- *Corresponding Author:
- Y. Murti
Institute of Pharmaceutical Research, GLA University, N.H.#2, Mathura-Delhi Road, P.O. Chaumuhan, Mathura-281 406, India
E-mail: ymurti@gmail.com
| Date of Submission | 20 May 2013 |
| Date of Revision | 22 January 2014 |
| Date of Acceptance | 27 January 2014 |
| Indian J Pharm Sci 2014;76(2):163-166 |
Abstract
A few flavanones were synthesised by cyclisation of corresponding 3-(heteroaryl)-1(2-hydroxyphenyl) prop-2-en-1-one with sodium acetate in alcohol-water and evaluated for activity. Synthesised compounds were assayed for their in vitro anticancer activity against three human cancer cell lines, mammary adenocarcinoma (MCF7), human colon adenocarcinoma (HT29) and human kidney adenocarcinoma (A498) using sulforhodamine B dye. Results indicated that most of the compounds exhibited significant in vitro anticancer potential. Among them, compound having furan ring showed most potent activity against all the tested cell lines.
Keywords
Flavanone, chalcone, anticancer activity, SRB dye, MCF7, HT29, A498
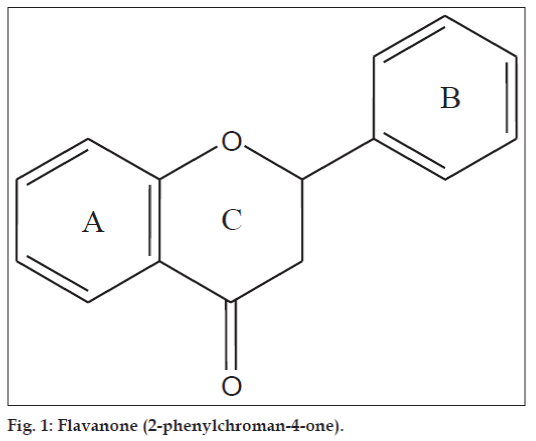
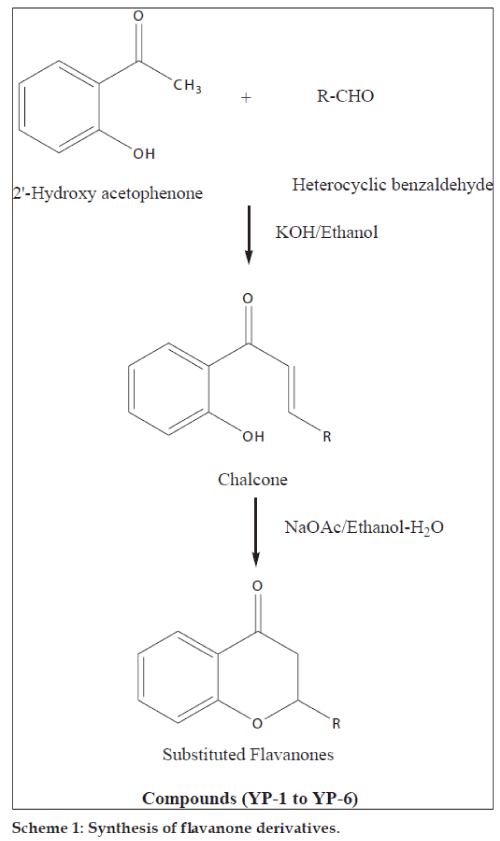
Flavanones (2-phenylchroman-4-ones) are a class of natural product that show extensive biological activities with low toxicity. Compounds with promising activity can be used as leads for synthesis of novel compounds with high efficacy and low side effects to improve drug therapy. Synthetic flavanones have attracted considerable attention because of their various pharmacological properties including antifungal [1,2], antibacterial [1,3,4], analgesic [4], antioxidant [4], vasorelaxant [5], AChE inhibitory [6] and antiVSMCs vegetation [7] activity. Flavanones have been a potential source in the search for lead compounds so investigation of synthetic routes and chemical modification is a new direction in flavanone research. Flavanones have been reported as a potent anticancer agent [8-14] also, but there were very few literature that reported the bioactivity of heteroaryl derivatives, which prompted us to synthesise a novel series of flavanones to investigate the effect by changing the B ring (fig. 1) to a heterocycle ring on in vitro anticancer activity. We herein, report the synthesis and evaluation of flavanones. The first step was Claisen–Schmidt reaction between heterocyclic benzaldehyde and 2-hydroxy acetophenone to form 2-hydroxy chalcone derivatives, which on intramolecular addition reaction, gave flavanone derivatives as shown in Scheme 1. The structures of target compounds are described in Scheme 2.
All the chemicals used for the synthesis of the compounds were obtained from Merck Ltd., Mumbai and SD Fine-Chem Ltd. Mumbai. Melting points were determined in open capillaries and are uncorrected. The compounds were analysed for elemental analysis. IR spectra were recorded on an FTIR spectrometer (Perkin Elmer) using KBr disc method. 1H NMR spectra were recorded on 1H NMR (Brucker AMx300 MHz) spectrometer in CDCl3.
General procedure for the synthesis of title compounds used was as follows: a mixture of the 2-hydroxy acetophenone (0.01 mol, 1.36 g) and corresponding heterocyclic aldehyde (0.01 mol) was taken in ethanol (30 ml) and stirred at 10-15°. To this solution, an aqueous solution of sodium hydroxide (40%, 5.0 ml) was added drop wise with continuous stirring. The mixture was kept overnight at room temperature and then it was poured into crushed ice and acidified with diluted hydrochloric acid. The chalcone precipitated as solid. The precipitated chalcone was collected and recrystallised from ethanol.
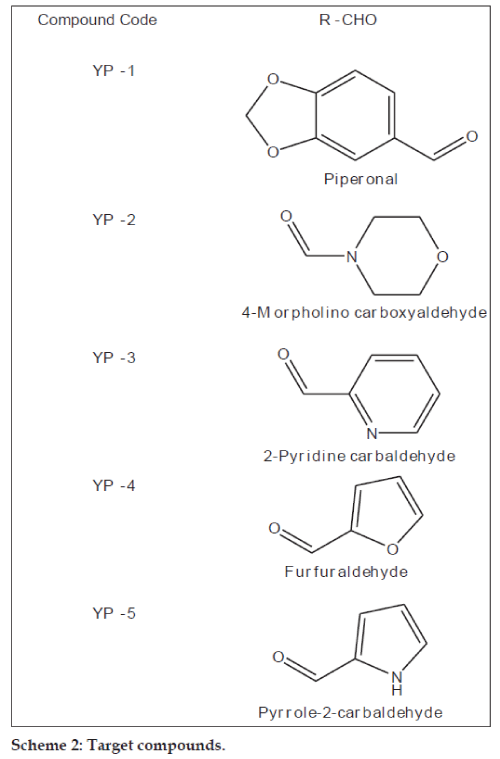
The synthesised chalcones (0.1 mmol) and sodium acetate (163 mg, 1.99 mmol) was added to a solution of ethyl alcohol (2.2 ml) and water (0.8 ml), the mixture was heated to reflux for 24 h, the solvent was evaporated under vacuum, 10 ml water was added to the residue and extracted with ethyl acetate (10 ml×3), the combined organic layer was washed with 2 N sodium hydroxide (brine), and dried over anhydrous sodium sulphate. The solvent was removed under reduced pressure and the residue was purified by silica gel column chromatography (petroleum ether:EtOAc; 4:1) to afford compounds YP-1 to YP-6. The purity of the synthesised compounds was established by TLC and melting point. The physical data of the compounds is listed in Table 1. The structures of the synthesised compounds YP-1 to YP-6 were characterised by IR and 1H NMR and the spectral data are summarised below.
| Code no. | Molecular formula (molecular weight) | % yield | M.P.(º) | Rf Value (CHCl3:EtOAc; 2:1) | Elemental analysis (%) calcd. (found) | ||
|---|---|---|---|---|---|---|---|
| C | H | N/S | |||||
| YP‑1 | C16H12O4 (268.26) | 39 | 131–132 | 0.68 | 71.64 (71.65) | 4.51 (4.50) | ‑ |
| YP‑2 | C13H15NO3 (233.26) | 36 | 170–172 | 0.66 | 66.94 (66.92) | 6.48 (6.50) | 6.00 (6.02) |
| YP‑3 | C14H11NO2 (225.24) | 48 | 147–148 | 0.73 | 74.65 (74.62) | 4.92 (4.91) | 6.22 (6.20) |
| YP‑4 | C13H10O3 (214.22) | 51 | 150–152 | 0.65 | 72.89 (72.90) | 4.71 (4.73) | ‑ |
| YP‑5 | C13H11NO2 (213.23) | 56 | 156–157 | 0.77 | 73.23 (73.25) | 5.20 (5.22) | 6.57 (6.56) |
| YP‑6 | C13H10O2S (230.28) | 43 | 120–122 | 0.73 | 67.80 (67.78) | 4.38 (4.40) | 13.92 (13.93) |
Table 1: Physical characterisation data of synthesised flavanones (yp‑1 to yp‑6)
Compound YP-1; FTIR (KBr, cm-1): 3010 (Ar=C-H str.), 1646 (C=O str.), 1589 (C-C str.), 1516, 1462, 1419 (Ar C=C str.), 1193 (C-O-C str.); 1H NMR (CDCl3, δ ppm): 3.43, 3.17 (d, 2H, methylene), 5.54 (t, 1H, methine), 5.88 (s, 2H, methylene dioxy), 6.54–7.80 (m, 7H, Ar-H). Compound YP-2; FTIR (KBr, cm-1): 3014 (Ar=C-H str.), 1686 (C=O str.), 1581 (C-C str.), 1519, 1460, 1415 (Ar C=C str.), 1239 (C-N str.), 1190 (C-O-C str.); 1H NMR (CDCl3, δ ppm): 2.35 (m, 4H, morpholine), 3.15, 2.93 (d, 2H, methylene), 3.65 (m, 4H, morpholine), 5.35 (t, 1H, methine), 6.83–7.53 (m, 4H, Ar-H).
Compound YP-3; FTIR (KBr, cm-1): 3065 (Ar=C-H str.), 1658 (C=O str.), 1600 (C-C str.), 1540 (C=N str.), 1559, 1458, 1420 (Ar C=C str.), 1196 (C-O-C str.); 1H NMR (CDCl3, δ ppm): 3.17, 2.90 (d, 2H, methylene), 5.52 (t, 1H, methine), 6.90–8.61 (m, 8H, Ar-H). Compound YP-4; FTIR (KBr, cm-1): 3012 (Ar=C-H str.), 1667 (C=O str.), 1598 (C-C str.), 1567, 1485, 1443 (Ar C=C str.), 1178 (C-O-C str.); 1H NMR (CDCl3, δ ppm): 3.18, 3.13, (d, 2H, methylene), 5.49 (t, 1H, methine), 6.19–7.38 (m, 7H, Ar-H).
Compound YP-5; FTIR (KBr, cm-1): 3117 (Ar=C-H str.), 1670 (C=O str.), 1612 (C-C str.), 1576, 1456, 1426 (Ar C=C str.), 1242 (C-N str.), 1167 (C-O-C str.); 1H NMR (CDCl3, δ ppm): 3.33, 3.09 (d, 2H, methylene), 5.10 (d, 1H, pyrrole N-H), 5.13 (t, 1H, methine), 5.72–6.36 (m, 3H, pyrrole), 6.90-7.75 (m, 4H, Ar-H). Compound YP-6; FTIR (KBr, cm-1): 3038 (Ar=C-H str.), 2846 (C-H str.), 1669 (C=O str.), 1587 (C-C str.), 1553, 1460, 1432 (Ar C=C str.), 1117 (C-O-C str.), 644 (C-S str.); 1H NMR (CDCl3, δ ppm): 3.38, 3.13 (d, 2H, methylene), 5.51 (t, 1H, methine), 6.62–7.79 (m, 7H, Ar-H).
The in vitro anticancer activity of synthesised flavanones was tested against human mammary adenocarcinoma (MCF7), human colon adenocarcinoma (HT29) and human kidney adenocarcinoma (A498) using sulforhodamine B dye [15]. 2-Phenyl chroman-4-one, a kind of flavanone, was tested together as a positive control in the assay. The in vitro anticancer activity results are summarised in Table 2. In accordance with the data from in vitro anticancer activity, all the synthesised heteroaryl flavanone derivatives have shown mild to good activity compared with that of positive control 2-phenyl chroman-4-one. The activity data indicate that the compound YP-4 with furan ring (B ring) had the strongest activity against MCF7, HT29 and A498 with IC50 values of 7.3, 4.9 and 5.7 μg/ml, respectively.
| Compound code | Cell lines (IC50, µg/ml)* | ||
|---|---|---|---|
| MCF7 | HT29 | A498 | |
| YP‑1 | >50 | >50 | >25 |
| YP‑2 | 10.2 (±0.4) | 6.7 (±2.3) | 8.1 (±0.7) |
| YP‑3 | 19.6 (±1.2) | 16.6 (±2.5) | 14.5 (±3.6) |
| YP‑4 | 7.3 (±0.3) | 4.9 (±0.5) | 5.7 (±0.9) |
| YP‑5 | 20.1 (±0.2) | 18.9 (±1.2) | 17.3 (±7.9) |
| YP‑6 | 12.6 (±1.5) | 7.8 (±1.4) | 9.4 (±1.4) |
| 2‑phenyl chroman‑4‑one | >25 | 28.3 (±0.6) | 20.7 (±0.6) |
*Values are mean of three experiments, standard deviation is given in parentheses
Table 2: in vitroanticancer activity ofsynthesised flavanones yp‑1 to yp‑6
In conclusion, a few number of new heterocycle containing flavanone derivatives were synthesised and studied for in vitro anticancer activity, and a furan ring containing analogue YP-4 had highest anticancer potency compared with those of other flavanones. These results suggested that furan ring may be crucial for anticancer activity and contribute to further understanding the critical molecular requirements that lead to anticancer property in the flavanones series. On the basis of the above findings, these flavanones scaffolds can be selected as skeleton for the development of heterocycle containing flavanones with potential as anticancer drugs.
References
- Fowler ZL, Shah K, Panepinto JC, Jacobs A, Koffas MA. Development of non-natural flavanones as antimicrobial agents. PLoS One 2011;6:1-5.
- Ali TE. Synthesis and fungicidal activity of some new 4H-chromen-4-ones containing some 1,3-thiazole, 1,3-thiazine, 1,2,4-triazole and 1,2,4-triazine moieties. Phosphorus Sulfur Silicon Relat Elem 2007;182:1717-21.
- Ahmed SK, Parveen A. A novel synthesis and antimicrobial activity of flavanone using environmental friendly catalyst H[bimBF4]. Res J Pharm BiolChemSci 2010;1:809-15.
- Joseph L, George M, Kassaye G. One pot method for the synthesis of arylideneflavanones and some of its activities. Afr J ClinExpMicrobiol 2008;9:147-51.
- Dong X, Liu Y, Yan J, Jiang C, Chen J, Liu T, et al. Identification ofSVM-based classification model, synthesis and evaluation of prenylatedflavanoids as vasorelaxant agents. Bioorg Med Chem2008;16:8151-60.
- Sheng R, Lin X, Zhang J, Chol KS, Huang W, Yang B, et al. Design,synthesis and evaluation of flavonoid derivatives as potent AChEinhibitors. Bioorg Med Chem 2009;17:6692-8.
- Lei S, Xiu-e F, Wen-han L, Lian-hua F, Guan-hua DU, Qing-shan L. Synthesis of new flavanone derivatives of farrerol and preliminary SAR studies on anti-VSMCs vegetation activity. Chem Res Chin Univ 2011;27:237-40.
- Choi EJ, Lee JI, Kim GH. Effects of 4’,7-dimethoxyflavanone on cell cycle arrest and apoptosis in human breast cancer MCF-7 cells. Arch Pharm Res 2011;34:2125-30.
- Lie S, Feng XE, Cui JR, Fang LH, Du GH, Li QS. synthesis and biological activity of flavanone derivatives. Bioorg Med ChemLett2010;20:5466-8.
- Poerwono H, Sasaki S, Hattori Y, Higashiyama K. Efficient microwave-assisted prenylation of pinostrobin and biological evaluation of its derivatives as antitumor agents. Bioorg Med ChemLett2010;20:2086-9.
- Ying H, Hu Y, He Q, Li R, Yang B. Synthesis and anticancer activity of a novel class of flavanoids: 2,4-Diarylchromane[4,3-d]-1,9b-1,2,3-thiadiazolines. Eur J Med Chem 2007;42:226-34.
- Shen S, Ko CH, Tseng S, Tsai S, Chen Y. Structurally related antitumor effects of flavanonesin vitroandin vivo: Involvement ofcaspase 3 activation, p21 gene expression, and reactive oxygen species production. ToxicolApplPharmacol 2004;197:84-95.
- Pouget C, Fagnere C, Basly J, Habrioux G, Chulia A. New aromatase inhibitors. Synthesis and inhibitory activity of pyridinyl-substituted flavanone derivatives. Bioorg Med ChemLett 2002;12:1059-61.
- Pouget C, Lauthier F, Simon A, Fagnere C, Basly J, Delage C, et al.Flavonoids: Structural requirements for antiproliferative activity on breast cancer cells. Bioorg Med ChemLett 2001;11:3095-7.
- Shah BA, Kumar A, Gupta P, Sharma M, Sethi VK, Saxena AK, et al. Cytotoxic and apoptotic activities of novel amino analogues ofboswellic acids. Bioorg Med ChemLett 2007;17:6411-6.