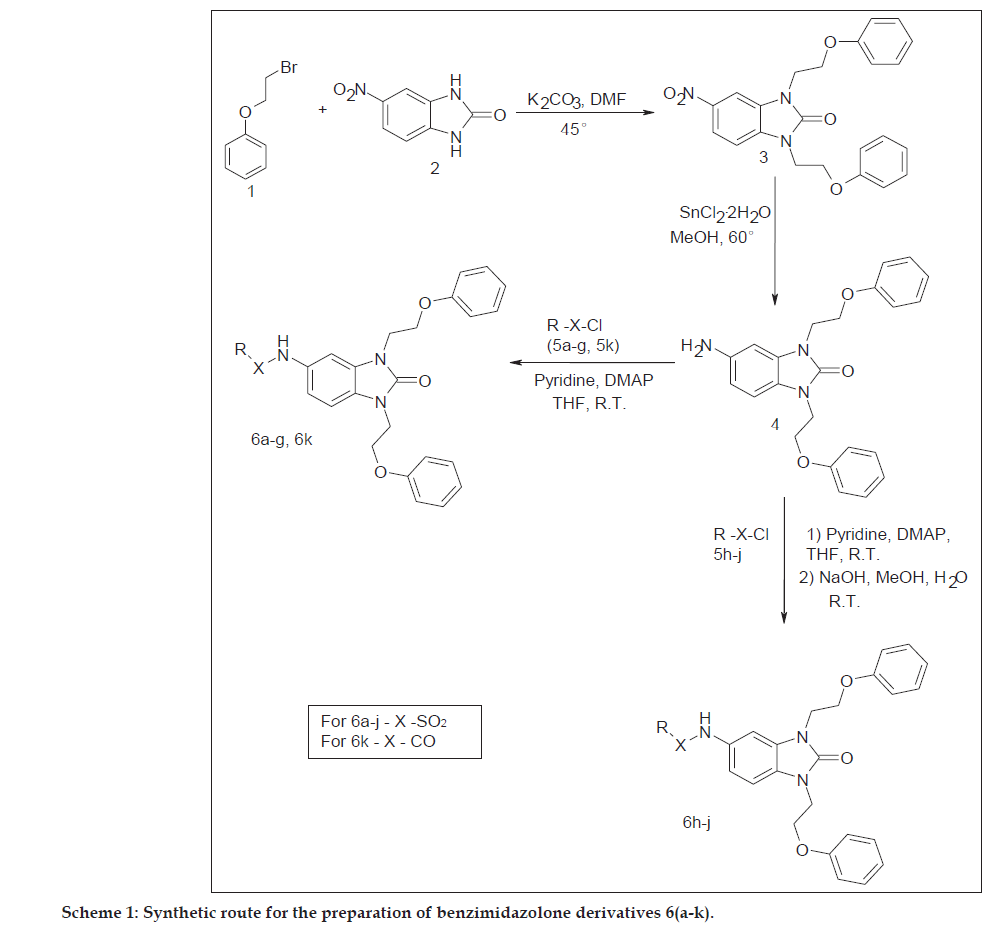
General procedure used for the synthesis of 6-nitro-1,3-bis(2-phenoxyethyl)-1,3-dihydro-2H-benzimidazol- 2-one (3) was as follows. Compound 1 (0.01 mol) and 2 (0.01 mol) were dissolved in DMF along with K2CO3. The reaction mixture was stirred at 45° for 14 h. The reaction mixture was cooled to room temperature and poured into water and extracted by ethyl acetate. The organic layer separated, dried over sodium sulphate and concentrated under vacuum. The crude product was recystallised from ethanol.
Compound (3) was obtained in a yield of 86%; yellow solid, mp: 90-92°. Analysis calculated for C23H21N3O5: C, 65.86; H, 5.05; N, 10.02. Found: C, 65.70; H, 5.01; N, 9.90. IR (KBr): 3432 s, 3111 s, 1678 s, 1560 s, 1498 s. 1H-NMR (400 MHz, DMSO): 8.27 d, 1H, J=2.4 (Ar-H); 8.10 dd, 1H, J=8.8, 2.0 (Ar-H); 7.53 d, 1H, J=8.8 (Ar-H), 7.21 m, 4H (Ar-H); 6.8 m, 6H (Ar-H); 4.35 m, 4H, (OCH2), 4.27 m, 4H (NCH2). MS m/z: 420 (M+1) with all isotopic and other peaks.
General procedure used for the synthesis of 6-amino- 1,3-bis(2-phenoxyethyl)-1,3-dihydro-2H-benzimidazol- 2-one (4) was as follows, to a solution of nitro derivative 3 (0.1 mol) in methanol (50 ml) was added 5 equivalent SnCl2:2H2O and the reaction mixture was heated at 60° for 4 h. The reaction mixture was cooled to room temperature and poured into liquid NH3 and filtered through hyflow. The filtrate was extracted by ethyl acetate. The organic layer was separated, dried over sodium sulphate and concentrated under vacuum. The product was recrystallized from ethanol.
Compound (4) was obtained in a yield of 80%; white solid, mp: 64-66°. Analysis calculated for C23H23N3O3: C, 70.93; H, 5.95; N, 10.79. Found: C, 70.74; H, 5.90; N, 10.70. IR (KBr): 3600 s, 3432 s, 3111 s, 1678 s, 1498 s. 1H-NMR (400 MHz, DMSO): 7.28 m, 4H (Ar-H); 6.96 m, 7H (Ar-H); 6.62 s, 1H (Ar-H), 6.39 d, 1H, J=8.0 (Ar-H); 4.91 s, 2H (NH2), 4.23 s, 4H (OCH2), 4.16 s, 4H (NCH2). MS m/z: 390 (M+1) with all isotopic and other peaks.
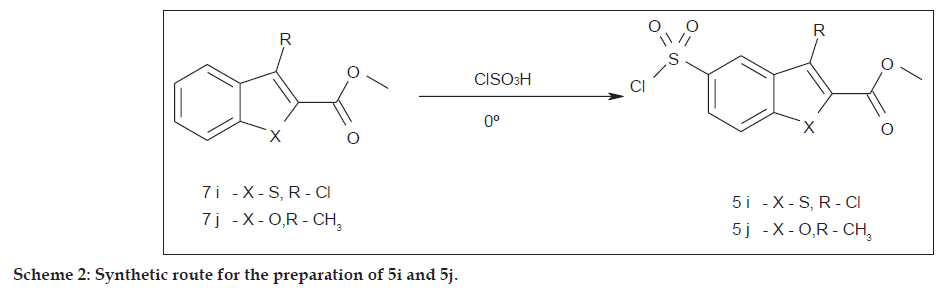
General procedure used for the synthesis of 5i-j was as follows; compound 7 (0.01 mol) was added in portions to the solution of chlorosulfonic acid (10 ml) at 0° and stirred for 1 h. The reaction mixture was poured into cold water and solid separated by filtration.
Compound 5i was obtained with an yield of 60%; white solid, mp: 182-184°. Analysis calculated for C10H6Cl2O4S2: C, 36.94; H, 1.86. Found: C, 36.85; H, 1.85. IR (KBr): 1735 s, 1598 s, 800 s. 1H-NMR (400 MHz, DMSO): 8.16 s, 1H (Ar-H); 8.10 d, 1H, J=8.4 (Ar-H); 7.84 d, 1H, J=8.4 (Ar-H); 3.92 s, 3H (OCH3). 13C-NMR (400 MHz, DMSO): 160.6, 146.3, 137.8, 135.4, 126.5, 126.2, 125.97, 123.2, 119.7, 52.8. MS m/z: 323 (M-1) with all isotopic and other peaks.
Compound 5j was obtained with an yield of 66%; white solid, mp:152-154°. Analysis calculated for C11H9ClO5S: C, 45.76; H, 3.14. Found: C, 45.60; H, 3.13. IR (KBr): 2937 s, 2840 s, 1735 s, 1598 s. 1H-NMR (400 MHz, DMSO): 8.39 d, 1H, J=1.6 (Ar-H); 8.14 dd, 1H, J=8.8, 2 (Ar-H); 7.75 d, 1H, J=8.8 (Ar-H); 4.00 s, 3H (OCH3); 2.64 s, 3H (Ar-CH3). 13C-NMR (400 MHz, DMSO): 159.9, 156.8, 143.6, 139.7, 129.6, 126.1, 125.8, 122.0, 113.7, 52.5, 9.3. MS m/z: 289 (M+1) with all isotopic and other peaks.
General procedure used for the synthesis of 6a-g and 6k was as follows, compound 4 (0.01 mol) and aryl sulphonyl chloride (in case of 6k, 6-chloronicotinyl chloride, 0.01 mol) were dissolved in THF along with dimethylaminopyridine (DMAP) and pyridine (0.03 mol). The reaction mixture was stirred at room temperature for 6 h. The reaction mixture poured into dilute HCl and extracted by ethyl acetate. The organic layer was washed by water, separated, dried over sodium sulphate and concentrated under vacuum. The crude product was purified by using silica gel column chromatography with hexane and ethyl acetate as solvent.
Compound 6a was obtained with an yield of 76%, yellow solid, mp: 148-150°. Analysis calculated for C28H25ClN4O5S: C, 59.52; H, 4.46; N, 9.92. Found: C, 59.46; H, 4.44; N, 9.88. IR (KBr): 3432 s, 3111 s, 1678 s, 1498 s. 1H-NMR (400 MHz, DMSO): 10.36 s, 1H (NH); 8.64 d, 1H, J=2.4 (Ar-H); 8.06 dd, 1H, J=8.4, 2.4 (Ar-H); 7.67 d, 1H, J=8.4 (Ar-H), 7.18 m, 6H (Ar-H); 6.8 m, 6H (Ar-H); 6.7 dd, 1H, J=8.4, 2 (Ar-H); 4.16 s, 8H, (CH2). MS m/z: 565 (M+1) with all isotopic and other peaks.
Compound 6b was obtained with an yield of 66%, brown microcrystalline, mp: 82-84°. Analysis calculated for C33H32N4O6S: C, 64.69; H, 5.26; N, 9.14. Found: C, 64.58; H, 5.25; N, 9.10. IR (KBr): 3430 s, 3119 s, 1645 s, 1677 s, 1499 s. 1H-NMR (400 MHz, DMSO): 9.97 s, 1H (NH); 7.51 m, 2H (Ar-H); 7.20 m, 5H (Ar-H); 7.10 m, 2H, (Ar-H); 6.80 m, 6H (Ar-H); 6.72 m, 1H (Ar-H); 4.24 s, 4H (OCH2); 4.09 s, 4H (NCH2); 4.04 t, 2H (NCH2); 3.03 t, 2H (CH2); 2.13 s, 3H (COCH3). MS m/z: 613 (M+1) with all isotopic and other peaks.
Compound 6c was obtained with an yield of 68%, yellow crystalline, mp: 85-87°. Analysis calculated for C32H32N4O7S2: C, 59.24; H, 4.97; N, 8.64. Found: C, 59.16; H, 4.95; N, 8.60. IR (KBr): 3435 s, 3109 s, 1678 s, 1500 s. 1H-NMR (400 MHz, DMSO): 10.19 s, 1H, (NH); 7.52 m, 2H (Ar-H); 7.22 m, 5H (Ar-H); 7.10 m, 2H, (Ar-H); 6.81 m, 6H (Ar-H); 6.74 m, 1H (Ar-H); 4.15 s, 8H (CH2); 3.82 t, 2H (NCH2); 3.12 s, 3H (SO2CH3); 3.07 t, 2H (indoline CH2). MS m/z: 648 (M+) with all isotopic and other peaks.
Compound 6d was obtained with an yield of 88%, yellow crystalline, mp: 83-85°. Analysis calculated for C32H27N3O7S: C, 64.31; H, 4.55; N, 7.03. Found: C, 64.25; H, 4.57; N, 7.06. IR (KBr): 3433 s, 3112 s, 1693 s, 1675 s, 1498 s. 1H-NMR (400 MHz, DMSO): 10.22 s, 1H (NH); 8.20 m, 2H (Ar-H); 7.86 d, 1H, J=10.8 (Ar-H); 7.50 d, 1H, J=10.8 (Ar-H); 7.20 m, 6H (Ar-H); 6.80 m, 7H (Ar-H); 6.55 m, 1H, (Ar-H); 4.14 s, 8H (CH2); MS m/z: 596 (M-1) with all isotopic and other peaks.
Compound 6e was obtained with an yield of 75%, yellow crystalline, mp: 92-94°. Analysis calculated for C35H34N4O5S: C, 67.51; H, 5.50; N, 9.00. Found: C, 67.44; H, 5.51; N, 8.97. IR (KBr): 3435 s, 3111 s, 1677 s, 1501 s, 1350 s. 1H-NMR (400 MHz, DMSO): 10.46 s, 1H, (NH); 8.40 q, 2H (Ar-H); 8.13 d, 1H, J=6.4 (Ar-H); 7.62 t, 1H (Ar-H); 7.48 t, 1H (Ar-H); 7.18 m, 5H (Ar-H); 7.04 m, 2H (Ar-H); 6.88 m, 2H (Ar-H); 6.75 m, 4H (Ar-H); 6.67 dd, 1H, J=8.4, 1.6 (Ar-H); 4.09 s, 8H (CH2); 2.76 s, 6H (NCH3). MS m/z: 623 (M+1) with all isotopic and other peaks.
Compound 6f was obtained with an yield of 78%, yellow crystalline, mp: 85-87°. Analysis calculated for C33H34N4O7S2: C, 59.80; H, 5.17; N, 8.45. Found: C, 59.72; H, 5.15; N, 8.48. IR (KBr): 3435 s 3109 s 1678 s, 1500 s. 1H-NMR (400 MHz, DMSO): 10.05 s, 1H, (NH); 7.64 d, 1H, J=2 (Ar-H); 7.51 m, 2H (Ar-H); 7.20 m, 6H (Ar-H); 6.81 m, 7H (Ar-H); 4.16 s, 8H (CH2); 3.64 t, 2H (tetrahydroquinoline NCH2); 3.09 s, 3H (SO2CH3) 2.70 t, 2H (tetrahydroquinoline CH2); 1.80 m, 2H (tetrahydroquinoline CH2). MS m/z: 663(M+1) with all isotopic and other peaks.
Compound 6g was obtained with an yield of 44%, yellow crystalline, mp: 89-91°. Analysis calculated for C41H40N6O8S2: C, 60.88; H, 4.98; N, 10.39. Found: C, 60.74; H, 4.96; N, 10.42. IR (KBr): 3455 s, 3050 s, 2927 s, 2830 s, 1677 s. 1H-NMR (400 MHz, DMSO): 10.04 s, 1H (NH); 8.25 s, 1H (N=CH); 7.85 d, 1H, J=8 (Ar-H); 7.68 m, 3H (Ar-H); 7.21 m, 7H (Ar-H); 6.85 m, 9H (Ar-H); 4.16 s, 8H (CH2); 4.10 t, 2H (indoline NCH2); 3.15 s, 3H (NCH3); 2.98 t, 2H (indoline CH2); 2.92 t, 3H (NCH3); MS m/z: 807 (M-1) with all isotopic and other peaks.
Compound 6k was obtained with an yield of 88%, yellow solid, mp: 110-112°. Analysis calculated for C29H25ClN4O4: C, 65.85; H, 4.76; N, 10.59. Found: C, 65.70; H, 4.74; N, 10.55. IR (KBr): 3432 s, 3111 s, 1668 s, 1498 s. 1H-NMR (400 MHz, DMSO): 10.26 s, 1H (NH); 8.60 d, 1H, J=2.4 (Ar-H); 8.00 dd, 1H, J=8.4, 2.4 (Ar-H); 7.66 d, 1H, J=8.4 (Ar-H), 7.21 m, 6H (Ar-H); 6.79 m, 6H (Ar-H); 6.7 dd, 1H, J=8.0, 2 (Ar-H); 4.12 s, 8H, (CH2). MS m/z: 527 (M-1) with all isotopic and other peaks.
General procedure used for the synthesis of 6h-j was as follows, compound 4 (0.01 mol) and aryl sulphonyl chloride (0.01 mol) were dissolved in THF along with DMAP and pyridine (0.03 mol). The reaction mixture was stirred at room temperature for 4 h. The reaction mixture poured into dilute HCl and extracted by ethyl acetate. The organic layer was washed by water, separated, dried over sodium sulphate and concentrated under vacuum. The product was dissolved in 50 ml MeOH and (1 mol) NaOH in water added to the reaction mixture and stirred at room temperature for 3 h. Reaction mixture poured in excess of water and acidified by dilute HCl to pH 2. Precipitate formed was filtered and washed by water, ether and Hexane.
Compound 6h was obtained with an yield of 86%, brown microcrystalline, mp: 68-70°. Analysis calculated for C32H31N3O7S: C, 63.88; H, 5.19; N, 6.98. Found: C, 63.79; H, 5.21; N, 6.96. IR (KBr): 3600 s, 3435 s, 3113 s, 1724 s, 1678 s, 1498 s. 1H-NMR (400 MHz, DMSO): 12.05 s, 1H (COOH); 10.05 s, 1H (NH); 7.62 d, 2H, J=8 (Ar-H); 7.32 d, 2H, J=8 (Ar-H); 7.22 m, 4H (Ar-H); 7.12 m, 2H (Ar-H); 6.90 m, 6H (Ar-H); 6.72 dd, 1H, J=8, 1.6 (Ar-H); 4.17 t, 2H (CH2); 4.15 s, 8H (CH2); 3.42 t, 2H (CH2). MS m/z: 602 (M+1) with all isotopic and other peaks.
Compound 6i was obtained with an yield of 70%, yellow crystalline, mp: 101-103°. Analysis calculated for C32H26ClN3O7S2: C, 57.87; H, 3.95; N, 6.33. Found: C, 57.70; H, 3.97; N, 6.36. IR (KBr): 3600 s, 3430 s, 3110 s, 1725 s, 1678 s, 1498 s. 1H-NMR (400 MHz, DMSO): 12.05 s, 1H (COOH); 10.67 s, 1H (NH); 8.24 m, 2H (Ar-H); 7.87 m, 1H (Ar-H); 7.20 m, 6H (Ar-H); 6.80 m, 7H (Ar-H); 4.15 s, 8H (CH2). MS m/z: 662 (M-1) with all isotopic and other peaks.
Compound 6j was obtained with an yield of 65%, yellow crystalline, mp: 103-105°. Analysis calculated for C33H29N3O8S: C, 63.15; H, 4.66; N, 6.69. Found: C, 63.00; H, 4.64; N, 6.66. IR (KBr): 3602 s, 3434 s, 3112 s, 1722 s, 1677 s, 1499 s. 1H-NMR (400 MHz, DMSO): 12.05 s, 1H (COOH); 10.14 s, 1H (NH); 8.16 s, 1H (Ar-H); 7.80 m, 2H (Ar-H); 7.17 m, 5H (Ar-H); 7.10 m, 1H (Ar-H); 6.87 m, 2H (Ar-H); 6.76 m, 5H (Ar-H); 4.12 s, 8H (CH2); 2.42 s, 3H (Ar-CH3); MS m/z: 628 (M+1) with all isotopic and other peaks.
The in vitro antimicrobial activity of test compounds was assessed against 24 h culture of several selected bacteria and fungi. The Gram +ve and Gram -ve bacteria used were, Escherichia coli, Pseudomonas aeruginosa, Streptococcus pyogenes and Staphylococcus aureus and the fungi used were Candida albicans, Aspergillus niger and Aspergillus clavatus. Antimicrobial activity of all the compounds was tested using Muller Hinton broth (Hi Media M 391) as nutrient medium for bacteria. Growth inhibition activities for test compounds were tested using disc diffusion method. The media were prepared using distilled deionized water and dispensed in 25 ml amounts into 100 mm petri dishes. One milliliter of inoculum suspension was used to inoculate by flooding the surface of Mueller-Hinton Agar petri dish. Excess liquid was air dried under a sterile hood. Different dilutions of test compounds and standard were loaded on 6 mm sterile disc. Dimethyl sulphoxide (DMSO) was used as negative control. The loaded disc was placed on the surface of medium and the compound was allowed to diffuse for 5 min and the plates were kept for incubation at 37° for 24 h. At the end of incubation, inhibition zones formed around the disc were measured with transparent ruler in mm.
Determination of antifungal activity of test compounds was accomplished by agar disc diffusion method on Sabouraud dextrose broth. Different dilutions of test compounds and standard were loaded on 6 mm sterile disc. The loaded disc was placed on the surface of medium and the compound was allowed to diffuse for 5 min and the plates were kept for incubation at 37° for 72 h. DMSO was used as the negative control. At the end of incubation, inhibition zones formed around the disc were measured with transparent ruler in mm.
All the compounds were screened for their in vitro antimycobacterial activity against M. tuberculosis by broth macro dilution method. The activity of compounds was confirmed by MIC determination against M. tuberculosis. A stock solution of each compound (1 mg/ml) was diluted in sterile distilled water to test the range. Each tube contained 4 ml sterile Middle brook 7H9 broth containing albumin-dextrose- catalase, Tween 80, glycerol and 4 ml of the compound solution was added to make serial double dilutions. Tubes were incubated at 37° for 7 days and then read visually. MIC was determined as the lowest concentration of antibiotic that prevented turbidity. Streptomycin, isoniazid, rifampicin and ethambutol were used as reference standards.
In the present work, 5-nitrobenzimidazolone (2) was treated with phenoxyethyl bromide (1) at 45° in DMF using potassium carbonate as a base to obtain nitro derivative (3), which was further reduced to amino derivative (4) using stannous chloride dihydrate. Amino derivative (4) was reacted with aromatic sulphonyl chloride (5a–g) in presence of pyridine and DMAP using THF as a solvent to get benzimidazolone derivatives 6a-g. Compounds 6h-j were synthesized by treating aromatic sulphonyl chlorides 5h-j, with amino compound (4) in presence of pyridine and DMAP in THF and further hydrolyzed using sodium hydroxide in methanol and water as shown in Scheme 1.
Compound (5i) was synthesized by the reaction of methyl-3-chlorobenzo [b]thiophene-2-carboxylate (7i) with chlorosulfonic acid. Similarly, compound (5j) was synthesized by treating methyl-3- methylbenzofuran-2-carboxylate (7j) with chlorosulfonic acid as shown in Scheme 2. The structure of (5i) and (5j) were confirmed by 13C-NMR and 1H-NMR spectroscopy. Compound (5g) was synthesized by chlorosulfonation of 1- [4- [(1E)- (dimethylamino)methylene]aminosulphonyl)benzoyl] indoline [7].
The structural data of the compounds 6a-k is given in Table 1. All the compounds, 6a-k were characterized by FTIR, 1H-NMR and mass spectroscopy. All of them were tested for their antibacterial, antifungal, and antituberculosis activities.
| Compound code | R‑X |
|---|---|
| 6a | 6‑Chloropyridine‑3‑sulfonyl |
| 6b | 1‑Acetyl‑5‑indolinesulfonyl |
| 6c | 1‑(Methylsulfonyl) indoline‑5‑sulfonyl |
| 6d | Coumarin‑6‑sulfonyl |
| 6e | 5‑Dimethylamino‑naphthalene‑1‑sulfonyl |
| 6f | 1‑Methanesulfonyl‑1,2,3,4‑tetrahydroquinoline‑6‑sulfonyl |
| 6g | 1‑ [4‑({ [(1E)‑(Dimethyl amino) methylene] amino} sulphonyl) benzoyl] indoline‑5‑sulphonyl |
| 6h | 3‑ [4‑(sulfonyl) Phenyl] Propanoic Acid |
| 6i | 5‑(sulfonyl)‑3‑chlorobenzo [b] thiophene‑2‑carboxylic acid |
| 6j | 5‑(sulfonyl)‑3‑methyl‑1‑benzofuran‑2‑carboxylic acid |
| 6k | 6‑Chloropyridine‑3‑carbonyl |
Table 1: Structural Data Of The Synthesized Compounds 6a-K
Except indoline derivatives 6b and 6c, all other compounds have shown very good antibacterial and antifungal activities as shown in Table 2. In order to evaluate the role of sulfonamide group in antimicrobial activity of these benzimidazolone derivatives, carboxamide analogue (6k) of one of the potent compound 6-chloropyridyl derivative (6a) was synthesized and tested for antibacterial and antifungal activity. Insignificant activity of 6-chloropyridyl derivative (6k) against bacterial and fungal strains, suggest that sulfonamide group plays vital role in antimicrobial activity of the tested compounds along with phenoxy group and nitrogen of benzimidazolone. Phenylpropionic acid derivative (6h, MIC 100 μg/ml) has shown promising antituberculosis activity. Chloropyridyl, dansyl, sulfamoylbenzoyl indoline and benzothiophene derivatives (6a, 6e, 6g and 6i) did exhibit low to moderate antituberculosis activity as shown in Table 3.
| Comp. No. | Concentration in µg/ml | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. niger | A. clavatus | E. coli | P. aeruginosa | S. aureus | |||||||||||||||||||
| 25 | 50 | 100 | 250 | 25 | 50 | 100 | 250 | 25 | 50 | 100 | 250 | 25 | 50 | 100 | 250 | 25 | 50 | 100 | 250 | ||||
| Ampicillin | 15 | 16 | 19 | 20 | 15 | 15 | 18 | 20 | 14 | 16 | 18 | 19 | |||||||||||
| Ciprofloxacin | 23 | 28 | 28 | 28 | 23 | 24 | 26 | 27 | 19 | 21 | 21 | 22 | |||||||||||
| Norfloxacin | 25 | 26 | 27 | 29 | 19 | 21 | 23 | 23 | 19 | 20 | 21 | 21 | |||||||||||
| Griseofulvin | 23 | 25 | 25 | 28 | 21 | 22 | 22 | 24 | |||||||||||||||
| Nystatin | 19 | 24 | 29 | 29 | 21 | 24 | 25 | 26 | |||||||||||||||
| 6a | 13 | 16 | 18 | 20 | 13 | 17 | 18 | 21 | 17 | 18 | 18 | 19 | 12 | 13 | 15 | 17 | 11 | 15 | 18 | 20 | |||
| 6b | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | 9 | ‑ | ‑ | ‑ | 10 | ‑ | ‑ | ‑ | 9 | |||
| 6c | ‑ | ‑ | ‑ | 9 | ‑ | ‑ | 9 | 10 | ‑ | ‑ | ‑ | 10 | ‑ | ‑ | ‑ | 9 | ‑ | ‑ | ‑ | 10 | |||
| 6d | 14 | 16 | 19 | 21 | 15 | 16 | 19 | 20 | 11 | 12 | 14 | 16 | 14 | 17 | 18 | 20 | 13 | 17 | 19 | 23 | |||
| 6e | 15 | 18 | 21 | 22 | 14 | 17 | 22 | 22 | 12 | 12 | 14 | 15 | 10 | 11 | 14 | 16 | 12 | 14 | 15 | 17 | |||
| 6f | 14 | 17 | 20 | 23 | 15 | 18 | 21 | 23 | 11 | 13 | 15 | 16 | 11 | 15 | 18 | 21 | 10 | 14 | 16 | 19 | |||
| 6g | 15 | 18 | 21 | 22 | 14 | 18 | 19 | 20 | 15 | 15 | 18 | 26 | 12 | 15 | 20 | 23 | 13 | 17 | 18 | 22 | |||
| 6h | 14 | 17 | 22 | 22 | 13 | 16 | 20 | 22 | 15 | 16 | 18 | 22 | 15 | 16 | 17 | 20 | 10 | 14 | 17 | 19 | |||
| 6i | 12 | 15 | 19 | 19 | 13 | 15 | 19 | 21 | 12 | 13 | 16 | 17 | 11 | 11 | 14 | 16 | 12 | 15 | 17 | 19 | |||
| 6j | 15 | 18 | 20 | 20 | 15 | 19 | 20 | 21 | 15 | 17 | 19 | 24 | 12 | 15 | 18 | 20 | 12 | 14 | 17 | 20 | |||
| 6k | ‑ | ‑ | 10 | 11 | ‑ | ‑ | 9 | 13 | ‑ | ‑ | ‑ | 13 | ‑ | ‑ | ‑ | 11 | ‑ | ‑ | ‑ | 12 | |||
Zone of inhibition (mm) excluding well size 6 mm
Table 2: Antifungal And Antibacterial Activity Of The Benzimidazolones
| Compound | MIC in μg/ml |
|---|---|
| Streptomycin | 4 |
| Isoniazid | 0.2 |
| Rifampicin | 40 |
| Ethambutol | 2 |
| 6a | 250 |
| 6b | ‑ |
| 6c | ‑ |
| 6d | 500 |
| 6e | 200 |
| 6f | 500 |
| 6g | 250 |
| 6h | 100 |
| 6i | 250 |
| 6j | 500 |
| 6k | >1000 |
| MIC: Minimum inhibitory concentration | |
Table 3: Antitubercular Activity Of The Benzimidazolones
In conclusion, a series of novel sulfonamide linked benzimidazolone derivatives were synthesized and subjected to various biological activities viz. antifungal, antituberculosis and antibacterial activity. Most of the compounds have shown very good antiinfective activity, which suggest that sulfonamide linked benzimidazolone derivatives are of very high therapeutic value and need to be explored for further studies.
References
- Chio LC, Bolyard LA, Nasr M, Queener SF. Identification of a Class of Sulfonamides Highly Active against Dihydropteroate Synthase from Toxoplasma gondii, Pneumocystis carinii, and Mycobacterium avium.Antimicrob Agents Chemother 1996;40:727-33.
- Starcevic K, Kralj M, Ester K, Sabol I, Grce M, Pavelic K, et al. Synthesis, antiviral and antitumor activity of 2-substituted-5-amidino-benzimidazoles. Bioorg Med Chem 2007;15:4419-26.
- O’Sullivan DG, Wallis AK. Antiviral benzimidazoles. Direct 1-substitution of 2-(.alpha.-hydroxybenzyl)benzimidazole and related compounds. J Med Chem 1972;15:103-4.
- Kazimierczuk Z, Upcroft JA, Uproft P, Gorska A, Starosciak B, Laudy A. Synthesis, antiprotozoal and antibacterial activity of nitro- and halogeno-substituted benzimidazole derivatives. ActaBiochim Pol 2002;49:185-95.
- Monforte AM, Rao A, Logoteta P, Ferro S, Luca LD, Barreca ML, et al. Novel N1-substituted 1,3-dihydro-2H-benzimidazol-2-ones aspotent non-nucleoside reverse transcriptase inhibitors. Bioorg Med Chem 2008;16:7429-35.
- Khalafi-Nezhad A, Soltani Rad MN, Mohabatkar H, Asrari Z, Hemmateenejad B. Design, synthesis, antibacterial and QSAR studies of benzimidazole and imidazole chloroaryloxyalkyl derivatives. Bioorg Med Chem 2005;13:1931-8.
- Rode MA, Rindhe SS, Karale BK. Synthesis and biological activities of some indoline derivatives. J Serb ChemSoc 2009;74:1377-87.