- *Corresponding Author:
- R. Kalirajan
Department of Pharmaceutical Chemistry, JSS College of Pharmacy, Udhagamandalam-643 001, India
E-mail: rkalirajan@ymail.com
| Date of Submission | 12 April 2017 |
| Date of Revision | 26 February 2018 |
| Date of Acceptance | 28 August 2018 |
| Indian J Pharm Sci 2018;80(5):921-929 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
A series of oxazine substituted 9-anilinoacridines were synthesized, characterized, and evaluated for antitumor activity against Daltons lymphoma ascites cells using in vitro and in vivo methods. Results indicated that these conjugates exhibited significant antitumour activity on Daltons lymphoma ascites cells. Among these agents, compounds 4b, 4c, 4e and 4j were the most cytotoxic with CTC50 value of 96.5-190 µg/ml (0.125-0.352 µM). 3D QSAR study was performed using PHASE module of Schrodinger suite.
Keywords
Acridine, Oxazine, Synthesis, Antitumour, 3D-QSAR
Chemotherapy is often the treatment of choice for many types of cancer and the search for new chemotherapeutic agents still plays a major role in the fight against cancer. A reasonable approach in this area deals with use of compounds interacting with DNA and/or inhibiting enzymes critical for cell survival and replication. Amsacrine is one such compound, a well-known antiproliferative agent used to treat some types of cancers including acute adult leukaemia [1]. The poisoning of topo II activity inhibits the relegation process and causes lethal double-strand breaks in DNA, leading to cell cycle arrest and apoptosis. The intercalative property was referred to the planar aromatic system of the acridine moiety [2].
In the same context, acridines have gained strong ground for various biological activities like antimicrobial [3], antioxidant [4], anticancer [5-8], antimalarial [9], antiinflammatory [10], analgesic [11], antileishmanial [12], antinociceptive [13], acetylcholinesterase inhibitory [14] and antiherpes [15]. Amsacrine is the best known compound of 9-anilinoacridines series. It was one of the first DNA-intercalating agents to be considered as a topoisomerase II inhibitor. The intercalation process is the strongest type of reversible binding to the double helical DNA in compounds with sufficiently large coplanar aromatic chromophore. Several detailed SAR studies of acridine-based DNA-intercalating agents suggest that the mode of binding is important and the chromophore intercalate with the DNA base pairs. The chemical modification of acridines such as the introduction of different substitutions or hetero cyclic rings were allowed expansion of research on the structure activity relationship to afford new insight into molecular interactions at the receptor level [16]. In fact, it is well-established that slight structural modification on 9-anilinoacridines may bring various pharmacological effects. Similarly oxazine derivatives also have various biological activities [17-20] like antimicrobial, anticancer. In this paper in vitro and in vivo antitumour activity against Daltons lymphoma ascites (DLA) cell lines were described. In continuation of our previous research work [21] on searching new potent cytotoxic agents, 9-anilinoacridine analogues bearing the oxazine residue on anilino rings were synthesized for antitumour evaluation. Acridine derivatives possessed a diverse range of pharmacological activities [22]. Hence the main objective of this study was to determine the antibacterial and antitumour activities of oxazine substituted 9-anilino acridine derivatives. The results revealed that the newly synthesized derivatives exhibited significant antitumour activities.
Materials and Methods
Melting points were obtained on Veego VMP-1 apparatus in open capillary tubes and are uncorrected. The reactions were monitored by thin-layer chromatography (TLC) on silica gel thin-layer plates. Compounds were analysed for C, H, N and analytical results obtained for these elements were within ± 0.5 % of the calculated values for the formula shown. All reagents were of commercially quality or were purified before use. Organic solvents were of analytical grade or were purified by standard procedures. IR spectra were obtained using a Perkin Elmer FT-IR spectrometer spectrum two model. 1H nuclear magnetic resonance (NMR) and 13C NMR were recorded on Bruker Avance III 500 MHz spectrometer. Chemical shifts are in parts per million (ppm). Mass spectra of the final compounds were recorded on a Jeol GC-Mate mass spectrometer.
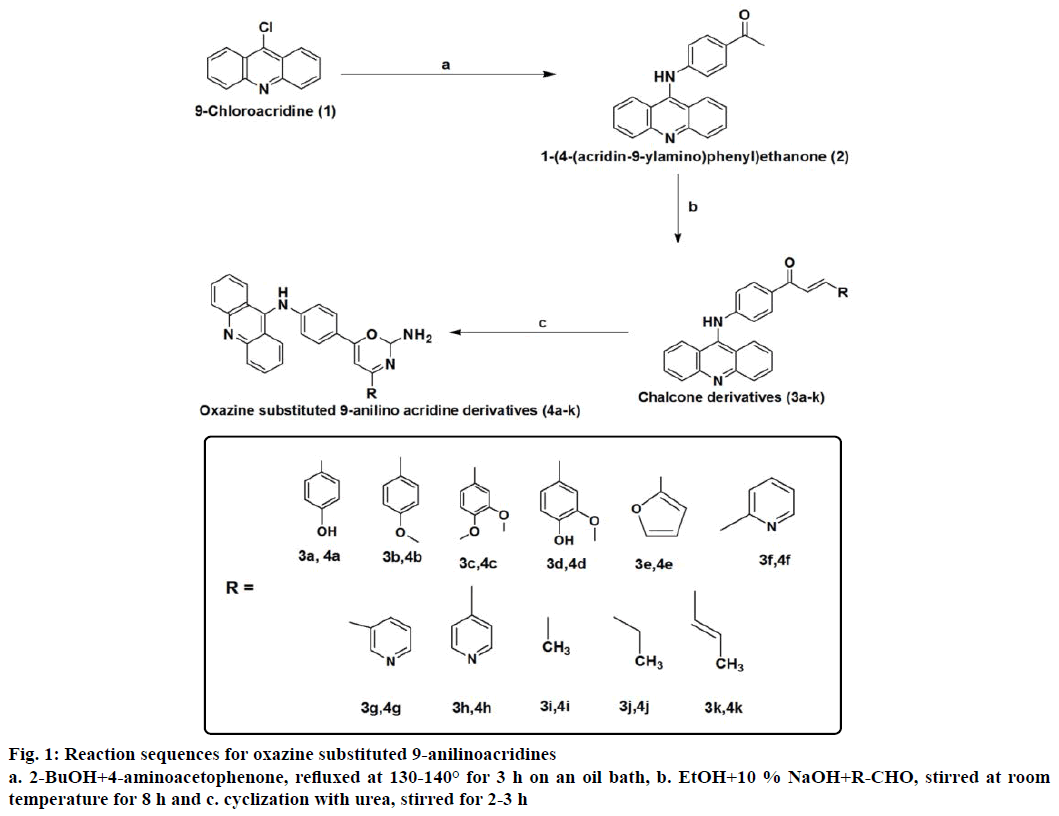
9-chloroacridine was synthesized by the cyclization of N-arylanthranilinic acid with phosporusoxy chloride as reported [23]. 1-[4-(Acridin-9-ylamino) phenyl]ethanone (2) was synthesized by the reaction of 4-aminoacetophenone was refluxed with 9-chloroacridine as reported [24].
General procedure for synthesis of chalcones (3a-k)
The chalcones were synthesized by using the general Claisen-Schmidt condensation [25]. In a 100 ml flat bottomed flask, 25 ml of the 10 % sodium hydroxide and 25 ml of ethanol were taken along with a magnetic stirring bar and was stirred on the magnetic stirrer. To this 0.01 mol of corresponding aldehyde was added, then 2.99 g (0.0096 mol) of 1-[4-(acridin-9-ylamino) phenyl]ethanone was added at the last. The solution was allowed to stir for 8 h at room temperature. After completion of the reaction, 100 ml of water was added, the precipitate formed was filtered, washed three times with 50 ml of water each time to remove sodium hydroxide, dried and crystallized from ethanol.
(E)-1-(4-(acridin-9-ylamino)phenyl)-3-(4-hydroxy phenyl)prop-2-en-1-one (3a)
Yellow powder, % yield: 59; melting point (MP): 195- 198°, IR (υ, cm-1): 3302 (N-H), 3100-3000 (Ar C-H), 1624 (α, β-unsaturated C=O), 1606 and 1518 (ArC=C), 1267 (C-N), 748 (ArC-H); MS: m/z 434.52 (M+); 1H NMR (DMSO-d6, δ ppm): 6.65-8.02 (16H, m, ArCH), 7.90 and 7.56 (2H, ss, CH=CH), 11.21 (1H s, NH); 13C NMR (DMSO-d6, δ ppm): 189 (C=O), 145.3, 120.4 (CH=CH), 153.5, 150.8, 148.4, 148.5, 143.2, 141.2, 136.3, 136.1, 132.4, 131.7, 130.8, 129.6, 130.5, 128.6, 127.2, 127.1, 127.2, 121.6, 119.5, 116.3. Anal. calcd. for C28H19ClN2O: C, 77.32; H, 4.42; N, 6.45; found: C, 77.25; H, 4.29; N, 6.62.
(E)-1-(4-(acridin-9-ylamino)phenyl)-3-(4-methoxy phenyl)prop-2-en-1-one (3b)
Yellow powder, % yield: 58; MP: 179-181°, IR (υ, cm-1): 3273 (NH), 3100-3000 (ArCH), 1626 (α, β-unsaturated C=O), 1607 and 1510 (ArC=C), 1260 (CN), 1168 (CO), 748 (Ar CH); MS: m/z 430.17 (100 %); 1H NMR (DMSO-d6) δ: 6.65-8.02 (16H, m, ArCH), 7.90 and 7.56 (2H, s, CH=CH), 11.21 (1H, s, NH), 3.73 (3H, t, CH3); 13C NMR (ppm): 189 (C=O), 148.8, 143.8, 141.4, 135.6, 135.5, 131.6, 131.5, 130.3, 129.6, 129.8, 128.9, 127.7, 126.8, 126.7, 121.9, 119.4, 115.7, 54.8 (CH3). Anal. calcd. for C29H22N2O2: C, 80.91; H, 5.15; N, 6.51; O, 7.43; found: C, 80.83; H, 5.23; N, 6.44.
(E)-1-(4-(acridin-9-ylamino)phenyl)-3-(3,4-di methoxyphenyl)prop-2-en-1-one (3c)
Yellow powder, % yield: 54; MP: 228-230°, IR (υ, cm-1): 3044 (NH), 3100-3000 (Ar st CH), 1626 (α, β-unsaturated C=O), 1577 and 1498 (ArC=C), 748 (ArCH); MS: m/z 460.58 (M+); 1H NMR (DMSO-d6, δ ppm): 6.65-8.02 (16H, m, ArCH), 7.90 and 7.56 (2H, s, CH=CH), 11.21 (1H, s, NH). 13C NMR (ppm): 181 (C=O), 53.47 (OCH3), 54.32 (OCH3), 104.8-158.8 (aromatic carbons). Anal. calcd. for C30H24N2O3: C, 78.29; H, 5.33; N, 6.13; found: C, 78.25; H, 5.36; N, 6.12.
(E)-1-(4-(acridin-9-ylamino)phenyl)-3-(4-hydroxy- 3-methoxyphenyl)prop-2-en-1-one (3d)
Yellow powder, % yield: 73; MP: 210-212°, IR (υ, cm-1): 3304 (NH), 3100-3000 (Ar st CH), 1626 (α, β-unsaturated C=O), 1587 and 1568 (ArC=C), 3327 (ArOH), 748 (Ar CH); MS: m/z 450.34 (M+); 1H NMR (DMSO-d6, δ ppm): 6.65-8.02 (16H, m, ArH), 7.90 and 7.56 (2H, s, CH=CH), 11.21 (1H, s, NH); 13C NMR (ppm): 178 (C=O), 52.15 (OCH3), 110.2- 169.3 (aromatic carbons). Anal. calcd. for C29H26N2O3: C, 77.42; H, 5.85; N, 6.33; found: C, 77.46; H, 5.92; N, 6.37.
(E)-1-(4-(acridin-9-ylamino)phenyl)-3-(furan-2-yl) prop-2-en-1-one (3e)
Yellow powder, % yield: 67; MP: 179-181°, IR (υ, cm-1): 3300 (NH), 3057-3034 (Ar CH), 1651 (α, β-unsaturated C=O), 1606 and 1512 (Ar C=C), 1230 (CN), 1176 (CO), 759 (Ar CH); MS (m/z): 391.14 (M++1); 1H NMR (DMSO-d6, δ ppm): 8.04 to 6.64 (15H, m, ArCH), 10.74 (1H, s, NH), 7.58 and 7.91 (2H, ss, CH=CH); 13C NMR (ppm): 180 (C=O), 53.17 (OCH3), 105.7-167.5 (aromatic carbons). Anal. calcd. for C26H18N2O2: C, 79.97; H, 4.64; N, 7.17; found: C, 79.88; H, 4.53; N, 7.25.
(E)-1-(4-(acridin-9-ylamino)phenyl)-3-(pyridin-2- yl)prop-2-en-1-one (3f)
Yellow powder, % yield: 48; MP: 174-177°; IR (υ, cm-1): 3028 (NH), 3100-3000 (ArCH), 1622 (α, β-unsaturated C=O), 1579 and 1498 (ArC=C), 1280 (CN), 744 (ArCH); MS: m/z 401.47 (M+); 1H NMR (DMSO-d6, δ ppm): 6.65-8.02 (16H, m, ArCH), 7.90 and 7.56 (2H, s, CH=CH), 11.21(1H, s, NH); 13C NMR (ppm): 183 (C=O), 112.5-153.6 (aromatic carbons). Anal. calcd. for C27H19N3O: C, 76.47; H, 4.30; N, 9.45; found: C, 76.26; H, 4.52; N, 9.51.
(E)-1-(4-(acridin-9-ylamino)phenyl)-3-(pyridin-3- yl)prop-2-en-1-one (3g)
Yellow powder, % yield: 63; MP: 124-126°, IR (υ, cm-1): 3290 (NH), 3100-3000 (ArCH), 1627 (α, β-unsaturated C=O), 1577 and 1498 (ArC=C), 1280 (CN), 748 (ArCH); MS (m/z): 402.15 (M++1); 1H NMR (DMSO-d6, δ ppm): 3.98 (s, 1H, NH), 7.58 (s, 1H, CH), 7.89 (s, 1H, CH), 8.1 to 6.63 (15H, m, ArCH). 13C NMR (ppm): 181 (C=O), 110.3-157.3 (aromatic carbons). Anal. calcd. for C27H19N3O (401.4): C, 80.80; H, 4.77; N, 10.48; found: C, 80.67; H, 4.60; N, 10.28.
(E)-1-(4-(acridin-9-ylamino)phenyl)-3-(pyridin-4- yl)prop-2-en-1-one (3h)
Yellow powder, % yield: 68; MP: 165-170°, IR (υ, cm-1): 3308 (NH), 3100-3000 (Ar st CH), 1626 (α, β-unsaturated C=O), 1587 and 1568 (ArC=C), 748 (ArCH); MS: m/z 401.48 (M+); 1H NMR (DMSO-d6, δ ppm): 6.60- 8.08 (16H, m, ArCH), 7.90 and 7.56 (2H, s, CH=CH), 11.21 (1H, s, NH); 13C NMR (ppm): 179 (C=O), 110.4-153.7 (aromatic carbons). Anal. calcd. for C27H19N3O: C, 78.42; H, 5.85; N, 6.33; found: C, 78.46; H, 5.92; N, 6.37.
(E)-1-(4-(acridin-9-ylamino)phenyl)but-2-en-1-one (3i)
Yellow powder, % yield: 61; MP: 188-190°, IR (υ, cm-1): 3347 (NH), 3100-3000 (Ar st CH), 1622 (α, β-unsaturated C=O), 1604 and 1473 (ArC=C); MS: m/z 338.18 (M+); 1H NMR (DMSO-d6, δ ppm): 3.72 (CH3), 6.65-8.02 (16H, m, ArCH), 7.90 and 7.56 (2H, s, CH=CH), 11.21 (1H, s, NH); 13C NMR (ppm): 185 (C=O), 54.32 (CH3), 114.8-154.5 (aromatic carbons). Anal. calcd. for C23H18N2O: C, 81.49; H, 5.33; N, 8.23; found: C, 81.53; H, 5.37; N, 8.13.
(E)-1-(4-(acridin-9-ylamino)phenyl)pent-2-en-1- one (3j)
Yellow powder, % yield: 54; MP: 195-200°; IR (υ, cm-1): 3044 (NH), 3100-3000 (Ar st CH), 1626 (α, β-unsaturated C=O), 1582 and 1496 (ArC=C), 754 (ArCH); MS: m/z 352.43 (M+); 1H NMR (DMSO-d6, δ ppm): 3.72 (CH3), 3.32 (CH3), 3.62 (CH2), 6.65-8.02 (16H, m, ArCH), 7.90 and 7.56 (2H, s, CH=CH), 11.21 (1H, s, NH). 13C NMR (ppm): 181 (C=O), 104.8-158.6 (aromatic carbons). Anal. calcd. for C24H20N2O: C, 74.29; H, 5.33; N, 6.13; found: C, 74.25; H, 5.36; N, 6.12.
(2E,4E)-1-(4-(acridin-9-ylamino)phenyl)hexa-2,4- dien-1-one (3k)
Yellow powder, % yield: 76; MP: 178-184°, IR (υ, cm-1): 3058 (NH), 3100-3000 (Ar st CH), 1632 (α, β-unsaturated C=O), 1595 and 1478 (ArC=C), 758 (ArCH); MS: m/z 364.48 (M+); 1H NMR (DMSO-d6, δ ppm): 3.32 (CH3), 6.65- 8.02 (16H, m, ArCH), 7.90 and 7.56 (2H, CH=CH), 11.21 (1H, s, NH). 13C NMR (ppm): 183 (C=O), 113.5-155.9 (aromatic carbons). Anal. calcd. for C25H20N2O: C, 76.29; H, 5.33; N, 6.13; found: C, 76.25; H, 5.36; N, 6.23.
General procedure for synthesis of oxazine substituted 9-anilinoacridines (4a-k)
A mixture of chalcone 3a-k (0.02 mol), urea (0.02 mol) were dissolved in sodium hydroxide in ethanol (10 ml), stirred for about for 2-3 h on a magnetic stirrer. This mixture was poured into 400 ml of cold water with continuous stirring for 1 h. This was kept in refrigerator for 24 h. The precipitate obtained was filtered, washed and recrystallized using petroleum ether:benzene (5:5). The reaction was monitored by TLC using methanol:water (5:3).
4-(6-(4-(acridin-9-ylamino)phenyl)-2-amino-2H- 1,3-oxazin-4-yl)phenol (4a)
Yellow powder, % yield: 54; MP: 108-111°, IR (υ, cm-1): 3389 (NH2), 3328 (NH), 1586 and 1437 (ArC=C), 1176 (ArC=N), 3223 (Ar-OH), 819 (ArCH); MS: m/z 458. 51 (M+); 1H NMR (DMSO-d6, δ ppm): 5.62 (s, 1H, OH), 6.85-7.94 (16H, m, ArCH), 7.46-7.48 (2H, d, CH), 7.95 (1H, s, NH), 6. 12 (2H, s, NH2); 13C NMR (ppm): 179.7 169.6, 163.7, 150.2, 148.3, 148.5, 145.3, 120.5, 142.5, 141.2, 136.2, 136.1, 132.5, 131.5, 130.8, 130.5, 129.6, 128.8, 127.3, 127.2, 127.1, 121.6, 119.5, 116.3, 116.2, 114.65, 112.43 (aromatic carbons). Anal. calc. for C29H22N4O2: C, 75.24; H, 4.83; N, 12.26; found: C, 75.18; H, 4.79; N, 12.31.
N-(4-(2-amino-4-(4-methoxyphenyl)-2H-1,3- oxazin-6-yl)phenyl)acridin-9-amine (4b)
Yellow powder, % yield: 72; MP: 142-145°, IR (υ, cm-1): 3014 (NH), 2961 (Ar st CH), 1588 and 1468 (ArC=C), 1177 (ArC=N), 1295 (C-O), 820 (ArCH); MS: m/z 472.54 (M+); 1H NMR (DMSO-d6, δ ppm): 3.36, (3H, OCH3), 6.12-7.39 (16H, m, ArCH), 7.46-7.48 (2H, m, CH), 11.25 (1H, s, NH), 6. 12 (2H, s, NH2); 13C NMR (ppm): 166.1, 164.7, 150.4, 149.3, 148.4, 145.1, 142.5, 140.2, 137.3, 136.5, 133.5, 132.1, 130.8, 130.2, 129.6, 128.5, 127.3, 127.2, 126.8, 121.2, 120.5, 119.1, 116.3, 116.1, 114.6, 112.4 (aromatic carbons), 54.63 (OCH3). Anal. calc. for C30H24N4O2: C, 76.27; H, 5.14; N, 11.87; found: C, 76.32; H, 5.17; N, 11.91.
N-(4-(2-amino-4-(3,4-dimethoxyphenyl)-2H-1,3- oxazin-6yl)phenyl)acridin-9-amine (4c)
Yellow powder, % yield: 67; MP: 162-165°, IR (υ, cm-1): 3352 (NH), 3005 (Ar st CH), 1600 and 1583 (ArC=C), 1642 (ArC=N), 1263 (CO), 744 (ArCH); MS: m/z 502.52 (M+); 1H NMR (DMSO-d6, δ ppm): 3.80, 3.86 (6H, d, OCH3), 6.85-7.97 (16H, s, ArCH), 7.48 (1H, s, NH), 6.12 (2H, s, NH2); 13C NMR (ppm): 185.96, 167.1, 153.66, 150.71, 148.9, 145.1, 142.5, 141.87, 137.3, 136.5, 133.6, 132.2, 130.8, 130.9, 129.6, 128.5, 127.3, 127.92, 125.55, 123.21, 120.5, 119.9, 112.7, 111.54, 110.49, (aromatic carbons), 55.69 (OCH3), 55.53 (OCH3). Anal. calc. for C31H26N4O3: C, 74.18; H, 5.16; N, 11.12; found: C, 74.23; H, 5.12; N, 11.18.
4-(6-(4-(acridin-9-ylamino)phenyl)-2-amino-2H- 1,3-oxazin-4yl)-2methoxyphenol (4d)
Yellow powder, % yield: 57; MP: 169-172°, IR (υ, cm-1): 3332 (NH), 2993 (Ar st CH), 1589 and 1563 (ArC=C), 3227 (Ar-OH), 1653 (ArC=N), 1178 (C-O), 750 (ArC-H); MS: m/z 488.47 (M+); 1H NMR (DMSO-d6, δ ppm): 3.36, (3H, d, OCH3), 5.34 (s, 1H, OH), 7.27-7.71 (18H, m, ArH), 8.28 (1H, s, NH), 6. 27 (2H, s, NH2); 13C NMR (ppm): 40.03 (OCH3), 163.9, 161.7, 151.3, 149.4, 147.1, 145.3, 141.5, 140.3, 138.5, 136.2, 133.1, 132.5, 130.8, 130.1, 129.3, 128.2, 127.3, 127.1, 126.3, 121.6, 120.2, 119.2, 117.3, 116.8, 114.6, 112.4 (aromatic carbons). Anal. calc. For C30H24N4O3: C, 73.68; H, 4.87; N, 11.36; found: C, 73.68; H, 4.87; N, 11.36.
N-(4-(2-amino-4-(furan-2-yl)-2H-1,3-oxazin-6-yl) phenyl)acridin-9-amine (4e)
Orange powder, % yield: 61; MP: 110-113°; yield: %, IR (υ, cm-1): 3415 (NH2), 3348 (NH), 1599 and 1477 (ArC=C), 1632 (ArC=N), 1259 (CO), 881 (ArCH); MS: m/z 432.47 (M+); 1H NMR (DMSO-d6, δ ppm): 6.24-7.98 (18H, m, ArH), 8.22 (1H, s, NH), 6.24 (2H, s, NH2); 13C NMR (ppm): 170.2, 168.1, 166.7, 151.7, 149.5, 148.1, 145.6, 142.2, 140.7, 138.2, 136.2, 134.5, 132.7, 130.5, 130.1, 129.3, 128.3, 127.7, 127.2, 126.6, 121.4, 120.4, 119.8, 116.7, 116.1, 115.6, 112.4, 108.8 (aromatic carbons). Anal. calc. for C27H20N4O2: C, 74.96; H, 4.68; N, 12.87; found: C, 74.93; H, 4.72; N, 12.83.
N-(4-(2-amino-4-(pyridin-2-yl)-2H-1,3-oxazin-6-yl) phenyl)acridin-9-amine (4f)
Brown powder, % yield: 55; MP: 200-203°; IR (υ, cm-1): 3349 (NH2), 3224 (NH), 1586 and 1473 (ArC=C), 1636 (ArC=N), 1259 (CO), 748 (ArCH). MS: m/z 443.47 (M+); 1H NMR (DMSO-d6, δ ppm): 6.06-8.49 (16H, m, ArCH), 8.40-8.43 (2H, m, CH), 5.45 (1H, s, NH), 4.54 (2H, s, NH2); 13C NMR (ppm): 168.4, 167.3, 153.7, 150.5, 147.3, 145.2, 142.1, 141.4, 138.6, 136.5, 134.3, 132.7, 131.3, 130.1, 129.5, 128.8, 127.4, 127.2, 126.2, 121.1, 120.7, 119.2, 116.7, 116.4, 114.2, 112.7, 109.3 (aromatic carbons). Anal. calc. for C28H21N5O: C, 75.87; H, 4.74; N, 15.79; found: C, 75.87; H, 4.74; N, 15.79.
N-(4-(2-amino-4-(pyridin-3-yl)-2H-1,3-oxazin-6-yl) phenyl)acridin-9-amine (4g)
Brown powder, % yield: 53; MP: 135-137°, IR (υ, cm-1): 3347 (NH2), 3221 (NH), 1587 and 1478 (ArC=C), 1632 (ArC=N), 1278 (CO), 827 (ArCH); MS: m/z 443.47 (M+); 1H NMR (DMSO-d6, δ ppm): 6.12- 8.47 (16H, m, ArCH), 8.38-8.42 (2H, m, CH), 5.75 (1H, s, NH), 4.76 (2H, s, NH2); 13C NMR (in ppm): 171.5, 167.4, 155.3, 151.3, 147.1, 145.2, 142.5, 141.5, 138.3, 137.5, 134.2, 132.5, 131.7, 130.4, 129.9, 128.5, 127.6, 127.3, 126.7, 121.5, 120.3, 119.6, 117.4, 116.7, 114.5, 112.2, 109.6, 107.4 (aromatic carbons). Anal. calc. for C28H21N5O: C, 75.81; H, 4.75; N, 15.78; found: C, 75.83; H, 4.74; N, 15.75.
N-(4-(2-amino-4-(pyridin-4-yl)-2H-1,3-oxazin-6-yl) phenyl)acridin-9-amine (4h)
Brown powder, % yield: 51; MP: 158-161°, IR (υ, cm-1): 3346 (NH2), 3219 (NH), 1587 and 1477 (ArC=C), 1632 (ArC=N), 1225 (CO), 822 (ArCH); MS: m/z 443.47 (M+); 1H NMR (DMSO-d6, δ ppm): 6.16-8.43 (16H, m, ArCH), 8.38-8.40 (2H, m, CH), 5.37 (1H, s, NH), 4.78 (2H, s, NH2); 13C NMR (ppm): 173.3 169.5, 167.6, 155.6, 151.8, 147.5, 144.2, 142.8, 141.1, 138.5, 136.7, 135.3, 134.6, 131.8, 130.3, 129.5, 128.5, 127.4, 126.8, 126.1, 122.5, 121.7, 119.6, 117.7, 116.4, 115.2, 113.1, 108.8 (aromatic carbons). Anal. calc. for C28H21N5O: C, 75.81; H, 4.79; N, 15.78; found: C, 75.83; H, 4.76; N, 15.75.
N-(4-(2-amino-4-(methyl)-2H-1,3-oxazin-6yl) phenyl)acridin-9-amine (4i)
Yellow powder, % yield: 51; MP: 173-176°, IR (υ, cm-1): 3343 (NH), 3067 (Ar st CH), 1589 and 1563 (ArC=C), 1159 (ArC=N), 1178 (CO), 742 (ArCH); MS: m/z 380.47 (M+); 1H NMR (DMSO-d6, δ ppm): 2.50 (3H, m, CH3), 7.23-7.85 (16H, m, ArCH), 7.59- 7.92 (2H, m, CH), 8.38 (1H, s, NH), 6.18 (2H, s, NH2); 13C NMR (ppm): 175.2, 167.3, 153.7, 147.3, 145.2, 142.1, 141.4, 138.6, 136.5, 134.3, 132.7, 132.3, 130.5, 129.2, 128.5, 127.6, 127.3, 126.2, 121.7, 119.3, 118.7, 112.6, 109.5 (aromatic carbons), 54.53 (CH3). Anal. calc. for C24H20N4O: C, 75.75; H, 5.34; N, 14.68; found: C, 75.68; H, 5.41; N, 14.62.
N-(4-(2-amino-4-(ethyl)-2H-1,3-oxazin-6yl)phenyl) acridin-9-amine (4j)
Brown powder, % yield: 49; MP: 118-121°, IR (υ, cm-1): 3428 (NH2), 3342 (NH), 1590 and 1442 (ArC=C), 1626 (ArC=N), 1279 (CO), 829 (ArCH); MS: m/z 394.47 (M+); 1H NMR (DMSO-d6, δ ppm): 2.55, (3H, m, CH3), 2.86, (2H, s, CH2), 6.58-7.62 (16H, m, ArH), 7.67-7.85 (2H, m, CH), 7.86 (1H, s, NH), 6.18 (2H, s, NH2); 13C NMR (ppm): 175.2, 168.5, 157.2, 151.3, 148.2, 143.2, 142.3, 141.3, 138.7, 136.4, 135.5, 134.2, 131.3, 130.7, 129.3, 128.2, 127.2, 126.5, 124.5, 121.3, 119.6, 115.2, 112.8 (aromatic carbons), 57.6 (CH2), 55.32 (CH3). Anal. calc. for C25H22N4O: C, 76.15; H, 5.64; N, 14.18; found: C, 76.18; H, 5.67; N, 14.15.
N-(4-(2-amino-4-(prop-1-enyl)-2H-1,3-oxazin-6yl) phenyl)acridin-9-amine (4k)
Brown powder, % yield: 53; MP: 165-167°; IR (υ, cm-1): 3345 (NH), 1591 and 1479 (ArC=C), 1649 (ArC=N), 1278 (CO), 823 (ArCH); MS: m/z 406.47 (M+); 1H NMR (DMSO-d6, δ ppm): 3.23 (3H, s, CH3), 2.85 (2H, CH), 7.23-7.85 (18H, m, ArH), 8.38 (1H, s, NH), 6.18 (2H, s, NH2); 13C NMR (ppm): 175.4, 169.5, 157.8, 153.4, 148.6, 143.7, 142.2, 141.1, 138.5, 136.1, 135.7, 134.3, 131.6, 130.3, 129.7, 127.9, 127.2, 126.8, 123.4, 121.1, 116.5, 114.2, 112.7 (aromatic carbons), 53.6, 54.1, 50.45 (aliphatic carbons). Anal. calc. for C26H22N4O: C, 76.79; H, 5.46; N, 13.78; found: C, 76.77; H, 5.48; N, 13.75.
Pharmacological evaluation
All the oxazine substituted 9-anilinoacridine derivatives 4a-k were screened for antibacterial activity and short term in vitro antitumour activity against DLA cells. All the synthesized final compounds 4a-k exhibited significant cytotoxic activities. The compounds 4b and 4h were further screened for in vivo antitumour activity against DLA cells.
Short-term study for in vitro antitumor activity [26]
Short term in vitro antitumor activity of the compounds was assayed by determining the percent viability of DLA cells using trypan blue dye exclusion technique. DLA cells were cultured in the peritoneal cavity of healthy albino mice weighing 25-30 g by injecting a suspension of DLA cells (1×106 cells/ml) intraperitoneally. The cells were aspirated aseptically from the peritoneal cavity of the mice on d 15. The cells were washed with Hank’s balanced salt solution (HBSS) and centrifuged for 10-15 min at 1500 rpm in the cooling centrifuge. The pellet was re-suspended with HBSS and the process was repeated three times. Finally the cells were suspended in a known quantity of HBSS and the cell count was adjusted to 2×106 cells/ml. 0.1 ml of the diluted cell suspension was distributed in to Eppendorf tubes and exposed 0.1 ml each of the different concentration of the drug in phosphate buffer saline and incubated at 37°, 5 % CO2 for 3 h. After 3 h, trypan blue dye exclusion test was performed to determine percent viability. For testing viability using dye exclusion method, the pooled cells from wells of each concentration were mixed with 0.4 % trypan blue in a ratio of 1:1 and the number of stained, nonstained and total number of cells were counted using haemocytometer. The percent inhibition and CTC50 values were calculated.
In vivo antitumor activity
In vivo antitumor activities of selected compounds were carried out using DLA tumor model in mice [26]. Male Swiss albino mice were divided into 7 groups of 6 animals each. Except normal group (group 1) all the animals were injected intraperitoneally (i.p.) with 1×10-6 DLA cells. Group-1 and group 2 animals received vehicle (0.5 % CMC, 10 ml/kg, p.o) and served as a normal and control, respectively. Group-3 animals received 5-fluorouracil (10 mg/kg, i.p) and treated as standard group. Group 4 and 5 animals received compound 4b at a dose of 10 and 20 mg/kg, p.o., respectively. Group 6 and 7 animals received compound 4h at a dose of 10 and 20 mg/ kg, p.o., respectively. The treatment was started 24 h after tumour inoculation and continued for a period of 24 d. Through body weight analysis in each group, mean survival time (MST) and increase in the life span was calculated. The treatment protocols received approval from the Institutional Animal Ethics Committee.
Results and Discussion
The reaction sequences leading to the various oxazine substituted 9-anilinoacridines were outlined in the Figure 1. This synthetic pathway was based on the preparation of oxazine substituted 9-anilino acridines 4a-k [25] from 9-chloroacridine 1. 1-(4-(acridine-9- ylamino)phenylethanone 2 [23] was prepared from compound 1, which was refluxed with p-aminoacetophenone. The various chalcone substituted 9-anilinoacridines 3a-k [21] were prepared by the reaction of 2 with various aldehydes and these chalcone derivatives were allowed to cyclized with urea afford the corresponding oxazine substituted 9-anilinoacridines 4a-k. Synthesis, characterization and evaluation of biological activities of novel oxazine substituted 9-anilino acridines are described in this paper. The synthesized compounds were purified by column chromatography. The final yield of the derivatives was in the range of 48-73 %. The compounds obtained were stable in the solid as well as in the solution state. The new compounds were completely characterized by IR, 1H NMR, 13C NMR, mass spectral data and elemental analysis. The IR spectra of compounds 4a-k showed intense bands in the region 1200-1300 cm-1 due to carbonyl stretching and broad bands in the region 3200-3400 cm-1 due to NH stretching. The 1H NMR spectra also support the structure of the compounds 4a-k. The NH proton appeared at 7.9-8.1 and NH2 proton at 5.9-6.3. The mass spectra of all compounds 4a-k showed molecular ion peaks confirming their molecular weight.
The pharmacological properties of the compounds greatly depended on the number and the chemical nature of the substituents. The synthesized final compounds 4a-k were subjected to short term study for in vitro antitumor activity against DLA cells. The compounds 4b, 4c, 4e-4j exerted significant antitumour activity against DLA cells at the concentration of 96.5-190 μg/ml (0.125-0.352 μM, Table 1).
| Compound No. | CTC50 (µg/ml) |
|---|---|
| 4a | 385 |
| 4b | 107 |
| 4c | 190 |
| 4d | 250 |
| 4e | 118 |
| 4f | 116 |
| 4g | 105 |
| 4h | 96.5 |
| 4i | 140 |
| 4j | 115 |
| 4k | 365 |
Table 1: Short Term In Vitro Anticancer Activity Against DLA Cells
The compounds 4b and 4h were screened further to evaluate in vivo antitumor activity against DLA cells. The in vivo study was carried out for 24 d. Body weight gradually increased for many groups. The body weight analysis, MST and % increase in life span at the dose of 10 and 20 mg/kg in Swiss albino mice inoculated with DLA cells (1×106) were calculated (Table 2).
| Group | Dose (mg/kg) | Compound | MST (In Days) |
% ILS |
|---|---|---|---|---|
| 2 (Control) | 10 | CMC (0.05 %) | 13 ± 2.7 | -- |
| 3 (Standard) | 10 | 5-Fluorouracil | 23.33 ± 0.8 | 79.46 |
| 4 | 10 | 5b | 17.33 ± 1.6 | 33.30 |
| 5 | 20 | 5b | 23.33 ± 1.0 | 79.46 |
| 6 | 10 | 5h | 19.33 ± 1.0 | 48.69 |
| 7 | 20 | 5h | 23.33 ± 1.1 | 79.46 |
Table 2: In Vivo Anticancer Activity Against DLA Cells
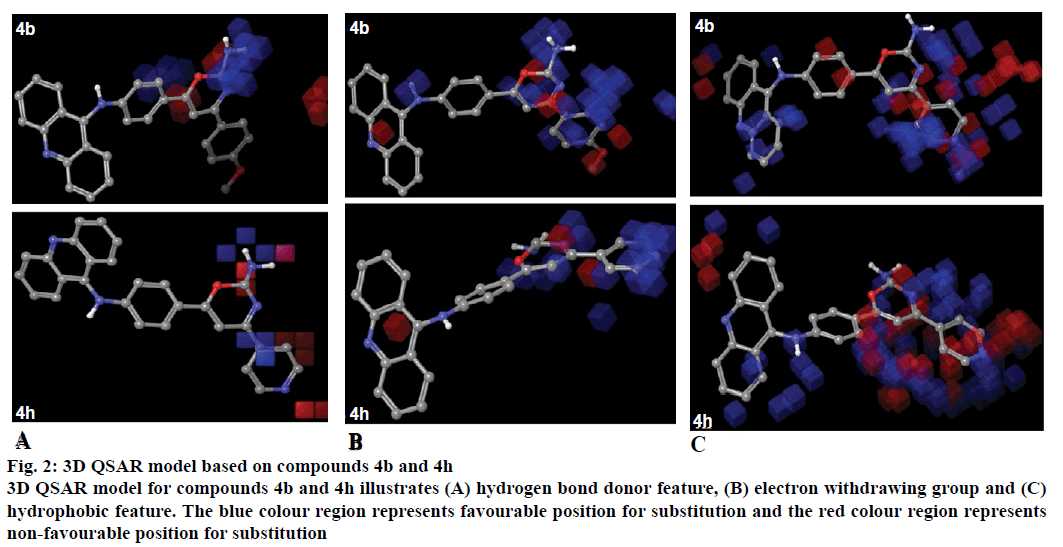
The 3D-QSAR model was generated by PHASE module of Schrodinger suite 2012. The predictive ability was analysed for the training set as well as the test set molecules. The features represented by the model with hydrogen bond donor, electron withdrawing group and hydrophobic/non-polar group (Figure 2). Blue colour region represented the favourable position for substitution and the red colour region represented the non-favourable position for substitution of groups.
Figure 2: 3D QSAR model based on compounds 4b and 4h
3D QSAR model for compounds 4b and 4h illustrates (A) hydrogen bond donor feature, (B) electron withdrawing group and (C) hydrophobic feature. The blue colour region represents favourable position for substitution and the red colour region represents non-favourable position for substitution
In conclusion, acridine family includes a wide range of tricyclic molecules with various biological properties. Considered as potential antitumour agents since the 1980s, numerous acridine derivatives have been synthesised and successfully assessed for their antitumour activity. On this basis, authors recently demonstrated that diverse compounds of the oxazine substituted 9-anilinoacridine series exerted potent antitumour activities. It was revealed that these agents exhibited significant cytotoxicity against DLA cell growth. Results observed in the present study clearly demonstrated that some derivatives of the oxazine substituted 9-anilinoacridine family could exert interesting antitumour activity. The compounds 4a-k showed significant antitumour activity and have the potential to be developed as useful drugs after further refinement. These derivatives certainly provide impetus to design future antitumour agents with greater therapeutic potential.
Acknowledgements
We thank All India Council for Technical Education, New Delhi for the financial support under Research Promotion Scheme. We also thank our Vice Chancellor Dr. B. Suresh, JSS University, Mysore, our principal S. P. Dhanabal, Department of Pharmaceutical analysis, Department of Pharmaceutical Biotechnology, Department of Pharmacology, JSS College of pharmacy, Ooty for the technical support.
Conflict of interest
There is no conflict of interest among authors.
References
- Fossé P, René B, Saucier JM, Hénichart JP, Waring MJ, Colson P, et al. Stimulation of Site-Specific Topoisomerase II-Mediated DNA Cleavage by an N-Methylpyrrolecarboxamide-anilinoacridine Conjugate: Relation to DNA Binding. Biochemistry 1994;33(33):9865-74.
- Chilin A, Marzaro G, Marzano C, Dalla Via L, Ferlin MG, Pastorini G, et al. Synthesis and antitumor activity of novel amsacrine analogs: The critical role of the acridine moiety in determining their biological activity. Bioorg Med Chem 2009;17:523-9.
- Nadaraj V, Selvi ST, Mohan S. Microwave-induced synthesis and anti-microbial activities of 7,10,11,12-tetrahydrobenzo[c]acridin-8(9H)-one derivatives. Eur J Med Chem 2009;44:976-80.
- Kalirajan R, Muralidharan V, Jubie S, Gowramma B, Gomathy S, Sankar S,et al. Synthesis of some novel pyrazole substituted 9-anilino acridine derivatives and evaluation for their antioxidant and cytotoxic activities. J Heterocycl Chem 2012;49:748-54.
- Santelli-Rouvier C, Barret JM, Farrell CM, Sharples D, Hill BT, Barbe J. Synthesis of 9-acridinyl sulfur derivatives: sulfides, sulfoxides and sulfones.Comparison of their activity on tumour cells. Eur J Med Chem 2004;39:1029-38.
- Rastogi K, Chang JY, Pan WY, Chen CH, Chou TC, Chen LT, et al. Antitumor AHMA linked to DNA minor groove binding agents: synthesis and biological evaluation. J Med Chem 2002;45(20):4485-93.
- Su TL, Chou TC, Kim JY, Huang JT, Ciszewska G, Ren WY, et al. 9-Substituted Acridine Derivatives with Long Half-Life and Potent Antitumor Activity: Synthesis and Structure-Activity Relationships. J Med Chem 1995;38:3226-35.
- Bacherikov VA, Chang JY, Lin YW, Chen CH, Pan WY, Dong H, et al. Synthesis and antitumor activity of 5-(9-acridinylamino)anisidine derivatives. Bioorg Med Chem 2005;13:6513-20.
- Gamage SA, Tepsiri N, Wilairat P, Wojcik SJ, Figgitt DP, Ralph RK, et al. Synthesis and in vitro evaluation of 9-anilino-3,6-diaminoacridines active against a multidrug-resistant strain of the malaria parasite Plasmodium falciparum. J Med Chem 1994;37(10):1486-94.
- Chen YL, Lu CM, Chen IL, Tsao LT Wang JP. Synthesis and antiinflammatory evaluation of 9-anilinoacridine and 9-phenoxyacridine derivatives. J Med Chem 2002;45(21):4689-94.
- Sondhi SM, Johar M, Nirupama S, Sukla R, Raghubir R Dastidar SG. Synthesis of sulpha drug acridine derivatives and their evaluation for anti-anflammatory, analgesic and anticancer activity. Indian J Chem 2002;41B:2659-66.
- Gamage SA, Figgitt DP, Wojcik SJ, Ralph RK, Ransijn A, Mauel J, et al. Structure-activity relationships for the antileishmanial and antitrypanosomal activities of 1'-substituted 9-anilinoacridines. J Med Chem 1997;40(16):2634-42.
- Llama EF, Campo CD, Capo M, Anadon M. Synthesis and antinociceptive activity of 9-phenyl-oxy or 9-acyl-oxy derivatives of xanthene, thioxanthene and acridine. Eur J Med Chem 1989;24:391-6.
- Recanatini M, Cavalli A, Belluti F, Piazzi L, Rampa A Bisi A, et al. SAR of 9-amino-1,2,3,4-tetrahydroacridine-based acetylcholinesterase inhibitors: synthesis, enzyme inhibitory activity, QSAR, and structure-based CoMFA of tacrine analogues. J Med Chem 2007;43(10):2007-18.
- Goodell JR, Madhok AA, Hiasa H, Ferguson DM. Synthesis and evaluation of acridine- and acridone-based anti-herpes agents with topoisomerase activity. Bioorg Med Chem 2006;14:5467-80.
- Harrison RJ, Cuesta J, Chessari G, Read MA, Basra SK, Reszka AP, et al. Trisubstituted acridine derivatives as potent and selective telomerase inhibitors. J Med Chem 2003;46(21):4463-76.
- Mathew BP, Kumar A, Sharma S, Shukla PK, Nath M. An eco-friendly synthesis and antimicrobial activities of dihydro-2H-benzo- and naphtho-1,3-oxazine derivatives. Eur J Med Chem 2010;45:1502-7.
- Kalirajan R, Sivakumar SU, Jubie S, Gowramma B. Suresh B. Synthesis and biological evaluation of some heterocyclic derivatives of chalcones. Indian J Chem Tech Res 2009;1(1):27-34.
- Shen G, Chen D, Zhang Y, Sun M, Chen K, Jin C, et al. Synthesis of benzoxazine and 1,3-oxazine derivatives via ligand-free copper(I)-catalyzed one-pot cascade addition/cyclization reaction. Tetrahedron 2012;68:166-72.
- Basappa, Murugan S, Kavitha CV, Purushothaman A, Nevin KG, Sugahara K, et al. A small oxazine compound as an antitumor agent: A novel pyranoside mimetic that binds to VEGF, HB-EGF, and TNF-a. Cancer Lett 2010;297:231-43.
- Kalirajan R, Rafick MH, Sankar S, Jubie S. Docking studies, Synthesis, Characterization and Evaluation of their Antioxidant and cytotoxic activities of some Novel Isoxazole substituted 9-anilinoacridine derivatives. Scientific World Journal 2011;2012:165258.
- Di Giorgio C, Shimi K, Boyer G, Delmas F, Galy JP. Synthesis and antileishmanial activity of 6-mono-substituted and 3,6-di-substituted acridines obtained by acylation of proflavine. Eur J Med Chem 2007;42:1277-84.
- Kalirajan R, Rathore L, Jubie S, Gowramma B, Gomathy S, Sankar S, et al. Microwave assisted synthesis and evaluation of pyrazole derivatives of benzimidazoles. Indian J Chem 2011;50B:1794-9.
- Kalirajan R, Kulshrestha V, Sankar S, Jubie S. Docking studies, synthesis, characterization of some novel oxazine substituted 9-anilinoacridine derivatives and evaluation for their anti-oxidant and anticancer activities as topo isomerase ii inhibitors. Eur J Med Chem 2012;56:217-24.
- Kalirajan R, Palanivelu M, Rajamanickam V, Vinothapooshan G, Anandarajagopal K. Synthesis and biological evaluation of some chalcone derivatives. Int J Chem Sci 2007;5(1):73-80.
- Vijayan P, Kumar S, Dhanaraj SA, Badami S, Suresh B. In vitro Cytotoxicity and Anti-tumor Properties of the Total Alkaloid Fraction of Unripe Fruits of Solanum pseudocapsicum. Pharm Biol 2002;40(6):456-60.