- Corresponding Author:
- P. Panneerselvam
Department of Pharmaceutical Chemistry, C. L. Baid Mehta College of Pharmacy, Jyothi Nagar, Old Mahabaliupuram Road, Thoraipakkam, Chennai-600 096, India
E-mail: pps2k2000@yahoo.co.in
| Date of Submission | 4 September 2006 |
| Date of Revision | 18 March 2009 |
| Date of Acceptance | 31 July 2009 |
| Indian J Pharm Sci, 2009, 71 (4): 428-432 |
Abstract
In the present study, a novel series of 4-(2-aminophenyl)morpholines were synthesized and characterized by IR, 1 H-NMR, 13 C NMR and mass spectral analysis. The synthesized compounds were screened for analgesic (100 and 200 mg/kg), antiinflammatory (200 and 400 mg/kg), antibacterial (Bacillus subtilis, Bacillus cereus, Staphylococcus epidermidis, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa and Escherichia coli) and antifungal (Candida albicans and Aspergillus niger) activities. The minimum inhibitory concentrations of the compounds were also ascertained by agar streak dilution method. N-benzylidine-2-morpholoino benzenamine (1) and N-(3-nitro benzylidine)-2-morpholino benzenamine (3) exhibited significant analgesic, antiinflammatory and antimicrobial activities.
Keywords
Morpholine, analgesic, antiinflammatory, antibacterial, antifungal
4-Phenyl morpholine derivatives have been reported to possess antiinflammatory[1-3] antimicrobial[4,5] and central nervous system activities[6,7]. Schiff bases have been reported to possess biological properties[8,9] apart from antimicrobial activities[10-13]. In our previous work[14], it was observed that substitution of 4-phenyl morpholine to quinazoline moiety results in potent analgesic, antiinflammatory, and antimicrobial activities. Linezolide also possess a 4-pheny1 morpholine substitution. Moreover morpholine as a substitution in many heterocyclic moieties has been reported to possess analgesic, antiinflammatory[14], central nervous system and antimicrobial activities[15]. In addition, in general nitro/chloro/furan substituted compounds have been reported to possess various biological activities.
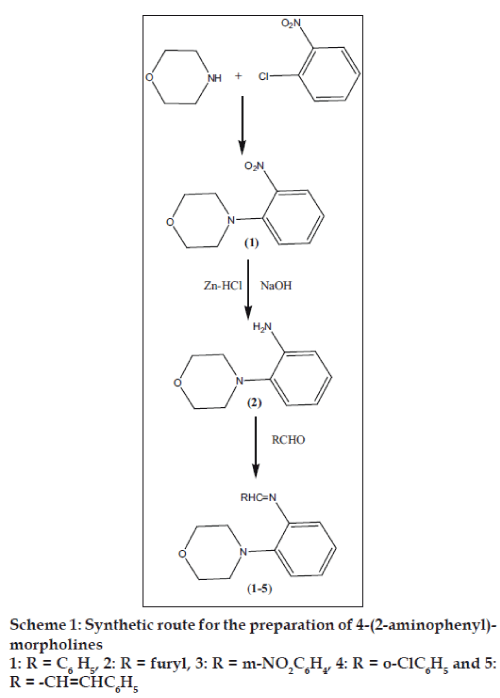
In continuation of our earlier work[14,15] on 2-methyl quiuazolin-4(3H)-ones we attempted to synthesize the new series of Schiff bases of 4-(2-aminophenyl)- morpholines and its characterization by IR, NMR and mass spectral analysis. The synthesized compounds were screened for analgesic activity by chemical writhing and Eddy’s hot plate method, antiinflammatory activity by paw edema method, anti-bacterial and anti-fungal activities. The minimum inhibitory concentrations of the compounds were also ascertained by agar dilution method.
Morpholine and 1-chloro-2-nitrobenzene was refluxed to yield 4-(2-nitrophenyl) morpholine, which was then reduced using zinc and hydrochloric acid affords 4-(2-aminophenyl) morpholine[16]. An equimolor mixture of 1.78 g (0.01 mol) of 4-(2-aminopheny1) morpholine and substituted aldehydes (aromatic/ heterocyclic) in absolute ethanol was refluxed using dean stark apparatus for 2 h (Scheme 1). The reaction mixture was cooled for a while and solid separated was filtered and recrystallised from ethanol (99%) yield the title compounds (1-5). The structural assignment of the products was based on their IR, 1H-NMR, 13C-NMR and mass spectral data.
IR (KBr) cm-1: 2950 (Ar-H), 2852 (N=CH), 1630 (C=N), 1503 (C=C), 1261 (C-N), 1114 (C-O-C), 848 (Ar-H), 1580 (N-O), 710 (C-Cl). 1H-NMR (CDCI3) δ: 8.12 (s, 1H: N=CH), 7.82-7.73 (m, 2H; 2”, 6”- H), 7.48-7.46 (m, 3H; 3”, 4”, 5”-H); 7.28-7.30 (d; J=8.9 Hz, 2H; 3’,5’-H), 6.97-6.99 (d, J=9.1 Hz , 2H; 4’,6’-H), 6.95-6.96 (d, J=6 Hz, 1H; CH=CH Ph), 3.89-3.92 (t, J=8.1 Hz, 4H, 2, 6-CH2), 3.23-3.25 (t, J=7.6 Hz, 4H; 3,5-CH2). 13C-NMR (CDC13) δ: 154.3, 139.7, 136.7, 131.3, 129.0, 128.2, 127.6, 127.3, 116.0, 66.9, 50.3. MS (E1) m/z: 266 (M+).
Melting points were determined in open capillary tubes and are uncorrected. IR spectra were recorded (in KBr) on an ABB Bomen MB-104. NMR spectra were recorded on 300 MHz-Bruker DPX200 using tetramethylsilane as internal standard. Mass spectra were recorded on a Shimadzu GC-MS QP5000. The purity of the synthesized compounds were checked by TLC using E-Merck TLC aluminum sheets silica gel 60F 254 (0.2 mm) using ethyl acetate:hexane (2:3) as eluent and visualized in an iodine chamber. All the chemicals used were of analytical grade. Unpaired student-t-test[17] was performed to ascertain the significance of the exhibited analgesic and antiinflammatory activities.
Swiss mice (15-25 g) and Wistar rats (150-200 g) were used for these studies[18]. They were kept in colony cages at 25±20, relative humidity of 45-55% under 12 h light and dark cycles. All the animals were acclimatized for a week before use. The animals were fed with standard animal feed and water ad libitum. The test compounds were administered orally using intragastric tube in the form of suspension using 5% Tween 80 as suspending agent. The experimental does was selected between the minimum effective dose and maximal non-lethal dose. All the animal experimentations were performed according to the protocols and recommendations of the Institutional Animal Ethics Committee. Unpaired student t-test was performed to ascertain the significance of the exhibited analgesic and antiinflammatory activities.
The analgesic activity[19] was determined by acetic acid-induced writhing method using Swiss mice (n=6) of either sex selected by random sampling technique was used for the study. Aspirin at a dose level of 100 mg/kg was administered as a standard drug for comparison. Test compounds at two dose levels (100 and 200 mg/kg) were administered orally 30 min prior to administration of the writhing agent (0.6% v/v aqueous acetic acid, 10 ml/kg). The writhing produced in the animal was observed for 30 min and percentage protection was calculated for analgesic activity. The results are presented in Table 1.
| Compounds | Dose (mg/kg) | Mean±SEM | % Protection |
|---|---|---|---|
| 1 | 100 | 34.33±1.616* | 50.6 |
| 200 | 23.00±1.252* | 66.9 | |
| 2 | 100 | 60.33±1.413* | 13.1 |
| 200 | 57.16±1.447* | 17.7 | |
| 3 | 100 | 38.66±1.781* | 44.3 |
| 200 | 27.83±1.839* | 59.9 | |
| 4 | 100 | 63.16±0.984* | 9.1 |
| 200 | 61.00±1.183* | 12.1 | |
| 5 | 100 | 66.5±2.152 | 4.3 |
| 200 | 63.16±1.559* | 9.1 | |
| Aspirin | 100 | 9.06±0.808 | 86.1 |
| Control (Aceticacid) | 0.1 ml / 10 g | 69.46 | - |
Table 1: Analgesic Activity of the Synthesized Compounds
The analgesic activity was also determined by Eddy’s hot plate method[20], using Swiss mice (n=6) of either sex selected by random sampling technique was used for the study. Pentazocine at the dose of 10 mg/kg (p.o.) was administered as standard drug for comparison. The test compounds at 2 dose levels (100 and 200 mg/kg) were administered orally. The temperature of the Eddy’s hot plate was maintained at 55±0.50. The animals for the experiment were chosen when the animal reaction (licking of the fore paws or jumping response) was within 10 s. After drug treatment every 30, 60, 90 and 120 min the reaction time was noted. The cutoff point of 30 s was observed to prevent the paw damage and data are presented in Table 2.
| Compounds | Dose (mg/kg) |
Pain reaction time (min) | ||||
|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | ||
| 1 | 100 | 3.2±0.31 | 5.2±0.47* | 11.3±0.58* | 11.1±0.63* | 10.8±0.49* |
| 200 | 3.4±0.36 | 6.4±0.53* | 14.1±0.68* | 14.3±0.72* | 13.9±0.63* | |
| 2 | 100 | 2.8±0.28 | 3.5±0.31 | 4.0±0.98 | 3.8±0.38 | 3.5±0.42 |
| 200 | 3.1±0.25 | 3.7±0.47 | 3.9±0.76 | 4.1±0.62 | 3.9±0.55 | |
| 3 | 100 | 3.3±0.35 | 7.9±0.51* | 9.4±0.54* | 9.1±0.41* | 8.7±0.47* |
| 200 | 3.4±0.37 | 8.7±0.57* | 12.1±0.78* | 11.5±0.71* | 11.1±0.61* | |
| 4 | 100 | 2.7±0.42 | 3.1±0.29 | 4.0±0.94 | 3.8±0.41 | 3.4±0.53 |
| 200 | 3.3±0.47 | 3.5±0.45 | 4.1±0.73 | 3.7±0.68 | 3.5±0.56 | |
| 5 | 100 | 3.2±0.39 | 3.4±0.5 | 3.9±0.57 | 3.5±0.54 | 3.6±0.47 |
| 200 | 3.5±0.49 | 3.3±0.48 | 4.2±0.63 | 3.3±0.57 | 3.4±0.52 | |
| Pentazocine | 10 | 3.3±0.47 | 9.4±0.89* | 12.1±0.62* | 14.9±0.71* | 14.6±0.59* |
Each value expressed as Mean±SEM (n=6) in the Eddy’s hot plate method using student- t-test followed by one way ANOVA and *P<0.05 compared to 0 min reaction time
Table 2: Analgesic Activity of the Synthesized Compounds
Antiinflammatory activity[21] was determined by carrageenan-induced paw edema method in Wistar rats (n=6) of either sex selected by random sampling technique. Indomethacin (20 mg/kg, p.o.) was administered as standard drug. The test compounds were administered at two dose levels (200 and 400 mg/kg) orally 30 min prior to the administration of carrageenan (0.1 ml) in the plantar region of the paw. The paw volumes were measured using plethysmograph at 1, 2, 3, 4 and 5 h after carrageenan administration. The results are presented in Table 3.
| Compound | Dose (mg/kg) | % Reduction of edema (h) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 1 | 200 | 16.7±0.07 | 29.0±0.03 | 38.2±0.04* | 21.2±0.06 | 18.2±0.03 |
| 400 | 22.3±0.04 | 34.2±0.06* | 47.3±0.07* | 49.4±0.02* | 43.4±0.03* | |
| 2 | 200 | 9.2±0.05 | 11.0±0.03 | 13.0±0.06 | 10.0±0.09 | 7.0±0.08 |
| 400 | 12.1±0.08 | 15.0±0.03 | 21.0±0.06 | 23.0±0.01 | 20.0±0.07 | |
| 3 | 200 | 19.9±0.05 | 28.2±0.09 | 45.6±0.07* | 39.2±0.06* | 21.3±0.02 |
| 400 | 25.3±0.07 | 31.3±0.04* | 52.3±0.09* | 46.7±0.04* | 28.4±0.08 | |
| 4 | 200 | 9.2±0.07 | 13.1±0.04 | 15.2±0.03 | 16.3±0.09 | 14.7±0.04 |
| 400 | 12.5±0.04 | 14.3±0.05 | 21.6±0.01 | 19.7±0.05 | 16.3±0.07 | |
| 5 | 200 | 11.2±0.09 | 17.3±0.03 | 24.7±0.07 | 15.4±0.04 | 11.5±0.05 |
| 400 | 17.2±0.05 | 21.4±0.07 | 26.4±0.08 | 28.1±0.02 | 20.5±0.07 | |
| Indomethacin | 20 | 31.3±0.06 | 64.5±0.01 | 79.4±0.04 | 80.1±0.03 | 81.2±0.02 |
Each value expressed as Mean±SEM (n=6) in the Eddy’s hot plate method using student- t-test followed by one way ANOVA and *P<0.05 compared to 0 min reaction time
Table 3: Antiinflammatory Activity of The Synthesized Compounds
Antibacterial activity[22] of the test compounds (10 μg/disc) were tested against B. subtilis, B. cereus, S. epidermidis, S. aureus K. pneumoniae and E. coil using nutrient agar medium (Hi-Media Laboratories, India). The antifungal activity of the compounds was tested against C. albicans and A. niger using Sabouraud dextrose agar medium (Hi-Media Laboratories, India). The sterilized (autoclaved at 1200 for 30 min) medium (40-500) was inoculated (1 ml/100 ml of medium) with the suspension (105 cfu/ ml) of the microorganism (matched of McFarland barium sulphated standard) and poured into a Petri dish to give a depth of 3-4 mm. The paper impregnated with the test compounds was placed on the solidified medium. The plates were pre-incubated for 1 h at room temperature and incubated at 370 for 24 h and 48 h for antibacterial and antifungal activity respectively. Ciprofloxacin (10 μg/disc) and ketoconazole (10 μg/disc) was used as standard for antibacterial and antifungal activity, respectively. The observed zone of inhibition is presented in Table 4.
Minimum inhibitory concentration (MIC)[23] of the test compounds were determined by agar dilution method. A stock solution of the test compounds (10 μg/ ml) in dimethylformamide was prepared and graded quantities of the test compounds were incorporated in specified quantity of molten sterile agar (nutrient agar for antibacterial activity and Sabouraud dextrose agar medium for antifungal activity). A specified quantity of the medium (40-500) containing the compound was poured into a Petri dish to give a depth of 3-4 mm and allowed to solidify. The microorganisms were then streaked on agar plate and the plates were incubated at 370 for 24 and 48 h for bacteria and fungi, respectively. The MIC was considered to be the lowest concentration of the test substance exhibiting no growth of bacteria or fungi. The observed MIC values are presented in Table 4.
| Compounds | In vitro Activity Zone of inhibition in mm (MIC in µg/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| S. aureus | S. epidermidis | B. cereus | B. subtilis | P. aureus | K. pneumonia | E. coli | A. niger | C. albicans | |
| 1 | 21 | 14 | 21 | 19 | 20 | 19 | 16 | 19 | 23 |
| (5.5) | (6.5) | (4.5) | (5.5) | (4.5) | (6) | (7) | (5.5) | (4.5) | |
| 2 | 18 | 17 | 14 | 17 | 16 | 13 | 14 | 28 | 21 |
| (6.5) | (5.5) | (7) | (6.5) | (5.5) | (7.5) | (7.5) | (3.5) | (5.5) | |
| 3 | 20 | 18 | 20 | 18 | 22 | 21 | 19 | 21 | 22 |
| (5) | (4) | (5.5) | (5.5) | (3.5) | (4.5) | (5.5) | (6) | (5.5) | |
| 4 | 19 | 16 | 15 | 16 | 15 | 16 | 17 | 19 | 20 |
| (6) | (5) | (6.5) | (7.5) | (6) | (5.5) | (6.5) | (6.5) | (6) | |
| 5 | 15 | 13 | 19 | 14 | 17 | 18 | 13 | 22 | 21 |
| (7.5) | (7.5) | (6) | (8) | (7) | (5) | (7) | (6) | (4.5) | |
| Ciprofloxacin | 29 | 22 | 28 | 27 | 26 | 25 | 30 | - | - |
| (10 µg/disc) | |||||||||
| Ketoconazole | - | - | - | - | - | - | - | 28 | 26 |
| (10 µg/disc) | |||||||||
Table 4: Antimicrobial Activity of The Synthesized Compounds
All the spectral data was consistent with the assigned structure of the compounds. The 1H-NMR spectral data of 1 consisted of one singlet (imine proton, N=CH), two doublets (2’-H, 6’-H with J=9.1 Hz; 3’-H, 5’-H with J = 8.9 Hz), two triplets (morpholino protons with J=7.6 and J=8.1 Hz) and multiplet (phenyl protons) peaks. The spectral data of 2 consisted of two doublet (5”-H, with J=7.2 Hz; 3’- 5’-H with 8.7 Hz) two triplet (morpholino protons with 7.8 Hz) and a multiplet (phenyl protons) peaks. The spectral data of 3 consisted of one singlet (2”-H), two doublets (3’,5’-H with 8.9 Hz; 2’,6’-H with 8.9 Hz) two triplet (morpholino protons with 7.7 Hz) and multiplet (phenyl protons) peaks. The spectral data of 4 consisted of a doublet, two triplet and multiplet (phenyl protons) peaks. The spectral data of 5 consisted of two doublets (-N=CH-CH with J=4.2 Hz, -CH=CH-ph, with J=6.0 Hz), triplets and multiplet (phenyl protons) peaks.
The 13C-NMR spectral data of all the compounds consistently exhibited peaks for the common functionalities present in the series of compounds such as C=N, morpholine and phenyl side chain carbons. The peak corresponding to C=N in 1, 2, 3, 4 and 5 was 154.3, 148.3, 159.9, 159.6 and 164.2, respectively. The peak corresponding to morpholino carbons representations in 1, 2, 3, 4 and 5 was 66.9, 50.3; 66.2, 50.8; 66.7, 50.5; 66.5, 50.7 and 66.1, 50.4, respectively. The peak corresponding to phenyl carbons (representative) in 1, 2, 3, 4 and 5 was 128.1, 128.6, 128.4, 127.9, and 128.7, respectively. The peak corresponding to C-Cl and C-NO2 was 134.8 and 147.7, respectively.
The mass spectral data revealed that all the compounds exhibited parent peak (M+) consistent with the assigned molecular formula. The fragmentation peaks correspond to the hypothesis of the fragmentation pattern of the compounds. The base peak of 1, 2, 3, 4 and 5 was 119, 169, 119, 139, and 131, respectively. The base peaks were found to be consistent with the proposed fragmentation hypothesis and according to the nature of the substituents.
N-benzylidine-2-morpholoinobenzenamine (1) and N-(3-nitrobenzylidine)-2-morpholino benzenamine (3) exhibited significant analgesic and antiinflammatory activities. Remaining compounds 2, 4, and 5 showed mild analgesic and antiinflammatory activities. All the synthesized compounds exhibited significant antibacterial and antifungal activity with an MIC range of 3.5-7.5 μg/ml. N-(3-nitrobenzylidine)-2- morpholino benzenamine (3) was found to exhibit the highest anti-microbial activity against S. aureus (5 μg/ml), S. epidermidis (4 μg/ml), B. cereus (5.5 μg/ ml), P. aeruginosa (3.5 μg/ml), K. pneumoniae (4.5 μg/ml), E. coli (5.5 μg/ml) and C. albicans (5.5 μg/ ml). Compound 2 exhibited highest activity against A. niger (3.5 μg/ml). The compounds were active against all the tested microorganism with a range of MIC values for S. aureus (5-7.5 μg/ml), S. epidermis (4-7.5 μg/ml), B. cereus (4.5-6.5 μg/ml), B. substilis (5.5-8.0 μg/ml), P. aeruginosa (3.5-7.0 μg/ml), K. pneumoniae (5-7.5 μg/ml), E. coli (5.5-7.5 μg/ml), A. niger (3.5-6.5 μg/ml) and C. albicans (4.5-6 μg/ml).
The results of the present study show that compound 1 containing unsubstituted phenyl ring in 2nd position of the 4-phenyl morpholine exhibits greater activity than the substituted one. Out of the synthesized compounds the nitro substituted compound 3 exhibits more activity than the chloro substituted compound 4, which in turn exhibits more activity than furyl substituted compound 2. Compound 5 exhibits lesser activity.
References
- Misra VS, Singh S, Agarwal R, Chandhary KC. Synthesis of 2-styryl-3,6,8-trisubstituted quinazolin-4(3H)-ones as anti-inflammatory agents. J ChemSoc Pak 1981;3;209-13
- Varma MG, Sharma VR, Saxena AK, Bhalla TN, Sinha JN, Bhargava KP, et al. Antiinflammatory activity of amino acylbenzoates. Pharmacol Res Comm 1984;16:9-20
- Agarwal R, Agarwal C, Singh C, Misra VS. Synthesis of 1-(2-arylindol-3-yl)-2[3-(p-morpholinophenyl)-6,8-disubstituted-quinazolin-4(3H)-ones. J ChemSoc Pak 1984;6:84-94.
- Varma RS, Prakash R, Khar MM, Ali MA. Synthesis of 1-(4-anilino)-morpholines/piperidine and related compounds potential biodynamic agents. Indian Drugs 1986;23:345-9.
- Hester JB, Nidy E, Eldon G, Perricone SC, Poel TJ. Preparation of thiocarboxylOxazolidines as antibacterial agents. PCT Appl W00144188, 2001.
- Agarwal R, Chandhary KC, Misra VS. Synthesis of 1-(4-morpholinophenyl)-4-(3,4-disubstituted benzylidine) imidazolidin- 5-ones as CNS active. Indian J Chem B 1983;22:308-10.
- Varma RS, Prakash R, Prasad CR. Synthesis of 1-(4-anilino)-morpholines/piperidine and related compondsas CNS active agents. J ChemSoc Pak 1986;8:117-23
- Popp FD. Synthesis of potent antineoplastic agents, compounds related to the 3-0-nitrophenylhydrazone of isatin. J Med Chem 1968;12:182-7
- Pou-Hsiung W, James GK, Eric JL, Michael MC. Design, synthesis, testing, and quantitative structural activity relationship analysis of substituted salicylaldehyde Schiff bases of 1-amino-3-hydroxyguanidine tosylate as new antiviral agents against coronavirus. J Med Chem 1990;33:608-14
- Germus F, Gurkhan P, Gunduz N, Abbasoglu A. Studied in vitro antibacterial activities of some Schiff bases. FABAD Farm BilimerDerg 1994;19:5-8
- Deshmukh MD, Doshi AG. Synthesis of new Schiff bases and their antimicrobial activity. Orient J Chem1995;11:85-6.
- Indreen M, Siddique M, Patil SD, Doshi AG, Raut AW. Synthesis of Schiff bases of thiophene-2-carboxaldehyde and its antimicrobial activity. Orient J Chem 2001;17:131-3.
- Sridhar SK, Saravanan M, Ramesh A. Synthesis and antibacterial screening of hydrazonesShiff and Mannich bases of isatin derivatives. Eur J Med Chem 2001;36:615-25.
- Pannerselvam P, PradeepChandran RV, Sridhar SK. Synthesis, characterization and biological Activities of Novel 2-Methyl – quinazolin-4(3H)-ones. Indian J Pharm Sci2003;65:268-73.
- Panneerselvam P, Rajasree RN, Vijayalakshmi G, Subramanian EH, Sridhar SK. Synthesis of Schiff bases of 4-(4-aminophenyl)-morpholineas potential antimicrobial agents. Eur J Med Chem 2005;40:225-9.
- Adams R, Schowaltes KA. Quinone dibezene sulfonamide. J Am ChemSoc 1952;74:2597-602.
- Spiegel MR, Meddis R. In; Probability and statistics. New York: Mcgraw-Hill Book Company; 1980. p. 142.
- Palanichamy S, Nagarajan SJ, Analgesic activity of Cassia alata leaf extract and kaemferol 3-0-sophoroside. Ethnopharmacol 1990;29:73-8.
- Writtin LB, Huebher GF, Galdif OE, Spitalctta P, Plummer AJ. Pharmacology of 2-amino-indane hydrochloride (SU-8629): A potent non-narcotic analgesic. J PharmacolExpTher 1961;133;400-8.
- Turner RA. Screening Methods in Pharmacology. 1st ed. New York: Academic press; 1985. p. 112-4
- Winter CA, Risley EA, Nus GN. Carragenan-induced oedema in hind paws of the rats as an assay for anti-inflammatory drugs. ProcSocExpBiol 1962;11:544-8
- Gillespie SH. Medical microbiology illustrated. 2nd ed. London: ButterworksHeiremark; 1994.p.234-9
- Hawkey PM, Lewis DA. Medical bacteriology: A Practical approach, 3rd ed. Oxford, UK: Oxford University Press; 1994. p. 181-5