- Corresponding Author:
- B. Yilmaz
Department of Analytical Chemistry, Faculty of Pharmacy, Ataturk University, 25240 Erzurum, Turkey
E-mail: yilmazb@atauni.edu.tr
| Date of Submission | 16 July 2014 |
| Date of Revision | 22 January 2015 |
| Date of Acceptance | 26 July 2015 |
| Indian J Pharm Sci 2015;77(4):413-421 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The purpose of the present research investigation was to synthesis, characterisation of chitosan conjugates and its effect on drug permeation from transdermal rate controlling membrane. Chitosan conjugate was synthesised by conjugation with thioglycolic acid. The prepared chitosan conjugate was characterised by determining the charring point, Fourier transmission-infrared and differential scanning calorimetric analysis. The rate controlling membranes were prepared by various proportions of chitosan and chitosan conjugate, to moderate drug permeation through rate controlling membrane. The membrane moderated transdermal system consists of reservoir to hold the drug gel was prepared by 20% w/v ethylcellulose with a cavity in its center. An impermeable backing layer was prepared by 2% w/v ethylcellulose. Gel consists of carvedilol was prepared by sodium alginate and sodium carboxymethylcellulose in ethanol:water solvent system The rate controlling membranes prepared were evaluated by various parameters like thickness, folding endurance, swelling index, moisture content, moisture uptake, water vapor transmission rate, tensile strength test, measurement of gel strength, in vitro permeation study, ex vivo permeation study, compatibility study using differential scanning calorimetry and stability studies. All physical parameters evident that prepared membranes have good folding endurance and sufficient tensile strength. As the proportion of chitosan conjugate increases in membrane swelling index, moisture content, moisture uptake and permeability coefficient increases. The gel strength of chitosan conjugate was considerable less compared with chitosan.
Keywords
Etodolac, cyclic voltammetry, linear sweep voltammetry, square wave voltammetry, differential pulse voltammetry
Nonsteroidal antiinflammatory drugs (NSAIDs) are widely used for the treatment of several rheumatic and inflammatory diseases[1−3]. Etodolac (fig. 1), which is 2−[(1RS)−1,8−diethyl−1,3,4,9−tetrahydropyrano[3,4−b] indol−1−yl] acetic acid[4], is a chiral NSAID with a single stereogenic carbon atom andexists in two enantiomeric forms; R and S. It is marketed as racemate and used as analgesic and for the treatment of the signs and symptoms of rheumatoid arthritis and osteoarthritis[5]. Etodolac decreases the synthesis of peripheral prostaglandins involved in mediating inflammation by inhibition of the cycloxygenase (COX) enzyme[5].
The drug is a selective inhibitor for COX−2 enzyme and it has been reported that its selectivity towards COX−2 enzyme is 10 times that of COX−1 enzyme[6]. It produces less gastrointestinal toxicity compared to the other NSAIDs. The racemate drug is rapidly absorbed after oral administration and is highly bound to plasma protein (99.3%)[7]. It is metabolized in the liver to inactive metabolites that are primarily eliminated via the renal route. Etodolac is administered orally for patients with rheumatoid arthritis at doses of 200−400 mg given twice daily in the form of tablets or capsules. The drug enantiomers possess virtually identical physical and chemical properties[8]. On the other hand, the pharmacological properties of the two enantiomers differ profoundly in which the S−form is responsible for the anti−inflammatory activity[8].
Most of the chiral NSAIDs mainly undergo in vivo unidirectional inversion mechanism; mediated by hepatic enzymes; from the inactive R−form to the active S−enantiomer[9]. Etodolac does not show in vivo inversion of its enantiomers[10]. Since enantiomers of racemate drugs exhibit pronounced pharmacokinetic differences, resulting from stereoselective pharmacokinetic processes such as absorption, distribution, metabolism and excretion, it should be noted that the degree of enantioselectivity in pharmacokinetics is markedly species dependent and the data cannot be transposed between species[11]. In view of these differences, studies of the pharmacokinetics of chiral NSAIDs should always measure and quantify the separate enantiomers and not “total drug”. The total drug concept fails to recognize that each enantiomer is a separate drug and when products are licensed as the racemic mixture they are products containing two drugs.
Several methods have been reported for the enantioselective determination of etodolac in pharmaceutical preparations and biological fluids. These methods included capillary electrophoresis[12,13], capillary electrochromatography[14], gas chromatography[15−17] and high performance liquid chromatography[18−25] methods.
An extensive literature survey revealed that there are several chromatographic methods for the determination of etodolac in blood plasma, whereas, there are few other literatures disclosed only for the quantitative determination of etodolac in pharmaceutical formulation samples. The reported methods were influenced by interference of endogenous substances and potential loss of drugs in the re−extraction procedure and involving lengthy, tedious and time−consuming plasma sample preparation and extraction processes and requiring a sophisticated and expensive instrumentation.
The development of a new method capable of determining drug amount in pharmaceutical dosage forms is important. Electroanalytical techniques have been used for the determination of a wide range of drug compounds with the advantages that there are, in most, instances no need for derivatization and that these techniques are less sensitive to matrix effects than other analytical techniques. Additionally, application of electrochemistry includes the determination of electrode mechanism. Redox properties of drugs can give insights into their metabolic fate or their in vivo redox processes or pharmacological activity[26−29]. Despite the analytical importance of the electrochemical behavior and oxidation mechanism of etodolac, no report has been published on the voltammetric study of the electrochemical oxidation of etodolac in nonaqueous media. It is well known that the experimental and instrumental parameters directly affect the electrochemical process and voltammetric response of drugs. Consequently, it would be interest to investigate the oxidation process of etodolac in aprotic media. Therefore, the goal of this work was the development of new linear sweep voltammetry (LSV), square wave voltammetry (SWV) and differential pulse voltammetry (DPV) methods for the direct determination of etodolac in pharmaceutical preparations without any time−consuming extraction or evaporation steps prior to drug assay. This paper describes fully validated simple, rapid, selective and sensitive procedures for the determination of etodolac employing LSV, SWV and DPV methods the platinum disc electrode. Besides, the methods were successfully applied for the quality control of three commercial etodolac tablets form to quantify the drug and to check the formulation content uniformity.
Materials and Methods
Etodolac was obtained from Sigma (St. Louis, MO, USA). Acetonitrile (Fluka for HPLC analysis) was purified by drying with calcium hyride, followed by distillation from phosphorus pentoxide. After purification in order to eliminate its water content as much as possible, it was kept over molecular sieves. Lithium perchlorate (LiClO4) was purchased from Fluka and used as received without further purification. Etol, Tadolak and Etodin tablets (200 mg etodolac) were obtained from pharmacy (Erzurum, Turkey).
Electrochemical instrumentation
Electrochemical experiments were performed on a Gamry Potentiostat Interface 1000 controlled with software PHE 200 and PV 220. All measurements were carried out in a single−compartment electrochemical cell with a standard three−electrode arrangement. A platinum disk with an area of 0.72 cm2 and a platinum wire were used as the working and the counter electrodes, respectively. The working electrode was successively polished with 1.0, 0.3 and 0.05 μm alumina slurries (Buehler) on microcloth pads (Buehler). After each polishing, the electrode was washed with water and sonicated for 10 min in acetonitrile. Then, it was immersed into a hot piranha solution (3:1, H2SO4, 30% H2O2) for 10 min, and rinsed copiously with water. All potentials were reported versus Ag/AgCl/ KCl (3.0 M) reference electrode (BAS Model MF−2078) at room temperature. The electrolyte solutions were degassed with purified nitrogen for 10 min before each experiment and bubbled with nitrogen during the experiment. Operating conditions for SWV were pulse amplitude 25 mV, frequency 10 Hz, potential step 4 mV; and for DPV were pulse amplitude 50 mV, pulse width 50 ms, scan rate 100 mV/s.
Preparation of the standard and quality control solutions
The stock standard solution of etodolac was prepared in 0.1 M LiClO4/acetonitrile to a concentration of 100 μg/ml. Working standard solutions were prepared from the stock solution. Standard solutions were prepared as 2.5−50 μg/ml for LSV, SWV and DPV. The quality control (QC) solutions were prepared by adding aliquots of standard working solution of etodolac to final concentrations of 7.5, 25 and 45 μg/ml for LSV, SWV and DPV.
Procedure for pharmaceutical preparations
A total 10 tablets of etodolac (Etol, Tadolak or Etodin) was accurately weighed and powdered. An amount of this powder corresponding to one tablet etodolac content was weighed and accurately transferred into 100 ml calibrated flask and 50 ml of 0.1 M LiClO4/acetonitrile was added and then the flask was sonicated to 10 min at room tempature. The flask was filled to volume with 0.1 M LiClO4/acetonitrile. The resulting solutions in both the cases were filtered through Whatman filter paper no 42 and suitably diluted to get final concentration within the limits of linearity for the respective proposed methods. The drug content of etodolac tablets were calculated from the current potential curves.
Results and Discussion
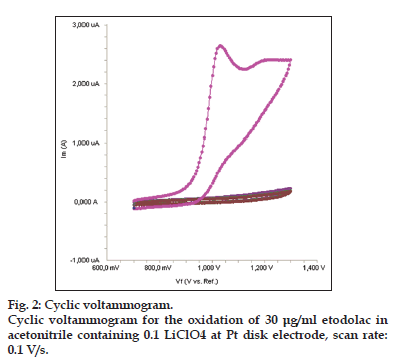
The electrochemical behavior of etodolac was investigated at the Pt disc electrode in acetonitrile solution containing 0.1 M LiClO4 as the supporting electrolyte by using cyclic voltammetry (CV). Fig. 2 shows a typical cyclic voltammogram of 30 μg/ml etodolac recorded under these conditions for the scan rate of 0.1 V/s. In the anodic sweep, an oxidation peak is seen at about potential of 1.03 V. Upon reversing the potential scan, no reduction peak corresponding to this oxidation wave is observed, indicating the irreversible nature of the electrode reactions.
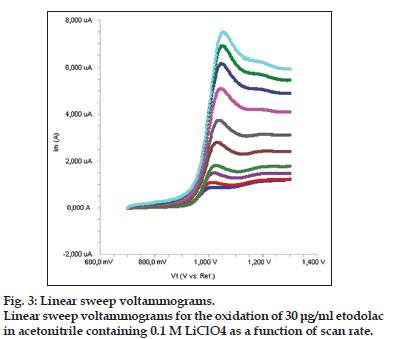
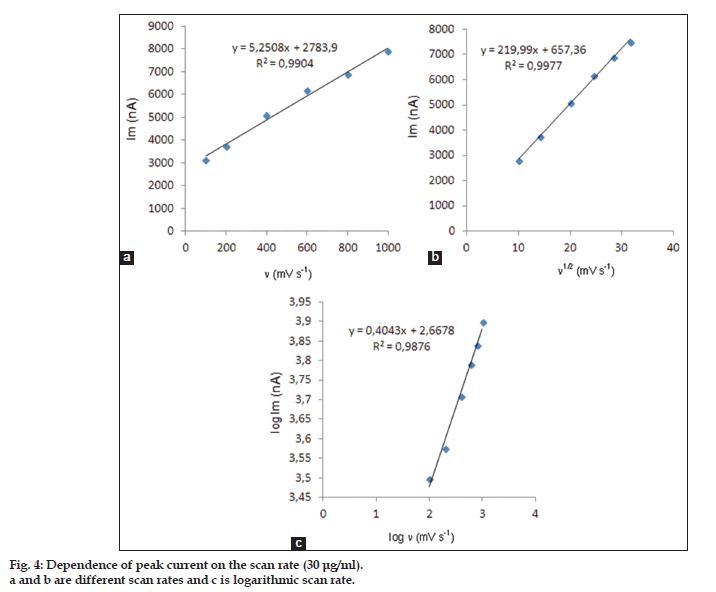
In order to gain a deeper insight into the voltammetric waves, the effect of scan rate on the anodic peak currents (Im) and peak potentials (Ep) was studied in the range of 0.01−1 V/s of the potential scan rates in acetonitrile solution containing 30 μg/ml concentration of etodolac (fig. 3). The representative linear sweep voltammograms obtained at Pt electrode for 30 μg/ml etodolac as a function of the scan rate are presented in fig. 4. Scan rate dependency experiments show that the peak currents for peak vary linearly with the scan rate (ν) (fig. 4a and b), which points out the adsorption−controlled process. However, the plots of logarithm of peak currents versus logarithm of scan rates for 30 μg/ml concentration of etodolac display straight lines with 0.4043 slope (fig. 4c), which are close to theoretical value of 0.5 expected for an ideal diffusion−controlled electrode process[30]. log Im−log ν curve is more eligible for this aim, therefore, a diffusional process for peak should be considered. These results suggest that the redox species are diffusing freely from solution and not precipitating onto the electrode surface. The reason for this behavior may be due to the solubility of the intermediate species in acetonitrile or poor adherence of products on the electrode surface.
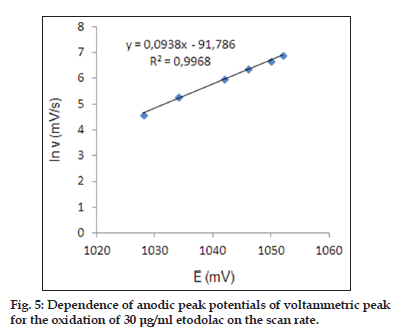
As shown in fig. 3, the oxidation peak potential (Epa) for peaks shift toward more positive values with increasing scan rate. The relationship between the peak potential and scan rate is described by the following equation[31],

and from the variation of peak potential with scan rate αna can be determined, where α is the transfer coefficient and na is the number of electrons transferred in the rate determining step. According to this equation, the plots of the peak potentials versus ln ν for oxidation peak show linear relationship (fig. 5). The slope indicate the value of αna is 0.61 for peak. Also, this value obtained indicate the total irreversibility of the electron transfer processes. This result show that the chemical step is a fast following reaction coupled to a charge transfer.
The validation was carried out by establishing specificity, linearity, accuracy, precision, limit of detection (LOD), limit of quantification (LOQ), recovery, ruggedness according to ICH Q2B recommendations[32,33].
The effects of common excipients and additives were tested for their possible interferences in the assay of etodolac. The simulated and placebo samples were prepared and analyzed. It has not been determined any interference of these substances at the levels found in dosage forms. Excipient that was used in this preparation was the most commonly used by the pharmaceutical industry. The presence of titanium dioxide, sodium chloride, talc, lactose, starch, and magnesium stearate did not appear interfere in the results of the analysis.
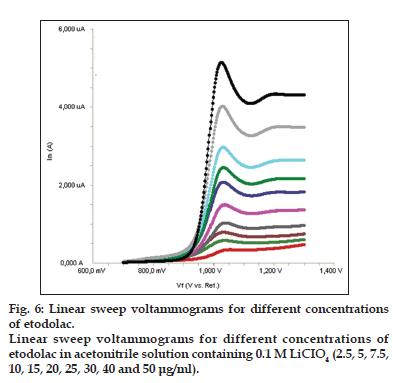
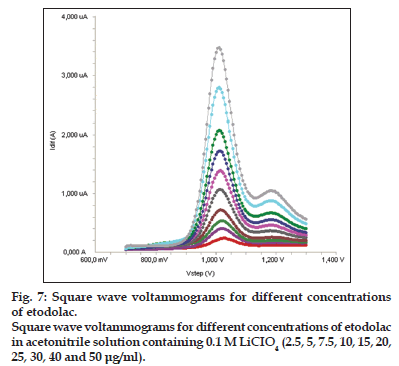
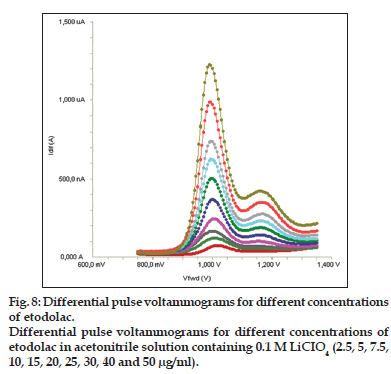
Standard solutions were prepared as 2.5−50 μg/ml (2.5, 5, 7.5, 10, 15, 20, 25, 30, 40 and 50 μg/ml) for LSV, SWV and DPV (figs. 6−8). Calibration curves were constructed for etodolac standard by plotting the concentration of compound versus peak current responses. The calibration curves were evaluated by its correlation coefficients. The correlation coefficients (r) of all the calibration curves were consistently greater than 0.99. The linear regression equations were calculated by the least squares method using Microsoft Excel® program and summarized in Table 1.
Accuracy of the assay methods were determined for both intra−day and inter−day variations using the six times analysis of the quality control (QC) samples. Precision of the assay was determined by repeatability (intra−day) and intermediate precision (interday). Repeatability refers to the use of the analytical procedure within a laboratory over a short period of time that was evaluated by assaying the QC samples during the same day. Intermediate precision was assessed by comparing the assays on different days (2 days). The intra−day accuracy ranged from 0.37 to 1.85% and precision from 1.58 to 4.69% (Table 2). The results obtained from intermediate precision (inter−day) also indicated a good method precision. All the values were within the acceptance criteria of 4.69%.
The Limits of detection (LOD) and quantification (LOQ) of etodolac by the proposed methods were determined using calibration standards. LOD and LOQ values were calculated as 3.3 σ/S and 10 σ/S, respectively, where S is the slope of the calibration curve and σ is the standard deviation of y−intercept of regression equation (n=6)[33]. The LOD and LOQ values of the methods were summarized in Table 1.
| Method | Range | LRa | R2 | LOD | LOQ |
|---|---|---|---|---|---|
| (µg/ml) | (µg/ml) | (µg/ml) | |||
| LSV | 2.5–50 | y=100.51x+25.207 | 0.9986 | 0.8 | 2.4 |
| SWV | 2.5–50 | y=68.52x+39.337 | 0.9997 | 0.4 | 1.2 |
| DPV | 2.5–50 | y=24.635x+0.7891 | 0.9994 | 0.5 | 1.5 |
Table 1: Linearity Of Etodolac.
| Method | Added | Intra-day | Inter-day | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (µg/ml) | Found±SDa | Precision | Accuracyc | Found±SDa | Precision | Accuracyc | |||
| RSD (%)b | RSD (%)b | ||||||||
| LSV | 7.5 | 7.45±0.10 | 1.41 | -0.67 | 7.38±0.12 | 1.58 | -1.55 | ||
| 25 | 24.67±0.82 | 3.31 | -1.33 | 24.50±0.55 | 2.23 | -2.00 | |||
| 45 | 45.17±0.98 | 2.18 | 0.37 | 44.67±1.21 | 2.71 | -0.74 | |||
| SWV | 7.5 | 7.38±0.12 | 1.58 | -1.55 | 7.47±0.10 | 1.38 | -0.44 | ||
| 25 | 25.00±0.89 | 3.58 | 0.04 | 24.83±0.98 | 3.96 | -0.67 | |||
| 45 | 45.33±1.03 | 2.28 | 0.74 | 45.67±1.03 | 2.26 | 1.48 | |||
| DPV | 7.5 | 7.47±0.14 | 1.83 | -0.44 | 7.37±0.08 | 1.11 | -1.78 | ||
| 25 | 25.33±1.19 | 4.69 | 1.33 | 24.67±0.82 | 3.31 | -1.33 | |||
| 45 | 45.83±1.17 | 2.55 | 1.85 | 45.17±0.98 | 2.18 | 0.37 | |||
Table 2: Precision And Accuracy Of Etodolac.
| Pharmaceutical | Added | SWV | DPV | |||||
|---|---|---|---|---|---|---|---|---|
| preparation | (µg/ml) | Found±SD | Recovery (%) | RSDa (%) | Found±SD | Recovery (%) | RSDa (%) | |
| Etol (5 µg/ml) | 5 | 4.9±0.11 | 98.0 | 2.24 | 5.1±0.18 | 102.0 | 3.53 | |
| 15 | 14.9±0.23 | 99.3 | 1.54 | 14.8±0.25 | 98.7 | 1.69 | ||
| 35 | 35.1±0.93 | 100.3 | 2.65 | 35.2±1.67 | 100.6 | 4.74 | ||
| Tadolak (5 µg/ml) | 5 | 4.8±0.20 | 96.0 | 4.17 | 4.9±0.13 | 98.0 | 2.65 | |
| 15 | 14.5±0.29 | 96.7 | 2.00 | 14.8±0.27 | 98.7 | 1.82 | ||
| 35 | 35.6±1.12 | 101.7 | 3.14 | 35.6±1.02 | 101.7 | 2.87 | ||
| Etodin (5 µg/ml) | 5 | 5.1±0.22 | 102.0 | 4.31 | 5.2±0.21 | 104.0 | 4.04 | |
| 15 | 14.7±0.34 | 98.0 | 2.31 | 14.6±0.28 | 97.3 | 1.92 | ||
| 35 | 35.9±1.24 | 102.6 | 3.45 | 35.4±0.73 | 101.1 | 2.06 | ||
Table 3: Recovery Of Etodolac In Pharmaceutical Preparations
To determine the accuracy of the LSV, SWV and DPV methods and to study the interference of formulation additives, the recovery was checked as three different concentration levels. Analytical recovery experiments were performed by adding known amount of pure drugs to pre−analyzed samples of commercial tablet forms. The recovery values were calculated by comparing concentration obtained from the spiked samples with actual added concentrations. These values are also listed in Table 3.
In this study, the LSV, SWV and DPV determination of etodolac were carried out by a different analyst in same instrument with the same standard (Table 4). The results showed no statistical differences between different operators suggesting that the developed method was rugged.
To evaluate the stability of etodolac, standard solutions were prepared separately at concentrations covering the low, medium and higher ranges of calibration curve for different temperature and times. These solutions were stored at room temperature, refrigeratory (4°) and frozen (−20°) temperature for 24 h and 72 h. Stability measurements were carried out with LSV, SWV and DPV methods. The results were evaluated comparing these measurements with those of standards and expressed as percentage deviation and etodolac was found as stable at room temperature, 4° and − 20° for at least 72 h. The stability of etodolac was obtained within the acceptance range of 90−110%.
LSV, SWV and DPV voltammetry methods were applied for the determination of the commercial tablets (Table 3). The results show that high reliability and reproducibility of three methods. The best results were statistically compared using the F−test. At 95% confidence level, the calculated F−values do not exceed the theoretical values (Table 5). Therefore, there are no significant difference between LSV, SWV and DPV voltammetry methods.
The proposed methods were applied to the direct determination of etodolac in tablet dosage forms, using the calibration straight line without sample preparation and after an adequate dilution. The drug was recently included in United States Pharmacopoeia[34]. Etodolac tablets were also determined with the official procedure, which involves a high−performance liquid chromatographic method[34]. Table 5 compares the results of the analysis of etodolac between the proposed and official methods. All methods showed similar accuracy and precision. According to the Student’s t−test, the calculated t values did not exceed the theoretical value for a significance level of 0.05. Statistical analysis of the results showed no significant difference between the performance of the official and proposed methods as regards to accuracy and precision.
On the other hand, the voltammetric assay is simple and rapid compared with the high performance liquid chromatographic assay. Moreover, the accuracy of the proposed methods was also evaluated by recovery studies after adding known amounts of the pure drug to various preanalysed formulations of etodolac and applying the procedure specified under experimental section. As Table 5 shows, good results demonstrate the validity of the proposed methods for the determination of etodolac in commercial dosage forms.
| Method | Added | Found (µg/ml) | Recovery | RSDa |
|---|---|---|---|---|
| (µg/ml) | mean±SD | (%) | (%) | |
| LSV | 5 | 4.9±0.13 | 98.0 | 2.65 |
| 15 | 14.8±0.27 | 98.7 | 1.82 | |
| 35 | 35.4±0.73 | 101.1 | 2.06 | |
| SWV | 5 | 5.1±0.18 | 102.0 | 3.53 |
| 15 | 14.8±0.25 | 98.7 | 1.69 | |
| 35 | 35.2±1.67 | 100.6 | 4.74 | |
| DPV | 5 | 5.2±0.21 | 104.0 | 4.04 |
| 15 | 14.6±0.28 | 97.3 | 1.92 | |
| 35 | 35.6±1.02 | 101.7 | 2.87 |
Table 4: The Results Of Analyses Of Etodolac By A Different Analyst.
| Parameters | LSV | SWV | DPV | Official method |
|---|---|---|---|---|
| Mean (recovery %) | 99.8 | 101.7 | 100.2 | 99.7 |
| SD | 2.66 | 2.49 | 1.81 | |
| RSD (%) | 2.67 | 2.45 | 1.81 | 0.78 |
| Variance | 3.20 | 5.95 | 7.08 | |
| F-test | 3.18 | 3.78 | 4.02 |
Table 5: Comparison Of The Proposed And Reported Methods For Determination Of Etodolac.
The electrochemical behavior of etodolac has been studied in nonaqueous media by CV, LSV, SWV and DPV voltammetry methods. It has concluded that there is a completely diffusion−controlled current process which isn’t affected by adsorption phenomenon. Besides, in the present report, a simple, rapid, sensitive, reliable, specific, accurate and precise LSV, SWV and DPV methods for the determination of etodolac in pharmaceutical preparations were developed and validated. The method described has been effectively and efficiently used to analyze etodolac pharmaceutical tablets without any interference from the pharmaceutical excipients. The voltammetric run time of 3 min allows the analysis of a large number of samples in a short period of time. Therefore, the methods can be used effectively without separation for routine analysis of etodolac in pure form and its formulations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Bruke A, Smyth E, Fitgerald GA. Analgesic-antipyretic agents; pharmacotherapy of gout. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2006. p. 671-715.
- Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18 820 patients. BMJ 2004;329:15-9.
- Magill P, Ridgway PF, Conlon KC, Neary P. A case of probable ibuprofen-induced acute pancreatitis. JOP 2006;7:311-4.
- Her Mayesty’s Stationary Office. The British Pharmacopoeia. London, UK: Her Mayesty’s Stationary Office; 2014.
- Craig CR, Stitzel RE, editors. Modern Pharmacology with Clinical Applications. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2004.
- Glaser K, Sung ML, O’Neill K, Belfast M, Hartman D, Carlson R, et al.Etodolac selectively inhibits human prostaglandin G/H synthase 2 (PGHS-2) versus human PGHS-1. Eur J Pharmacol 1995;281:107-11.
- Lee HS, Kang IM, Lee HW, Seo JH, Ryu JH, Choi SJ, et al. Development and validation of a high performance liquid chromatography-tandem mass spectrometry for the determination of etodolac in human plasma. J Chromatogr B AnalytTechnol Biomed Life Sci 2008;863:158-62.
- Boni J, Korth-Bradley J, McGoldrick K, Appel A, Cooper S. Pharmacokinetic and pharmacodynamic action of etodolac in patients after oral surgery. J ClinPharmacol 1999;39:729-37.
- Jamali F, Lovlin R, Aberg G. Bi-directional chiral inversion of ketoprofen in CD-1 mice. Chirality 1997;9:29-31.
- Young JW, Gray NM, Wechter WJ. Antipyretic and analgesic methods and compositions containing optically pure R-etodolac. US Patent, 5561151; 1996.
- Lees P, Taylor PM, Landoni FM, Arifah AK, Waters C. Ketoprofen in the cat: Pharmacodynamics and chiral pharmacokinetics. Vet J 2003;165:21-35.
- dePablos RR, García-Ruiz C, Crego AL, Marina ML. Separation of etodolac enantiomers by capillary electrophoresis. Validation and application of the chiral method to the analysis of commercial formulations. Electrophoresis 2005;26:1106-13.
- Dilek DA, Özlem BK, Muzaffer T, Hassan YA. Capillary electrophoretic method for the determination of etodolac in pharmaceutical tablet formulation. J LiqChromatogrRelatTechnol 2010;24:773-80.
- Fanali S, Catarcini P, Presutti C. Enantiomeric separation of acidic compounds of pharmaceutical interest by capillary electrochromatography employing glycopeptide antibiotic stationary phases. J Chromatogr A 2003;994:227-32.
- Singh NN, Jamali F, Pasutto FM, Coutts RT, Russell AS. Stereoselective gas chromatographic analysis of etodolac enantiomers in human plasma and urine. J Chromatogr 1986;382:331-7.
- Srinivas NR, Shyu WC, Barbhaiya RH. Gas chromatographic determination of enantiomers as diastereomers following pre column derivatization and applications to pharmacokinetic studies: A review. Biomed Chromatogr 1995;9:1-9.
- Giachetti C, Assandri A, Zanolo G, Brembilla E. Gas chromatography-mass spectrometry determination of etodolac in human plasma following single epicutaneous administration. Biomed Chromatogr 1994;8:180-3.
- Caccamese S. Direct high-performance liquid chromatography (HPLC) separation of etodolac enantiomers using chiral stationary phases. Chirality 1993;5:164-7.
- 19.Ali I, Aboul-Enein HY. Enantioseparation of some clinically used drugs by HPLC using cellulose Tris (3,5-dichlorophenylcarbamate) chiral stationary phase. Biomed Chromatogr 2003;17:113-7.
- Ghanem A, Aboul-Enein MN, El-Azzouny A, El-Behairy MF. Lipase-mediated enantioselective kinetic resolution of racemic acidic drugs in non-standard organic solvents: Direct chiral liquid chromatography monitoring and accurate determination of the enantiomeric excesses. J Chromatogr A 2010;1217:1063-74.
- Jin YX, Tang YH, Zeng S. Analysis of flurbiprofen, ketoprofen and etodolac enantiomers by pre-column derivatization RP-HPLC and application to drug-protein binding in human plasma. J Pharm Biomed Anal 2008;46:953-8.
- Guo CC, Tang YH, Hu HH, Yu LS, Jiang HD, Zeng S. Analysis of chiral nonsteroidalantiinflammatory drugs flurbiprofen, ketoprofen and etodolac binding with HSA. Pharm Anal 2011;1:184-90.
- Jamali F, Mehvar R, Lemko C, Eradiri O. Application of a stereospecific high-performance liquid chromatography assay to a pharmacokinetic study of etodolac enantiomers in humans. J Pharm Sci 1988;77:963-6.
- Higashi T, Kawasaki M, Tadokoro H, Ogawa S, Tsutsui H, Fukushima T, et al.Derivatization of chiral carboxylic acids with (S)-anabasine for increasing detectability and enantiomeric separation in LC/ESI-MS/MS. J Sep Sci 2012;35:2840-6.
- 25. Ogawa S, Tadokoro H, Sato M, Hanawa T, Higashi T. (S)-1-(4-Dimethylaminophenylcarbonyl)-3-aminopyrrolidine: A derivatization reagent for enantiomeric separation and sensitive detection of chiral carboxylic acids by LC/ESI-MS/MS. J Chromatogr B AnalytTechnol Biomed Life Sci 2013;940:7-14.
- El-Hefnawey GB, El-Hallag IS, Ghoneim EM, Ghoneim MM. Voltammetric behavior and quantification of the sedative-hypnotic drug chlordiazepoxide in bulk form, pharmaceutical formulation and human serum at a mercury electrode. J Pharm Biomed Anal 2004;34:75-86.
- Corti P, Corbini G, Gratteri P, Furlanetto S, Pinzauti S. Determination of some quinolones in tablets, human plasma and urine by differential-pulse polarography. Int J Pharm 1994;111:83-7.
- Radi A, Elmogy T. Differential pulse voltammetric determination of carvedilol in tablets dosage form using glassy carbon electrode. Farmaco 2005;60:43-6.
- Dogan B, Ozkan SA. Electrochemical behavior of carvedilol and its adsorptive stripping determination in dosage forms and biological fluids. Electroanalysis 2005;17:2074-83.
- Laviron E, Roullier L, Degrand C. A multilayer model for the study of space distributed redox modified electrodes: Part II. Theory and application of linear potential sweep voltammetry for a simple reaction. J ElectroanalChem 1980;112:11-23.
- Yilmaz B, Ekinci D. Voltammetric behavior of carvedilol in non-aqueous media and its analytical determination in pharmaceutical preparations. Rev Anal Chem 2011;30:187-93.