- Corresponding Author:
- Gupta N
Department of Pharmaceutical Chemistry, Delhi Institute of Pharmaceutical Sciences and Research (DIPSAR), Sector-3, Pushp Vihar, New Delhi-110 017, India
E-mail: namitagupta12@gmail.com
| Date of Submission | 16 October 2010 |
| Date of Revision | 03 November 2011 |
| Date of Acceptance | 09 November 2011 |
| Indian J Pharm Sci, 2011, 73 (6): 674-678 |
Abstract
A series of N-substituted imidazole derivatives was synthesized. Imidazole nucleus was reacted with ethylchloroacetate to form imidazole ester. Reaction of the imidazole ester (I) with different amines yields the desired products (1a- 1e). The compounds were characterized by FT-IR, 1H-NMR and mass spectra. The synthesized compounds were evaluated for the antimicrobial activity against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Candida albicans and Aspergillus niger by determination of MIC (minimum inhibitory concentration) using tube dilution method. Compound (1b) was found to be the most active antimicrobial compound amongst others in the series.
Keywords
1H-NMR, FT-IR, imidazole, minimum inhibitory concentration, tube dilution method
Development of drug resistance in microorganisms is a tussle between science and nature. An increasing public health problem is disease-causing microbes that have become resistant to antibiotic drug therapy. The current antimicrobial therapy is increasingly compromised by the emergence and spread of bacteria resistant to commonly used antimicrobial agents [1].
There is growing evidence of high levels of antibiotic resistance among important pathogens, including vancomycin resistant enterococci (VRE), methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-intermediate/resistant S. aureus (VISA/ VRSA), and multidrug-resistant (MDR) Pseudomonas aeruginosa, which have emerged in the past 20 years [2]. Since 2000 for the treatment of Grampositive infections only few new classes have been introduced; these are the oxazolidinones (linezolid), cyclic lipopeptides (daptomycin), and glycylcyclines (tigecycline) [2].
Various nuclei which have been studied for antimicrobial activity are benzothiazepine [3,4], triazole [5,6], benzoxazole [7,8], indazole [9,10], quinazoline[11,12], pyrazole [13,14], imidazole [15-19], benzimidazole [20-24] etc.
As healthcare providers we have a responsibility to acknowledge the issue of increasing resistance and to develop strategies for combating this continuing challenge to the management and treatment of the infectious diseases. Thus, in the present study we aim to synthesize a new chemical class of heterocyclic compounds to overcome the problem of microbial resistance.
The incorporation of imidazole nucleus is an important strategy in drug discovery. The high therapeutic properties of the imidazole related drugs have encouraged the medicinal chemists to synthesize a large number of novel chemotherapeutic agents based on imidazole nucleus[19].
The melting points were taken in open glass capillary tubes using Lab India visual melting point apparatus and are uncorrected. IR spectra of compounds were recorded on Jasco-410 FTIR spectrometer. 1H-NMR spectra of compounds were recorded on Bruker 300 MHz NMR spectrophotometer using CDCl3 as a solvent and TMS (tetramethylsilane) as an internal standard. Mass spectra of compounds were recorded on Waters micromass Q-Tof micro spectrometer. The purity of the compounds was checked by thin layer chromatography using silica gel G as adsorbent and spots were visualized by exposure to iodine vapours.
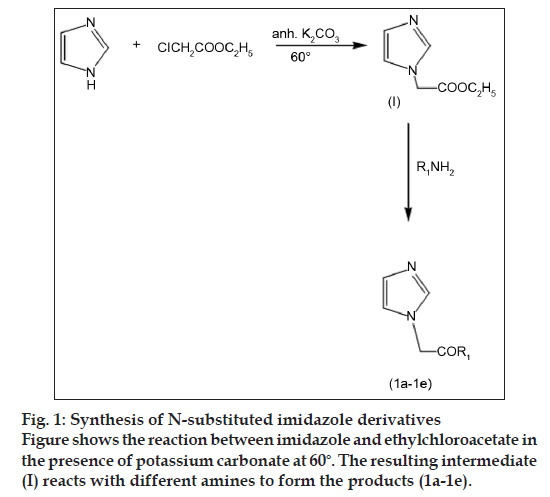
Synthesis of ethyl 1H-imidazol-1-yl acetate I: Ethylchloroacetate (0.075 mol) was added to a solution of imidazole (0.05 mol) in dry acetone (50 ml). To the mixture anhydrous K2CO3 (0.05 mol) was added and the reaction mixture was refluxed till the starting material disappeared. Acetone was evaporated in vacuo and the residue extracted with carbon tetrachloride. Organic layer was separated, dried over sodium sulphate and solvent evaporated to obtain semi liquid product. The product obtained was used for next step without further purification, yield 82%.
General procedure for the synthesis of compounds 1a-1e: The mixture of compound I (0.02 mol) and desired amine (0.03 mol) was heated till the reactants disappeared. The reaction mixture was extracted with chloroform. Organic layer was separated, dried over sodium sulphate and solvent evaporated to obtain solid product which was recrystallized using chloroform/hexane.
N-benzyl-2-(1H-imidazol-1-yl)acetamide (1a). The following spectral data were recorded for the compound 1a: IR (KBr cm-1): 3212 (N-H str.), 3030 (Ar C-H str.), 2940 (C-H str.), 1683 (C=O str.), 1597 (C=N str.). 1H-NMR (CDCl3): 7.462 (s, -N=CH-, 1H), 7.333-7.348 (t, -NH-, 1H), 7.183-7.294 (m, Ar-H, 5H), 6.952-7.076 (d, imidazole-H, 2H), 4.672 (s, -N-CH2-, 2H), 4.412-4.431 (d, -CH2-, 2H). MS-ES: 216 (M+ + 1).
N-cyclohexyl-2-(1H-imidazol-1-yl)acetamide (1b). The following spectral data were recorded for the compound 1b: IR (KBr cm-1): 3297 (N-H str.), 2854 (C-H str.), 1654 (C=O str.), 1550 (C=N str.). 1H-NMR (CDCl3): 7.537 (s, -N=CH- , 1H), 7.179- 7.269 (d, -NH-, 1H), 6.964-7.179 (d, imidazole-H, 2H), 4.635 (s, -N-CH2-, 2H), 3.729- 3.827 (m, -NH-CH, 1H), 1.164-1.854 (m, -CH2-, 10H). MS-ES: 208 (M++1).
2-(1H-imidazol-1-yl)-N-(naphthalen-1-ylmethyl) acetamide (1c). The following spectral data were recorded for the compound 1c: IR (KBr cm-1): 3279 (N-H str.), 3041 (Ar C-H str.), 2926 (C-H str.), 1650 (C=O str.), 1556 (C=N str.). 1H-NMR (CDCl3): 7.875-7.916 (t, -NH-, 1H), 7.261-7.572 (m, Ar-H, 7H), 7.484 (s, -N=CH-, 1H), 6.892-7.080 (d, imidazole-H, 2H), 4.889-4.908 (d, -CH2-, 2H), 4.702 (s, -N-CH2-, 2H). MS-ES: 266 (M++1).
2-(1H-imidazol-1-yl)-N-phenylacetamide (1d). The following spectral data were recorded for the compound 1d: IR (KBr cm-1): 3267 (N-H str.), 3080 (Ar C-H str.), 2926 (C-H str.), 1671 (C=O str.), 1544 (C=N str.). 1H-NMR (CDCl3): 7.644 (s, -NH-, 1H), 7.127-7.418 (m, Ar-H, 5H), 7.151 (s, -N=CH-, 1H), 7.035-7.072 (d, imidazole-H, 2H), 4.824 (s, -N-CH2-, 2H), 4.412-4.431 (d, -CH2-, 2H). MS-ES: 202 (M++1).
2-(1H-imidazol-1-yl)-1-(piperidin-1-yl)ethanone (1e). The following spectral data were recorded for the compound 1e: IR (KBr cm-1): 3127 (Imidazole C-H str.), 2853(C-H str.), 1643 (C=O str.), 1509 (C=N str.), 1255 (C-N str.). 1H-NMR (CDCl3): 7.522 (s, -N=CH-, 1H), 6.963-7.099 (d, imidazole-H, 2H), 4.768 (s, -N-CH2-, 2H), 3.391-3.579 (t, piperidine-H, 4H), 1.256-1.908 (m, piperidine-H, 6H). MS-ES: 194 (M+ + 1).
The antimicrobial activity was performed against Gram-positive bacteria: S. aureus, B. subtilis; Gramnegative bacteria: E. coli, P. aeruginosa and fungal strains: C. albicans and A. niger. The standard and test samples were dissolved in DMSO (dimethyl sulphoxide) to give a concentration of 100 μg/ ml. The minimum inhibitory concentration (MIC) was determined by tube dilution method. Two fold dilutions of test and standard compounds were prepared in double strength nutrient broth IP (bacteria) or Sabouraud dextrose broth IP (fungi). The samples were incubated at 37o (bacteria) for 24 h, 25o for 7 days (A. niger) and 37o for 48 h (C. albicans), respectively, and the results were recorded in terms of MIC (the lowest concentration of test substance which inhibited the growth of microorganism). The procedure was repeated twice.
A series of amides of imidazole were synthesized by reaction of ester of imidazole (fig. 1) with corresponding amines. The ester of imidazole was prepared by reacting it with ethylchloroacetate in the presence of anhydrous potassium carbonate. The physicochemical characteristics of synthesized derivatives are given in the Table 1. Compounds were synthesized in moderate to good yield. Purity of the compounds was determined by TLC on silica gel G plates. The spots were detected by exposure to iodine vapours. Synthesized compounds were characterized by spectral analysis (FT-IR, 1H-NMR and Mass spectra). The spectra were found to be in agreement with the assigned molecular structure.
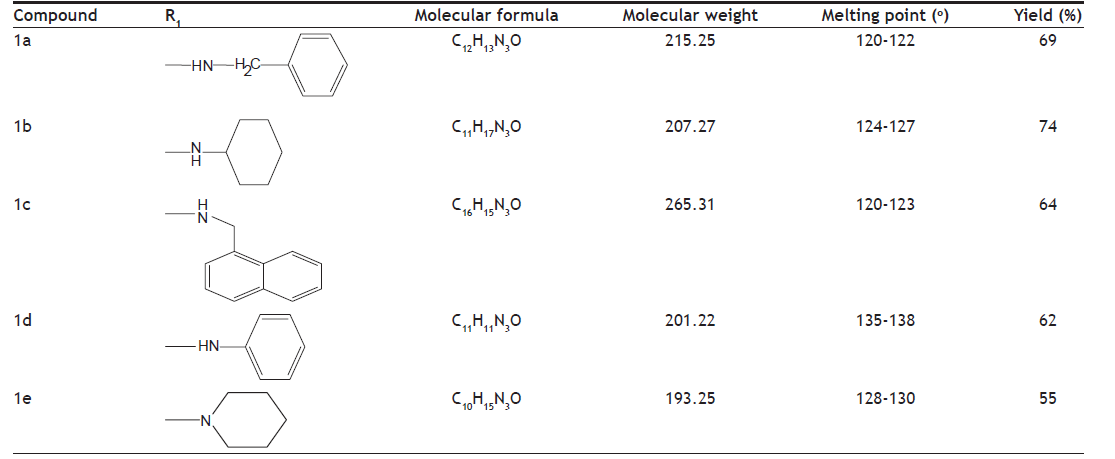
Table shows the properties like structure of the substituent present, molecular formula, molecular weight, melting point and their percentage yield
Table 1: Physicochemical Properties Of The Compounds.
The synthesized compounds (1a-1e) were evaluated for in vitro antimicrobial activity against Staphylococcus aureus, Bacillus subtilis (Gram positive); Escherichia coli, Pseudomonas aeruginosa (Gram negative) and Candida albicans and Aspergillus niger by tube dilution method. MIC (mg/ml) was the concurrent reading obtained from the experiment which was done thrice and is given in Table 2. N-cyclohexyl-2-(1H-imidazol-1-yl)acetamide (1b) was found to be more active antibacterial compound than other compounds.
| Compound | E. | P. | S. | B. | C. | A. |
| coli | aeruginosa | aureus | subtilis | albicans | niger | |
| 1a | 25 | 50 | 25 | 25 | 25 | 25 |
| 1b | 50 | 50 | 12.5 | 12.5 | 25 | 25 |
| 1c | 25 | 50 | 25 | 25 | 50 | 50 |
| 1d | 50 | 25 | 25 | 25 | 25 | 25 |
| 1e | 50 | 25 | 25 | 12.5 | 25 | 25 |
| Std | 0.78a | 0.78a | 0.78a | 0.78a | 1.56b | 1.56b |
Minimum inhibitory concentration of the synthesized compounds against Staphylococcus aureus, Bacillus subtilis (Gram positive); Escherichia coli, Pseudomonas aeruginosa(Gram negative) and Candida albicansandAspergillusnigerusing standards as a: Ciprofloxacin; b: Fluconazole
Table 2: Mic (µG/Ml) Of The Synthesized compounds
Acknowledgements
The authors would like to sincerely thank Arbro Pharmaceuticals ltd.; Department of Chemistry, Indian Institute of Technology, Delhi and Sophisticated Analytical Instrumentation Facility, Panjab University, Chandigarh for providing spectral data; Vice President, Pharma Research, Ranbaxy Research Laboratories, for providing gift samples of ciprofloxacin and fluconazole for activity purpose and Director, Institute of Microbial Technology, Chandigarh, for providing bacterial and fungal strains.
References
- Rice LB. Unmet medical needs in antibacterial therapy. BiochemPharmacol 2006;71:991-5.
- Song JH. What’s new on the antimicrobial horizon? Int J Antimicrob Agents 2008;32 Suppl4:S207-13.
- Wang L, Zhang P, Zhang X, Zhang Y, Li Y, Wang Y. Synthesis and biological evaluation of a novel series of 1,5-benzothiazepine derivatives as potential antimicrobial agents. Eur J Med Chem 2009;44:2815-21.
- Zhang P, Wang LZ, Wu HS, Lan JM, Li Y, Wang YX. The synthesis and biological evaluation of a series of novel 2-COOC 2H5/COONa substituted 1,5-benzothiazepine derivatives as antimicrobial agents. Chin ChemLett 2009;20:660-2.
- Sztanke K, Tuzimski T, Rzymowska J, Pasternak K, Kandefer-Szerszen M.Synthesis, determination of the lipophilicity, anticancer and antimicrobial properties of some fused 1,2,4-triazole derivatives. Eur J Med Chem 2008;43:404-19.
- Turan-Zitouni G, Kaplancikli ZA, Yildiz MA, Chevallet P, Kaya D.Synthesis and antimicrobial activity of 4-phenyl/cyclohexyl-5-(1-phenoxyethyl)-3-[N-(2-thiazolyl)acetamido]thio-4H-1,2,4-triazole derivatives. Eur J Med Chem 2005;40:607-13.
- Ertan T, Yildiz I, Tekiner-Gulbas B, Bolelli K, Temiz-Arpaci O, Ozkan S, et al. Synthesis, biological evaluation and 2D-QSAR analysis of benzoxazoles as antimicrobial agents. Eur J Med Chem 2009;44:501-10.
- Rida SM, Ashour FA, El-Hawash SA, ElSemary MM, Badr MH, Shalaby MA. Synthesis of some novel benzoxazole derivatives as anticancer, anti-HIV-1 and antimicrobial agents. Eur J Med Chem 2005;40:949-59.
- Schenone S, Bruno O, Ranise A, Brullo C, Bondavalli F, Filippelli W, et al. 2-Aryl-3-phenylamino-4,5-dihydro-2h-benz[g]indazoles with analgesic activity. II Farmaco 2003;58:845-9.
- Lorand T, Koscis B, Emody L, Sohar P. 2-Substituted indazoles: Synthesis and antimicrobial activity. Eur J Med Chem 1999;34:1009-18.
- Kuyper LF, Baccanari DP, Jones ML, Hunter RN, Tansik RL, Joyner SS, et al. High-affinity inhibitors of dihydrofolatereductase: antimicrobial and anticancer activities of 7,8-dialkyl-1,3-diaminopyrrolo[3,2-f] quinazolines with small molecular size. J Med Chem 1996;39: 892-903.
- Rohini R, Muralidhar Reddy P, Shanker K, Hu A, Ravinder V. Antimicrobial study of newly synthesized 6-substituted indolo[1,2-c] quinazolines. Eur J Med Chem 2010;45:1200-5.
- Bekhit AA, Abdel-Aziem T. Design, synthesis and biological evaluation of some pyrazole derivatives as antiinflammatory-antimicrobial agents. Bioorg Med Chem 2004;12:1935-45.
- Bekhit AA, Ashour HM, Abdel Ghany YS, BekhitAel-D, Baraka A. Synthesis and biological evaluation of some thiazolyl and thiadiazolyl derivatives of 1H-pyrazole as antiinflammatory antimicrobial agents. Eur J Med Chem 2008;43:456-63.
- Jain AK, Ravichandran V, Sisodiya M, Agrawal RK. Synthesis and antibacterial evaluation of 2-substituted-4,5-diphenyl-N-alkyl imidazole derivatives. Asian Pac J Trop Med 2010;471-4.
- Karakurt A, Ozalp M, Isık S, Stables JP, Dalkara S. Synthesis, anticonvulsant and antimicrobial activities of some new 2-acetylnaphthalene derivatives. Bioorg Med Chem 2010;18:2902-11.
- Sharma V, Khan MS. Synthesis of novel tetrahydroimidazole derivatives and studies for their biological properties. Eur J Med Chem 2001;36:651-8.
- Khabnadideh S, Rezaei Z, Khalafi-Nezhad A, Bahrinajafi R, Mohamadi R, Farrokhroz AA. Synthesis of N-Alkylated derivatives of imidazole as antibacterial agents. Bioorg Med ChemLett 2003;13: 2863-5.
- Sharma D, Narasimhan B, Kumar P, Judge V, Narang R, De Clercq E, et al. Synthesis, antimicrobial and antiviral evaluation of substituted imidazole Derivatives. Eur J Med Chem 2009;44:2347-53.
- Ansari KF, Lal C. Synthesis, physicochemical properties and antimicrobial activity of some new benzimidazole derivatives. Eur J Med Chem 2009;44:4028-33.
- Ansari KF, Lal C. Synthesis and evaluation of some new benzimidazole derivatives as potential antimicrobial agents. Eur J Med Chem 2009;44:2294-9.
- He Y, Wu B, Yang J, Robinson D, Risen L, Ranken R, et al. 2-Piperidin-4-yl-benzimidazoles with broad spectrum antibacterial activities. Bioorg Med ChemLett 2003;13:3253-6.
- Güven ÖÖ, Erdoğan T, Göker H, Yildiz S. Synthesis and antimicrobial activity of some novel phenyl and benzimidazole substituted benzyl ethers. Bioorg Med ChemLett 2007;17:2233-6.
- Sharma D, Narasimhan B, Kumar P, Jalbout A. Synthesis and QSAR evaluation of 2-(substituted phenyl)-1Hbenzimidazoles and [2-(substituted phenyl)-benzimidazol-1-yl]-pyridin-3-yl-methanones. Eur J Med Chem 2009;44:1119-27