- Corresponding Author:
- B. Padmashali
Department of Chemistry, Sahyadri Science College (Autonomous), Shimoga-577 203, India
E-mail: basavarajpadmashali@yahoo.com
| Date of Submission | 05 July 2013 |
| Date of Revision | 21 June 2014 |
| Date of Acceptance | 25 June 2014 |
| Indian J Pharm Sci 2014;76 (4): 332-338 |
Abstract
Thiophene substituted chalcones (1a-e) were cyclised with thiourea in presence of potassium hydroxide to get 4-substituted-6-(thiophen-2-yl)pyrimidine-2-thiols (2a-e) which were then stirred with methyl iodide to obtain 4-substituted-2-(methylsulfanyl)-6-(thiophen-2-yl)pyrimidines (3a-e). Compounds (3a-e) were refluxed with different N-methylpiperazine and N-phenylpiperazine to afford 4-substituted-2-(4-methylpiperazin-1-yl)-6-(thiophen-2-yl)pyrimidines (4a-e) and 4-substituted-2-(4-phenylpiperazin-1-yl)-6-(thiophen-2-yl)pyrimidines (5a-e). The structures of all the newly synthesised compounds 4b, 4d, 5a and 5b showed good antibacterial activity at 40µg/mlconcentration. Compounds 4a, 4d, 4e, 5c and 5e showed significant antifungal activity at 40 µg/ml concentration compared with standard drugs.
Keywords
2-Acetylthiophene, thiourea, piperazine, pyrimidine, antibacterial, antifungal
Piperazines have a long and distinguished history extending from the days of their discovery as important pharmacophore in chemotherapy. The medicinal value of piperazine derivatives is significant among various heterocycles, as they are found to possess various biological activities [1-9]. Moreover, several bis (heteroaryl) piperazine derivatives (BHAP) have been introduced as potent antiHIV drugs [10,11]. In addition, fused pyrimidines have been reported to exhibit important biological activities [12,13]. These facts contemplated us for the synthesis of novel pyrimidine derivatives incorporating piperazine moiety to explore their biological activities resulting in efficient bio molecules with minimum side effects.
Materials and Methods
Melting points are determined in an open capillary and are uncorrected (Tables 1 and 2). IR spectra were recorded on Bruker alpha FTIR spectrophotometer, 1H NMR and 13C NMR spectra were measured on Bruker AV 400MHz using CDCl3 and DMSO as solvent, respectively. Chemical shifts are expressed in δ ppm. Mass spectra were recorded on a Joel JMS-D 300 mass spectrometer operating at 70 eV. The reactions were followed and checked by TLC and further purification was done by column chromatography. The reagents used were of AR grade and they have been purified by distillation.
| Compound | Yield (%) |
m.p.(º) | Mol. formula (Mol.Wt.) |
Found % (Calculated) | ||
|---|---|---|---|---|---|---|
| C | H | N | ||||
| 1a | 80 | 170-175 | C14H12O2S | 68.53 (68.57) | 4.85 (4.89) | - |
| 1b | 91 | 166-170 | C13H10OS | 72.84 (72.89) | 4.61 (4.67) | - |
| 1c | 68 | 184-186 | C13H9ClOS | 62.62 (62.65) | 3.53 (3.61) | - |
| 1d | 59 | 215-220 | C16H10ClNOS | 63.93 (64.00) | 3.30 (3.33) | 4.61 (4.66) |
| 1e | 70 | 189-192 | C19H14O2S | 74.47 (74.50) | 4.48 (4.57) | - |
| 2a | 60 | 181-185 | C15H12N2OS2 | 59.92 (60.00) | 4.58 (4.66) | 9.27 (9.33) |
| 2b | 45 | 170-174 | C14H10N2S2 | 62.22 (62.28) | 3.63 (3.70) | 10.37 (10.47) |
| 2c | 53 | 184-189 | C14H9ClN2S2 | 55.02 (55.08) | 2.79 (2.95) | 9.11 (9.18) |
| 2d | 42 | 202-207 | C17H10ClN3S2 | 57.15 (57.30) | 2.80 (2.85) | 11.78 (11.79) |
| 2e | 47 | 190-194 | C20H14N2OS2 | 66.22 (66.29) | 3.82 (3.86) | 7.83 (7.73) |
Table 1: Physical Data of Synthesized Compounds 1a-2e
| Compound | Yield (%) |
m.p.(º) | Mol. formula (Mol.Wt.) |
Found % (calculated) | ||
|---|---|---|---|---|---|---|
| C | H | N | ||||
| 3a | 75 | 219-225 | C16H14N2OS2 | 61.07 (61.14) | 4.40 (4.45) | 8.85 (8.91) |
| 3b | 60 | 212-217 | C15H12N2S2 | 63.27 (63.38) | 4.26 (4.22) | 8.66 (8.77) |
| 3c | 47 | 195-202 | C15H11ClN2S2 | 56.38 (56.42) | 3.38 (3.44) | 9.70 (9.85) |
| 3d | 58 | 215-220 | C18H12ClN3S2 | 58.29 (58.37) | 3.20 (3.24) | 11.28 (11.35) |
| 3e | 67 | 205-211 | C21H16N2OS2 | 66.98 (67.02) | 4.27 (4.25) | 7.33 (7.44) |
| 4a | 67 | 212-213 | C20H22N4OS | 65.44 (65.48) | 5.94 (6.00) | 15.24 (15.28) |
| 4b | 75 | 199-202 | C19H20N4S | 67.70 (67.76) | 5.88 (5.94) | 16.59 (16.64) |
| 4c | 71 | 212-214 | C25H24N4OS | 69.94 (70.00) | 5.55 (5.60) | 13.00 (13.06) |
| 4d | 72 | 198-200 | C24H22N4S | 72.20 (72.26) | 5.47 (5.52) | 14.00 (14.05) |
| 4e | 63 | 200-203 | C25H24N4OS | 69.96 (70.00) | 5.54 (5.60) | 13.00 (13.06) |
| 5a | 71 | 212-214 | C25H24N4OS | 69.94 (70.00) | 5.55 (5.60) | 13.00 (13.06) |
| 5b | 72 | 198-200 | C24H22N4S | 72.20 (72.26) | 5.47 (5.52) | 14.00 (14.05) |
| 5c | 61 | 210-212 | C24H21ClN4S | 66.45 (66.51) | 4.80 (4.85) | 12.87 (12.93) |
| 5d | 55 | 220-221 | C27H22ClN5S | 66.88 (66.94) | 4.50 (4.54) | 14.40 (14.46) |
| 5e | 91 | 229-230 | C30H26N4OS | 73.32 (73.37) | 5.24 (5.29) | 11.36 (11.41) |
Table 2: Physical Data of Synthesized Compounds 3a-5e
Synthesis of (2E)-3-(4-methoxyphenyl)-1-(thiophen-2-yl)prop-2-en-1-one(1a)
A mixture of 2-acetylthiophene (1.26 g, 0.01 mol) and anisaldehyde (1.36 ml, 0.01 mol) were stirred in ethanol (15 ml), then an aqueous solution of 40% potassium hydroxide (10 ml) was added and stirring was continued for 2 h. The mixture was kept overnight at room temperature, poured into crushed ice and then acidified with dilute hydrochloric acid. The solid separated was filtered and recrystallized from ethyl acetate, ethanol mixture (8:2) gave pure yellow crystalline solid 1a. Same procedure was followed for compounds 1b-e.
Synthesis of 4-(4-methoxyphenyl)-6-(thiophen-2-yl) pyrimidine-2-thiol(2a)
A mixture of (2E)-3-(4-methoxyphenyl)-1-(thiophen- 2-yl)prop-2-en-1-one (1a) (2.44 g, 0.01 mol) and thiourea (0.76 g, 0.01 mol) in 1,4-dioxane (10 ml) and a catalytic amount of acetic acid are taken in a round bottomed flask and refluxed for about 24 h. The progress of the reaction was monitored by TLC.
The reaction mixture was cooled, and poured into ice cold water with stirring. The product was filtered, dried and recrystallized using ethanol gave pure crystalline solid 2a. Same procedure was followed for compounds 2b-e from 1b-e.
Synthesis of 4-(4-methoxyphenyl)-2- (methylsulfanyl)-6-(thiophen-2-yl)pyrimidine (3a)
To a solution of 4-(4-methoxyphenyl)-6-(thiophen- 2-yl)pyrimidine-2-thiol (2a)(3.0 g. 0.01 mol) in dimethyl formamide (20 ml), potassium carbonate (2.76 g, 0.02 mol) and methyl iodide (2.84 g, 0.02 mol) were added and stirred for 4 h. Reaction time and completion of reaction was monitored by TLC. Then reaction mixture was diluted with cold water and neutralised by glacial acetic acid. The product was filtered off, dried and recrystallized from ethanol to give pure crystalline solid 3a. Same procedure was followed for compounds 3b-e from 2b-e.
Synthesis of 4-(4-methoxyphenyl)-2-(4- methylpiperazin-1-yl)-6-(thiophen-2-yl)pyrimidine (4a)
4-(4-Methoxyphenyl)-2-(methylsulfanyl)-6-(thiophen- 2-yl)pyrimidine (3a)(0.314 g, 0.001 mol) and N-methylpiperazine ( 0.1 ml, 0.001 mol) in dry ethanol (15 ml) were taken in a round bottomed flask and the reaction mixture was refluxed for 12 h in presence of catalytic amount of potassium hydroxide. After completion of reaction, it was then poured into crushed ice. The solid separated was filtered, dried and recrystallized using ethanol to give pure crystalline solid 4a. Same procedure was followed for compounds 4b-e from 3b-e.
Synthesis of 4-(4-methoxyphenyl)-2-(4- phenylpiperazin-1-yl)-6-(thiophen-2-yl)pyrimidine (5a)
4-(4-Methoxyphenyl)-2-(methylsulfanyl)-6-(thiophen- 2-yl)pyrimidine (3a)(0.314 g, 0.001 mol) and N-phenylpiperazine (0.16 ml, 0.001 mol) in dry ethanol (15 ml) were taken in a round bottomed flask and the reaction mixture was refluxed for 12 h in presence of catalytic amount of potassium hydroxide. After completion of reaction, it was then poured into crushed ice. The solid separated was filtered, dried and recrystallized using ethanol to give pure crystalline solid 5a. Same procedure was followed for compounds 5b-e from 3b-e.
Characteristics of 1a-e
1a;IR (KBr)cm-1: 1614 (CH=CH), 1644 (C=O);1H NMR δ: 6.93-6.94 (dd, 2H, CH=CH), 6.95-7.86 (m, 7H, aromatic protons), 3.87 (s, 3H, OCH3); MS: m/z 244. 1b; IR (KBr)cm-1: 1610 (CH=CH), 1658 (C=O); 1H NMR δ: 6.75-6.70 (dd, 2H, CH=CH), 7.17-7.82 (m, 8H, aromatic protons); MS m/z: 214. 1c; IR (KBr)cm-1: 1625 (CH=CH), 1650 (C=O), 730 (C-Cl); 1H NMR δ: 6.75-6.70 (dd, 2H, CH=CH), 6.95-7.82 (m, 7H, aromatic protons); MS m/z: 249. 1d; IR (KBr)cm-1: 1622 (CH=CH), 1647(C=O), 785 (C-Cl); 1H NMR δ: 6.83-6.90 (dd, 2H, CH=CH), 7.10-7.76 (m, 8H, aromatic protons); MS m/z: 300. 1e; IR (KBr)cm-1: 1629 (CH=CH), 1640 (C=O);1H NMR δ:6.81-6.88 (dd, 2H, CH=CH), 7.04-7.70 (m, 12H, aromatic protons); MS m/z: 306.
Characteristics of 2a-e
2a; IR (KBr)cm-1: 2363 (S-H), 1250 (C-O-C); 1H NMR δ: 12.50 (b, 1H, SH), 3.86 (s, 3H, OCH3), 7.17-8.19 (m, 8H, aromatic protons); MS: m/z 300. 2b; IR (KBr)cm-1: 2375 (S-H); 1H NMR δ: 13.10 (b, 1H, SH), 7.02-8.22 (m, 9H, aromatic protons); MS: m/z 270. 2c; IR (KBr)cm-1: 2380 (S-H), 790 (C-Cl); 1H NMR δ: 12.87 (b, 1H, SH), 7.10-8.12 (m, 8H, aromatic protons); MS: m/z 305. 2d;IR (KBr) cm-1:2365 (S-H), 790 (C-Cl);1 H NMR δ: 12.95 (b, 1H, SH), 7.17-8.10 (m, 9H, aromatic protons); MS: m/z 356. 2e; IR (KBr) cm-1: 2360 (S-H); 1H NMR δ: 13.04 (b, 1H, SH), 7.16-8.24 (m, 13H, aromatic protons); MS: m/z 362.
Characteristics of 3a-e
3a;IR (KBr)cm-1: 827(C-S-C), (C-O-C), 1645(C=N);1H NMR δ: 2.17 (s, 3H, SCH3), 6.64-7.70 (m, 8H, aromatic protons), 3.85 (s, 3H, OCH3);MS: m/z 314.3b;IR (KBr)cm-1: 780 (C-S-C), 1630 (C=N);1H NMR δ: 2.20 (s, 3H, SCH3), 6.80-7.88 (m, 9H, aromatic protons); MS: m/z 284. 3c; IR (KBr)cm-1: 815 (C-S-C), 752 (C-Cl), 1644 (C=N); 1H NMR δ: 2.04 (s, 3H, SCH3), 7.02-7.12 (m, 8H, aromatic protons); MS: m/z 319.3d;IR (KBr)cm-1: (C-S-C), 1604 (C=N); 1H NMR δ: 2.44 (s, 3H, SCH3), 6.68- 8.11 (m, 9H, aromatic protons); MS: m/z 370. 3e; IR (KBr)cm-1: 821 (C-S-C), 1634 (C=N); 1H NMR δ: 2.15 (s, 3H, SCH3), 6.66-8.25 (m, 13H, aromatic protons); MS: m/z 376.
Characteristics of 4a-e
4a;IR (KBr)cm-1: 1120 (C-N-C), 1607 (C=N); 1H NMR δ: 2.45 (s, 3H, NCH3), 2.80 (m, 4H, 2CH2), 3.25 (m, 4H, 2CH2), 6.65-6.90 (m, 4H, aromatic protons) 6.95-7.60 (m, 4H, aromatic protons), 3.88 (s, 3H, OCH3); 13C NMR 164.9, 161.5, 159.6, 150.9, 144.2, 131.3, 129.1, 126.9, 126.1, 125.6, 95.9, 55.9, 55.8, 51.4, 45.8; MS m/z: 366. 4b; IR (KBr)cm-1: 1125 (C-N-C), 1628 (C=N); 1H NMR δ: 2.42 (s, 3H, NCH3), 2.70 (m, 4H, 2CH2), 3.20 (m, 4H, 2CH2), 6.60-6.92 (m, 4H, aromatic protons) 6.95-7.59 (m, 5H, aromatic protons); 13C NMR 163.8, 162.4, 159.2, 151.1, 141.8, 133.9, 130.1, 128.5, 127.2, 126.4, 125.1, 96.6, 55.1, 50.3, 46.3; MS m/z: 336. 4c; IR (KBr)cm-1:1126 (C-N-C), 1622 (C=N); 1H NMR δ: 2.40 (s, 3H, NCH3), 2.85 (t, 4H, 2CH2), 3.29 (t, 4H, 2CH2), 6.90-7.20 (m, 4H, aromatic protons), 7.30-7.80 (m, 4H, aromatic protons); 13C NMR 163.1, 160.2, 152.1, 141.4, 134.8, 129.9, 128.6, 127.4, 126.1, 125.1, 95.6, 54.1, 50.4, 46.1; MS m/z: 371. 4d; IR (KBr) cm-1: 1125 (C-N-C), 1627 (C=N), 765 (C-Cl); 1H NMR δ: 2.37 (s, 3H, NCH3), 3.25 (m, 4H, 2CH2), 2.70 (m,4H, 2CH2), 6.99-7.26 (m, 4H, aromatic protons). 7.35-7.96 (m, 5H, aromatic protons); 13C NMR 163.3, 160.5, 161.2, 151.3, 146.9, 141.4, 137.3, 131.5, 129.4, 128.8, 127.8, 126.1, 125.3, 94.2, 53.9, 49.8, 45.2; MS m/z: 422. 4e; IR (KBr)cm-1: 1129 (C-N-C), 1620 (C=N); 1H NMR δ: 2.30 (s, 3H, NCH3), 2.75 (m, 4H, 2CH2), 3.20 (m, 4H, 2CH2), 6.99-7.30 (m, 6H, aromatic protons), 7.40-8.20 (m, 7H, aromatic protons); 13C NMR 163.5, 162.7, 159.8, 158.1, 141.1, 129.9, 128.4, 127.4, 126.4, 125.6, 122.8, 119.1,117.9, 96.2, 54.3, 50.2, 46.1; MS m/z: 428.
Characteristics of 5a-e
5a;IR (KBr)cm-1: 1125 (C-N-C), 830 (C-S-C), 1650 (C=N); 1H NMR δ: 2.85 (m, 4H, 2CH2), 3.25 (m, 4H, 2CH2), 7.26-7.66 (m, 6H, aromatic protons), 7.80-8.14 (m, 7H, aromatic protons), 3.80 (s, 3H, OCH3); 13C NMR 165.6, 165.2, 161.1, 159.4, 151.6, 143.4, 130.8, 129.6 128.7, 128.6,126.6, 126.0, 118.8, 116.4, 116.1, 98.6, 56.7, 49.8;MS m/z: 428.5b; IR (KBr)cm-1:1133 (C-N-C), 1635 (C=N); 1H NMR δ: 2.89 (m, 4H, 2CH2), 3.20 (m, 4H, 2CH2), 7.14-7.54 (m, 6H, aromatic protons), 7.60-8.20 (m, 8H, aromatic protons); 13C NMR 163.2, 162.6, 159.9, 151.1, 141.4, 134.3, 129.4, 128.8, 127.4, 126.1, 125.1, 124.1, 118.1, 114.3, 98.6, 51.1, 49.3; MS m/z: 398.
5c;IR (KBr)cm-1: 1230 (C-N-C), 760 (C-Cl); 1H NMR δ: 2.90 (m, 4H, 2CH2), 3.25 (m, 4H, 2CH2), 7.13- 7.53 (m, 6H, aromatic protons), 7.59-7.99 (m, 7H, aromatic protons); 13C NMR 163.5, 159.9, 152.1, 141.4, 134.3, 129.4, 128.8, 127.4, 126.1, 125.1, 124.1, 119.1, 115.3, 95.6, 52.1, 49.9; MS m/z: 433. 5d; IR (KBr)cm-1: 1120 (C-N-C), 755 (C-Cl); 1H NMR δ: 2.85 (m, 4H, 2CH2), 3.20 (m, 4H, 2CH2), 7.04- 7.49 (m, 6H, aromatic protons), 7.61-7.79 (m, 8H, aromatic protons); 13C NMR 163.5, 160.1, 159.9, 150.8, 146.4, 141.4, 137.3, 131.5, 129.4, 128.8, 127.8, 126.1, 125.3, 118.1, 115.8, 94.2, 51.1, 49.6; MS m/z: 484. 5e; IR (KBr)cm-1: 1125 (C-N-C), 1638 (C=N); 1H NMR δ: 3.00 (m, 4H, 2CH2), 3.22 (m, 4H, 2CH2), 7.21-7.28 (m, 8H, aromatic protons), 7.35-7.88 (m, 10H, aromatic protons); 13C NMR 163.8, 162.9, 160.1, 158.9, 150.8, 140.5, 129.9, 128.4, 127.5, 126.2, 125.3,122.1, 118.1,117.4, 115.1, 95.2, 51.3, 49.7; MS m/z: 490.
Antibacterial activity
Some selected compounds were screened for their in vitro antibacterialactivityagainst Gram positive bacteria namely Staphylococcus aureus (NCIM2492), Bacillus subtilis (NCIM2088) and Gram negative bacteria Escherichia coli (NCIM2138) and Salmonella paratyphi-A (ATCC3220). The activity was carried out using the cup-plate agar diffusion method [14]. The test compounds were dissolved in dimethyl formamide to obtain a solution of 40 μg/ml concentration. The inhibition zones of microbial growth produced by different compounds were measured in millimetres at the end of an incubation period of 48 h at 38º dimethyl formamide alone showed no inhibition zone. Choramphenicol was employed as reference standard (40 μg/ml) to evaluate the potency of tested compounds. The results are illustrated inthe Table 3.
| Compound | Diameter of zone of inhibition (mm) | |||
|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | S. paratypi-A | |
| 4a | 14 | 08 | 10 | 12 |
| 4b | 16 | 13 | 15 | 17 |
| 4c | 11 | 12 | 14 | 18 |
| 4d | 18 | 14 | 16 | 15 |
| 4e | 11 | 12 | 15 | 16 |
| 5a | 19 | 13 | 15 | 15 |
| 5b | 17 | 13 | 17 | 17 |
| 5c | 14 | 10 | 15 | 15 |
| 5d | 10 | 10 | 12 | 18 |
| 5e | 12 | 10 | 13 | 11 |
| DMF | 00 | 00 | 00 | 00 |
| Chloramphenicol | 20 | 14 | 18 | 22 |
Chloramphenicol was as use as standard and dimethylformamide was as used as control, zone of inhibition expressed in mm. The cup plate method was followed and chloramphenicol was used as standard. The concentration of the drug used is 40 μg/ml, DMF: dimethyl formamide
Table 3: Antibacterial Activity of The Compounds
Antifungal activity
Some selected compounds were screened for theirantifungal activity against fungi Aspergillus niger (ATCC9687), Pencillium notatum (NCIM746), Aspergillus fumigates (ATCC9389) and Candida albicans (NCIM3466). The activity was carried out using the cup-plate agar diffusion method [14]. The test compounds were dissolved in dimethyl formamide to obtain a solution of 40 μg/ml concentration. The inhibition zones of fungal growth produced by different compounds were measured in millimetres at the end of an incubation period of 48 h at 38º. Dimethyl formamide alone showed no inhibition zone. Fluconazole was employed as reference standard and results were shown in Table 4. Most of the tested compounds showed significant antifungal activity comparable with that of the standard drug fluconazole. The investigation of antibacterial and antifungal screening data revealed that the some selected compounds showed moderate to good inhibition at 40 μg/ml concentration. However the activity was less compared to the standard drugs.
| Compound | Diameter of zone of inhibition (mm) | |||
|---|---|---|---|---|
| A. niger | P. notatum | A. fumigatus | C. albicans | |
| 4a | 22 | 21 | 23 | 17 |
| 4b | 19 | 12 | 15 | 16 |
| 4c | 20 | 20 | 19 | 14 |
| 4d | 20 | 22 | 22 | 16 |
| 4e | 23 | 21 | 23 | 15 |
| 5a | 19 | 19 | 20 | 16 |
| 5b | 12 | 19 | 18 | 14 |
| 5c | 22 | 20 | 19 | 15 |
| 5d | 15 | 18 | 15 | 13 |
| 5e | 23 | 23 | 18 | 19 |
| DMF | 00 | 00 | 00 | 00 |
| Fluconazole | 25 | 25 | 25 | 19 |
Fluconazole was use as standard and dimethylformamide was used as control, zone of inhibition expressed in mm. The cup plate method was followed and fluconazole was used as standard. The concentration of the drug used is 40 μg/ml, DMF: dimethyl formamide
Table 4: Antifungal Activity Data of Compounds
Results and Discussion
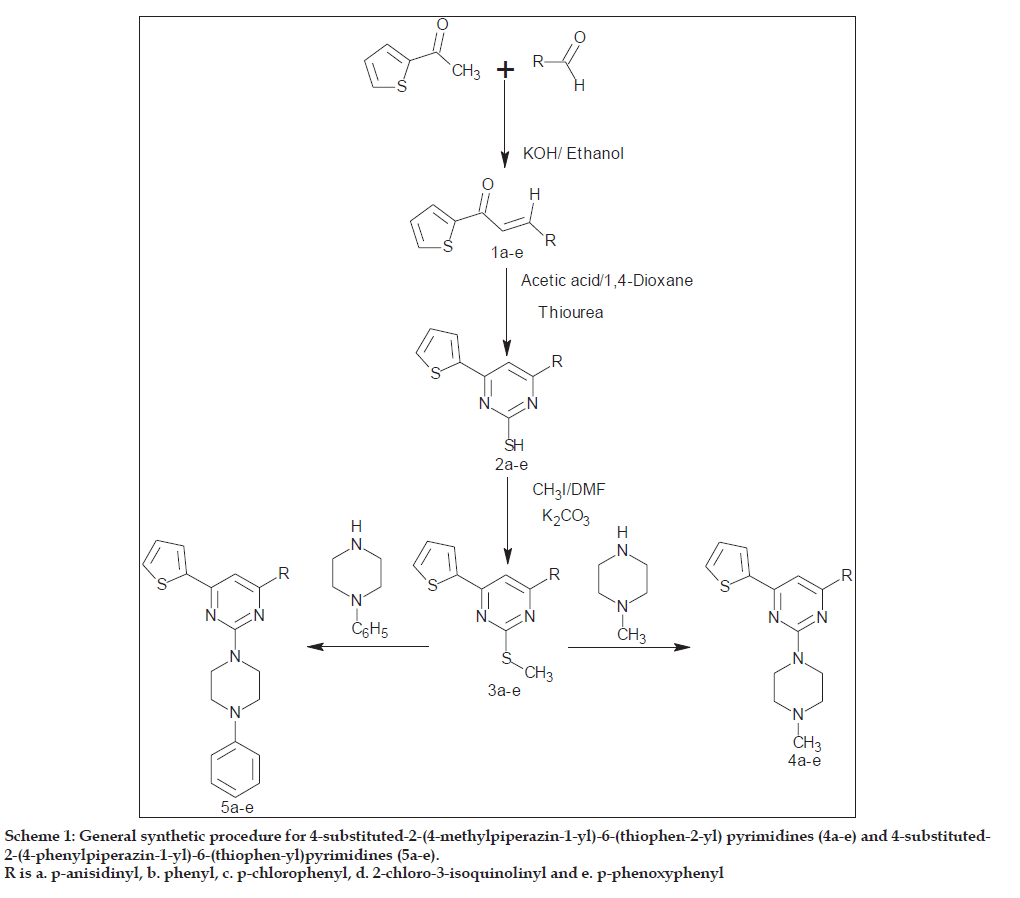
In this article, we reported the synthesis and biological properties of some pyrimidine linked piperazine derivatives (Scheme 1). The chalcones (1a-e) have been prepared by refluxing 2-acetylthiophene with aromatic aldehydes in presence of potassium hydroxide in ethanol medium. All the chalcones have been characterised by elemental analysis and spectral studies.
Scheme 1: General synthetic procedure for 4-substituted-2-(4-methylpiperazin-1-yl)-6-(thiophen-2-yl) pyrimidines (4a-e) and 4-substituted-2-(4-phenylpiperazin-1-yl)-6-(thiophen-yl)pyrimidines (5a-e).
R is a. p-anisidinyl, b. phenyl, c. p-chlorophenyl, d. 2-chloro-3-isoquinolinyl and e. p-phenoxyphenyl
Compounds (1a-e) were then refluxed with thiourea in presence of acetic acid in 1,4-dioxane solvent to afford 4-substituted-6-(thiophen-2-yl)pyrimidine-2-thiols (2a-e) in good yield. The formation of (2a-e) was monitored by TLC. Compound(2a) exhibited absorption band at 2363 cm-1 corresponding to SH stretching in its IR spectrum. The 1H NMR spectrum showed a singlet at 3.86 δ due to three protons of OCH3 group and a singlet at 12.50 δ due to one protons of SH group. Further, a molecular ion peak at m/z 300 in its mass spectrum is in agreement with the structure.
4-Substituted-2-(methylsulfanyl)-6-(thiophen- 2-yl)pyrimidines (3a-e) were prepared by the treatment of (2a-e) with methyliodide in presence of potassium carbonate in dimethyl formamide medium. The IR spectrum of (3a) exhibited an absorption band at 827 cm-1 due to C-S-C group.
The 1H NMR spectrum of compound (3a) showed a singlet at 3.85 δ due to three protons of OCH3 group and singlets at 2.17 δ for three protons of one SCH3 group. Further, it showed a molecular ion peak at m/z 314 in its mass spectrum is in agreement with the structure.
Compounds (3a-e) on refluxion with N-methylpiperazine and N-phenylpiperazine in presence of catalytic amount of potassium hydroxide produced 4-substituted-2-(4-methylpiperazin-1-yl)-6- (thiophen-2-yl)pyrimidines (4a-e) and 4-substituted-2- (4-phenylpiperazin-1-yl)-6-(thiophen-2-yl)pyrimidines derivatives (5a-e). The reactions were monitored by TLC. In confirmation, the IR spectrum of (4a) absorption band at 1120cm-1 due to C-N-C group. The 1H NMR spectrum showed a singlet at 3.88 δ due to three protons of OCH3 group, a singlet at 2.45 δ due to three protons NCH3 group and two triplets in the range 2.80-3.25 δ for eight piperazine protons. Its mass spectrum showed a molecular ion peak at m/z 366 which is in agreement with the structure. The IR spectrum of (5a) absorption band at 1125 cm-1 due to C-N-C group. The 1H NMR spectrum showed a singlet at δ 3.80 due to three protons of OCH3 group, two triplets in the range δ 2.85-3.25 for eight piperazine protons. Its mass spectra showed a molecular ion peak at m/z 428 which is in agreement with the structure.
The compounds 4b, 4d, 5a and 5b showed good antibacterial activity against Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Salmonella paratyphi-A at 40 μg/ml concentration. Compounds 4a, 4d, 4e, 5c and 5e showed significant antifungal activity against Aspergillus niger, Pencillium notatum, Aspergillus fumigates and Candida albicans at 40 μg/ml concentration. However the activity was less compared to the standard drug. The data of these studies is summarized in Tables 3 and 4.
The standard drugs chloramphenicol and fluconazole have structural analogues of synthesized piperazine derivatives. Due to the presence of electronegativity functionality such as nitrogen, oxygen and sulphur containing heterocyclic ringsin the synthesized compounds, it enhances the antimicrobial activity.
In conclusion, in the present work, thiophene substituted chalcones (1a-e) were cyclised with thiourea in presence of potassium hydroxide to get 4-substituted-6-(thiophen-2-yl)pyrimidine-2-thiols (2a-e) which were then stirred with methyl iodide to obtain 4-substituted-2-(methylsulfanyl) (thiophen- 2-yl)pyrimidines (3a-e). Compounds (3a-e) were refluxed with different N-methylpiperazine and N-phenylpiperazine to afford 4-substituted-2-(4- methylpiperazin-1-yl)-6-(thiophen-2-yl) pyrimidines (4a-e) and 4-substituted-2-(4-phenylpiperazin-1-yl)-6- (thiophen-2-yl) pyrimidines (5a-e). The synthesized compounds were characterised by analytical data, IR, 1HNMR, 13C NMR and mass spectral studies, then compounds were screened for antibacterial and antifungal activities.
Acknowledgements
Authors thank the Principal, Sahyadri Science College (Autonomous), Shimoga and the Chairman, Department of chemistry, Sahyadri Science College (Autonomous), Shimoga, for providing necessary laboratory facilities. Authors are also thankful to the Principal, SCS College of Pharmacy, Harapanahalli, for providing facilities to conduct biological activities.
References
- Dyck B, Parker J, Phillips T, Carter L, Murphy B, Summers R, et al. Aryl piperazine melanocortin MC4 receptor agonists. Bioorg Med Chem Lett 2003;13:3793-6.
- Johan FK, Nicholas AM, Tao W, Zhiwei Y, Zhongxing Z. Piperazine enamines as antiviral agents. Patent no EP2029532, 2012.
- Tominaga M, Yo E, Ogawa H, Yamashita S, Yabuuchi Y, Nakagawa K. Synthesis of 3,4-dihydro-6-[4-(3,4-dimethoxybenzoyl)-1-piperizinyl]-2(1H)-quinolone and related compounds. Chem Pharm Bull 1984;32:2100-10.
- El-Emam AA, Al-Deeb OA, Al-Omar M, Lehmann. Synthesis, Antimicrobial, and AntiHIV-1 Activity of Certain 5-(1-Adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-Adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. J Bioorg Med Chem 2004;12:5107-13.
- Ayman EF, Mercedes AU, Jose AE, Fernando A. Use of N-methylpiperazine for the preparation of piperazine-based unsymmetrical bis-ureas as antiHIV agents. Chem Med Chem 2008;3:1034-7.
- Taverne T, Diouf O, Depreux P, Poupaert JH, Lesieur D, Guardiola LB, et al. Novel benzothiazolin-2-one and benzoxazin-3-one arylpiperazine derivatives with mixed 5HT1A/D2 affinity as potential atypical antipsychotics. J Med Chem 1998;41:2010-8.
- Lyon RA, Titeler M, McKenney JD, Magee PS, Glennon RA. Synthesis and evaluation of phenyl and benzoylpiperazines as potential serotonergic agents. J Med Chem 1986;29:630-4.
- Patel HS, Desai HD, Mistry HJ. Synthesis an antimicrobial activity of some new piperazine derivatives containing aryl sulfonyloxy group. Eur J Chem 2004;1:93-8.
- Romero DL, Morge RA, Biles C, Berrios PN, May PD, Palmer JR, et al. Discovery, synthesis and bioactivity of bis(heteroaryl)piperazines. A Novel class of non-nucleoside HIV-1 reverse transcriptase inhibitors. J Med Chem 1994;37:999-1014.
- Romero DL, Olmsted RA, Poel TJ, Morge RA, Biles C, Keiser BJ, et al. Targeting delavirdine/atevirdine resistant HIV-1: Identification of (alkylamino)piperidine-containing bis(heteroaryl)piperazinesas broad-spectrum HIV-1 reverse transcriptase inhibitors. J Med Chem 1996;39:3769-89.
- Hussein AM, Ahmed OM. Regioselective one-pot synthesis and anti-proliferative and apoptotic effects of some novel tetrazolo[1,5-a] pyrimidine derivatives. J Bioorg Med Chem 2010;18:2639-44.
- Chen CN, Chen Q, Liu YC, Zhu XL, Niu CW, Xi Z, et al. Syntheses and herbicidal activity of new triazolopyrimidine-2-sulfonamides as acetohydroxyacid synthase inhibitor. J Bioorg Med Chem 2010;18:4897-904.
- Khobragade CN, Bodade RG, Konda SG, Dawane BS, Manwar AV. Synthesis and antimicrobial activity of novel pyrazolo[3,4-d]pyrimidin derivatives. Eur J Med Chem 2010;45:1635-8.
- Barry AL. The Antimicrobial Susceptibility Test: Principle and Practice. Chapter 21. Philadelphia, USA: Lea and Febiger; 1976. p. 180.