- *Corresponding Author:
- Hema A. Nair
Department of Pharmaceutics, Bombay College of Pharmacy, Kalina, Mumbai, India
E-mail: hemastudent@gmail.com
| Date of Submission | 06-May 2010 |
| Date of Revision | 27 December 2010 |
| Date of Acceptance | 28 December 2010 |
| Indian J Pharm Sci, 2010, 72 (6): 766-774 |
Abstract
Modification of polymers by covalent attachment of thiol bearing pendant groups is reported to impart many beneficial properties to them. Hence in the present study, sodium alginate-cysteine conjugate was synthesized by carbodiimide mediated coupling under varying reaction conditions and the derivatives characterized for thiol content. The thiolated alginate species synthesized had bound thiol content ranging from 247.8±11.03-324.54±10.107 Îůmol/g of polymer depending on the reaction conditions. Matrix tablets based on sodium alginate-cysteine conjugate and native sodium alginate containing tramadol hydrochloride as a model drug were prepared and mucoadhesive strength and in vitro drug release from the tablets were compared. Tablets containing 75 mg sodium alginate-cysteine conjugate could sustain release of 10 mg of model drug for 3 h, whereas 90% of the drug was released within 1 h from corresponding tablets prepared using native sodium alginate. An approximately 2-fold increase in the minimal detachment force of the tablets from an artificial mucin film was observed for sodium alginate-cysteine conjugate as compared to native sodium alginate. In vitro cytotoxicity studies in L-929 mouse fibroblast cells studied using an MTT assay revealed that at low concentrations of polymer, sodium alginate-cysteine conjugate was less toxic to L-929 mouse fibroblast cell line when compared to native sodium alginate. Hence, thiolation is found to be a simple route to improving polymer performance. The combination of improved controlled drug release and mucoadhesive properties coupled with the low toxicity of these new excipients builds up immense scope for the use of thiolated polymers in mucoadhesive drug delivery systems.
Keywords
Cytotoxicity, mucoadhesion, sodium alginate, thiolation
The development of mucoadhesive systems are of great interest in drug delivery science. These systems increase the residence time of formulations by localization at the mucosal surface and can also be postulated to increase the bioavailability of drugs by providing an intimate contact of delivery system to the absorbing membrane [1]. By tailoring the duration and extent of mucoadhesion, intelligent drug delivery systems can be developed.
Despite the above advantages, developments in the area of mucoadhesive drug delivery systems, most of which depend on the use of mucoadhesive polymers, has not reached its full potential. Conventional mucoadhesive polymers such as carbomers and chitosan are believed to bind with mucosa via hydrogen bonding and ionic interaction, respectively. Therefore, these polymers provide weak adhesion and are unable to localize a delivery system for sufficient period of time to achieve the advantages stated above [2].
Various approaches have been used to improve the adhesion strength of the polymer. Target specific bioadhesive polymers such as plant derived lectins that bind to specific sugars [3] and bacteria derived polymeric materials such as those from the fimbriae [4] are some of the newer mucoadhesive components under investigation for drug delivery.
A class of polymeric excipients introduced in pharmaceutical literature in the recent past is the thiolated polymers- also called thiomers. These are chemically modified mucoadhesive polymers containing sulfhydryl (thiol) groups in the backbone structure [5]. Thiolated polymers adhere to the mucosal membrane by the formation of covalent disulfide bonds between numerous cysteine residues of the mucin molecule and thiol groups of the polymers [6]. Formation of covalent bonds improves the mucoadhesive strength and results in a superior ‘Pharmaceutical Glue’ for improved drug delivery.
In previous reports various thiolated polymers have been synthesized including polycarbophilcysteamine [7], polyacrylic acid-cysteine [8], and chitosan- 2-iminothiolane [9]. Thiolation has been found to impart a number of beneficial properties to the polymers including improved mucoadhesion, extended drug release, enzyme inhibitory potential and permeation enhancing effect with varying degrees of success.
Sodium alginate is a naturally occurring hydrophilic polymer obtained from marine algae. Alginates are linear unbranched polysaccharides containing varying proportions of β-d-mannuronic acid and α-l-guluronic acid residues with good mucoadhesive and release retardant properties [10]. Sodium alginate has been widely investigated as oral drug delivery carrier for abundant therapeutic agents including vaccines, proteins and small molecular weight drugs in the form of tablets and microparticles [11,12].
Recently, Bernkop et al. demonstrated an improvement of mucoadhesive properties of sodium alginate due to covalent linkage of cysteine [13]. In addition, thiolation can also result in an improvement in the release retardant properties of this polymer due to intrapolymer chain crosslinking via disulfide bond formation. Further, for in vivo drug delivery applications, biocompatibility of the thiolated polymer needs to be established. The aim of the present paper therefore was twofold, first to synthesize and characterize thiolated sodium alginate and second to evaluate sustained release potential and safety of the newly synthesized polymer. Hence, sodium alginate-cysteine conjugates were synthesized and evaluated for thiol content, in vitro drug release and mucoadhesive strength. Biocompatibility of native and thiolated sodium alginate was evaluated in vitro using L-929 mouse fibroblast cells.
Tramadol HCl (TH), an opioid analgesic- was used as the model drug to study the controlled release properties. TH has a short plasma half life (4.5-5.0 h) and needs to be administered 3-4 times daily to achieve steady state plasma concentration [14].
Materials and Methods
Sodium alginate (Protanal® LF 240 D) was obtained as a gift sample from Signet Chemical Corporation, Mumbai, India. Cysteine hydrochloride monohydrate and sodium borohydride were purchased from S. D. Fine Chemicals, Mumbai, India. 1-Ethyl-3-(3- dimethylaminopropyl) carbodiimide hydrochloride (EDAC), Ellman’s Reagent (5,5-dithiobis (2-nitro benzoic acid), 2,4,6-trinitrobenzenesulfonic acid (TNBS), foetal calf serum, trypsin, 3-(4,5-dimethylthiazol–2- yl) -2,5-dimethyl tetrazolium bromide (MTT) and artificial porcine mucin were purchased from Sigma-Aldrich. Tramadol hydrochloride (TH) was received as a gift sample from Unichem Lab Ltd., Mumbai, India. Dialysis membrane (cut off size MW 12-16 KDa) and Dulbeccos Modified Eagle’s Medium (DMEM) were purchased from Himedia, Mumbai, India. L-929 mouse fibroblast cell line was procured from NCCS, Pune, India.
Synthesis of sodium alginate cysteine conjugates (SA-cys)
Sodium alginate (200 mg) was hydrated overnight in 10 ml water. Five millilitres of freshly prepared 200 mM EDAC solution was added to the polymer solution in order to activate the carboxyl groups of the polymer. pH of the reaction mixture was adjusted to 6.0 with 0.1M HCl and it was incubated for specified period as indicated in Table 1. After incubation with EDAC, 5 ml of an aqueous solution of cysteine hydrochloride monohydrate resulting in a weight ratio of 2:1 (polymer: Cysteine) was added to the reaction mixture. Reaction was allowed to proceed at room temperature at pH values as specified in Table 1 for a total of 3 h under continuous stirring with the help of a magnetic needle.
| Batch code | Incubation time (min) | pH of reaction with cysteine | |
|---|---|---|---|
| First 2 h | Remaining 1 h | ||
| B1 | 20 | 4 | 6 |
| B2 | 45 | 4 | 6 |
| B3 | 45 | 5 | 5 |
| B4 | 45 | 6 | 6 |
Different batches of thiolated alginate prepared by varying pH of reaction and time of incubation.
Table 1: Experimental Conditions For Preparation Of Sa-Cys
At the end of the reaction time, the aqueous solution was dialyzed to isolate the alginate -cysteine conjugate. Solution was tied up in a dialysis membrane and dialyzed against 1mM HCl (pH 4) for two days. Next the dialyzing fluid was replaced with fresh medium, now containing, in addition, 1% NaCl, twice, for 24 h each. Finally, dialysis was continued against 1mM HCl for two more days. Preparation and purification of the polymer was carried out in the dark as far as possible to minimize disulfide bond formation. Controls to polymers were prepared in the same way except that EDAC was omitted. The samples were frozen at -40º for 4 h and frozen samples were lyophilized by sequentially drying at -30º for 10 h, at -20º for 6 h, at -5º for 12 h, at 5º for 12 h, at 25º for 3 h and finally at 35º for 6 h (Vertex E9-91 freeze dryer).
Estimation of bound thiol content of sodium alginate-cysteine conjugates
The conjugate was characterized for its thiol content using Ellman’s reagent [9] in two steps. Thiol content was first determined before reduction of disulfide bonds. Initially, 10 mg of each SA-cys and control were hydrated in 5 ml of distilled water. Phosphate buffer (250 μl, 0.5 M, pH 8.0) and 500 μl of Ellman’s reagent were added to polymer solution (250 μl). The mixture was incubated for 2 h under ambient conditions. At the end of 2 h, absorbance of the above solutions was measured at a wavelength of 450 nm using Jasco V-530 UV/Vis spectrophotometer. The amount of free thiol groups were calculated from the standard plot prepared by treating increasing concentrations of cysteine hydrochloride monohydrate solutions as described above.
Next, the thiol content after reduction of disulfide bonds was quantified. To determine the total amount of bound thiol functions, oxidized thiol moieties were first reduced using sodium borohydride. Both SA-cys and control (10 mg each) were hydrated in 5 ml of distilled water. Phosphate buffer (750 μl, 0.05 M, pH 6.8) and freshly prepared sodium-borohydride solution (1 ml, 4% w/v) were added to the polymer solution (250 μl). The mixture was incubated for 1 h at room temperature. Thereafter, HCl (200 μl, 5 M) was added and the reaction mixture was agitated for 10 min. The solution was then neutralized by the addition of phosphate buffer (1 ml, 1M, pH 8.5) followed immediately by addition of 100 μl of freshly prepared Ellman’s reagent. After incubation for 2 h at room temperature absorbance was measured at 450 nm and thiol content was quantified.
The amount of unreacted cysteine in the SA-cys and control was estimated using 2,4,6-trinitrobenzenesulfonic acid (TNBS) reagent [7]. At first, 10 mg each of conjugate and control were hydrated with 1380 μl of water and then incubated with sodium bicarbonate solution (600 μl, 4% w/v) and TNBS reagent (20 μl, 5% w/v) for 2 h at 37º. Absorbance was measured at 450 nm using UV/Vis spectrophotometer and amount of free amino groups was quantified from a standard plot prepared by treating solutions containing increasing concentrations of cysteine hydrochloride monohydrate.
Formulation of matrix tablets
Tablets were compressed to evaluate mucoadhesive strength and controlled release properties of SA-cys and native polymer. In order to ensure homogenous mixing of drug and polymer, an aqueous solution of TH was added to the dialyzed polymer from batch B2 and to the control in the ratio of 1:7.5 (drug:polymer) and stirred. Above aqueous solution containing drug and polymer were then freeze dried as described earlier.
Matrix tablets of both SA-cys and control polymer each containing 10 mg TH were prepared by compressing 85 mg of the above freeze dried product using a 7 mm punch on a rotary tableting machine. No other excipients were added and tablets comprised of only polymer and drug.
Evaluation of tablets
For estimation of drug content, tablets prepared using SA-cys and control were deformed by crushing under a pestle head. Crushed tablet mass (25 mg) was placed on an agitating water bath under ambient conditions for 20 min with 5 ml methanol successively four times to effect complete drug extraction from the polymer matrix. Extracts were pooled and volume was made upto 25 ml with methanol. Absorbance of the above solution was measured at 271 nm. Amount of drug was calculated from a standard plot and expressed as a percentage of theoretical content of 10 mg TH per tablet.
In vitro release studies were carried out using USP apparatus I at 50 rpm in 100 ml of phosphate buffer, pH 6.8. Dissolution medium contained in a 100 ml beaker and placed on suitable glass support was immersed into dissolution vessel containing water. The dissolution medium was equilibrated at 37±0.2º and TH tablets based on SA-cys and control were placed in the basket. Aliquots of 5 ml each were withdrawn at different time points, filtered and the amount of drug released was measured using UV/Vis spectrophotometer at 271 nm. Each in vitro release study was performed in triplicate. The cumulative amounts of drug released at 2 h from both formulations were compared using t-test.
Measurement of mucoadhesive strength
To examine the effect of thiolation, in vitro mucoadhesion studies were carried out using a modified two pan balance. The left side of the balance was provided with Teflon® blocks attached at the top and in place of the pan at the bottom and the right side had a receptacle for water.
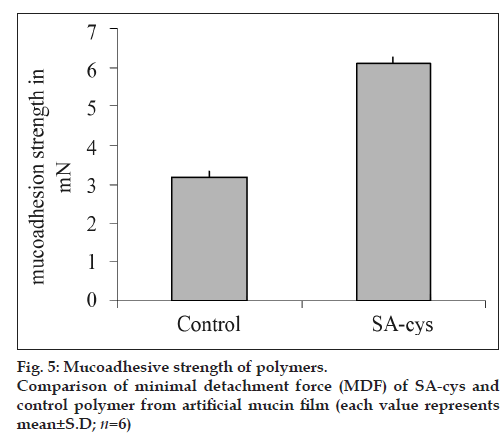
A film of 100 μl of artificial mucin solution (3% w/v in phosphate buffer pH 6.8) was prepared on the cover slip and dried over night. This coverslip was adhered on the upper block of the left side of the balance by two faced adhesive tape with the mucin coated side facing downward and balanced with the right pan containing a weight of 5 g. Tablets were initially hydrated by placing 10 μl phosphate buffer pH 6.8 on one lateral surface. The unhydrated surface of the tablet was adhered to the lower block of the balance using two faced tape, and hydrated surface was allowed to adhere with the mucin film for 30 s. At the end of 30 s water was transferred to the receptacle on the right pan at a rate of 1 ml/min using a peristaltic pump, until tablet was detached from the mucin film. Mucoadhesive strength was measured in terms of weight of water required to detach the mucin film from the tablet and reported in terms of Minimal Detachment Force (MDF), expressed in mN [weight in g×9.8 ms-2]. Five tablets each based on SA-cys (batch B2) and control polymer were evaluated.
In vitro cytotoxicity studies
L-929 mouse fibroblast cells were used to compare the cytotoxicity/biocompatibility of newly synthesized SA-cys and unmodified sodium alginate via an MTT test. Cells were subcultured in Dulbecco’s minimum essential medium containing 10% foetal calf serum and maintained within a carbon dioxide incubator (Heraceus BB15, India) under a humidified atmosphere of 5% CO2/95% O2. On confluence, cells were trypsinized and collected by centrifugation. The resultant cell suspension was quantified using haemocytometry followed by dilution with culture medium appropriately to a cell density of 2×105 cells/ ml. Above cell suspension was seeded into 96 well flat bottomed plates with 20000 cells in each well. The plates were incubated at 37° in a humidified atmosphere of 5% CO2/95% O2 for 24 h. Different concentrations including 0.1, 0.05, 0.01, 0.005 and 0.001% (w/v) of polymeric material was added into the wells as 100 μl quantities in triplicate. Blank wells contained only 100 μl of medium. The plates were incubated for 24 h. The test samples included similar dilutions of sodium alginate and sodium alginate–cysteine conjugate (SA-cys).
The test samples were prepared by dissolving 1.0 g of polymer in 10 ml of distilled water. The pH of the solution was adjusted to neutral and serial dilutions were prepared using distilled water. All the polymeric test solutions were sterilized by autoclaving at 121° for 15 min. The thiol content of the polymer was estimated post sterilization as described earlier.
At the end of 24 h the supernatant medium and test material were removed using fine gauze needle. Following this, 100 μl of a 2 mg/ml solution of MTT in phosphate buffered saline was added in each well. The plates were wrapped in aluminum foil for protection from light and incubated for 4 h in a humidified atmosphere of 5% O2/95% CO2. The MTT solution was removed using fine gauze needle and 100 μl of DMSO was added to dissolve the formazan crystals in each well. The absorbance was recorded at 540 nm on an ELISA plate reader.
The absorbance of the blank wells containing the cells in medium was considered as that corresponding to 100% proliferation and the relative cell proliferations in the treated wells were calculated. The relative percent proliferation in the wells treated with identical concentrations of thiolated and native sodium alginate were statistically compared using the student’s t-test at P<0.05.
Results
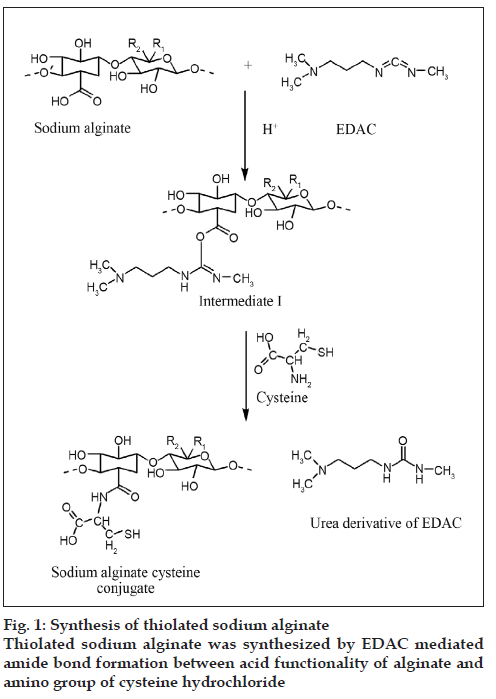
SA-cys was synthesized by amide bond formation between carboxylic acid groups of sodium alginate and primary amino groups of cysteine as shown fig 1. In order to arrive at optimal conditions for reaction, the derivatization was carried out under different conditions of pH and the products were compared in terms of the amount of immobilized thiol groups on the polymer.
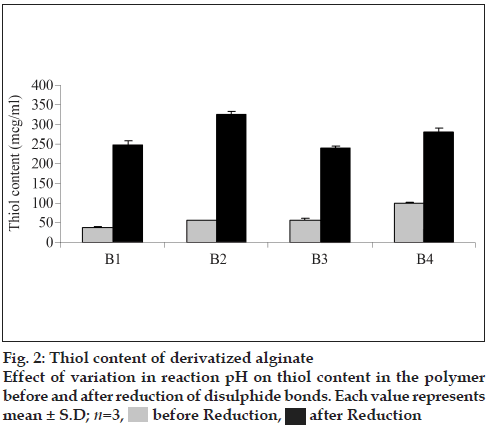
The amount of thiol groups per gram of polymer derivatized under different conditions is shown in fig. 2. Before reduction a maximum thiol content of 97.45 μmol/g was found to be present in batch B4 of SA-cys wherein a pH of 6 was maintained throughout the reaction. However, the thiol content determined after reduction with sodium borohydride demonstrated that optimal conditions for synthesis of sodium alginate cysteine-conjugate amongst the experiments carried out in the present study are: 45 min incubation of alginate with EDAC at pH 6 and reaction with cysteine at pH 4 for first 2 h and then at a pH of 6 for another hour which led to the highest yield of total covalently bound cysteine of 324.54 μmol/g (indicative of a polymer: cysteine ratio of 1: 0.039 for batch B2). The thiol content of this optimal batch is consistent with that of a report in literature for preparation of SA-cys conjugates under identical conditions [13]. Under all reaction conditions, the control polymer was found to have <0.028 μmol/g thiol content (i.e. the absorbance was less than the lowest point on the linearity curve for cysteine).
As observed from fig. 2, time of activation by EDAC as well as the pH of the reaction was found to influence the extent of thiolation. A 1.31 fold increment thiol content was obtained when the activation time of polymer with EDAC was increased from 20 to 45 min under identical reaction conditions. The extent of oxidation of thiol to disulfide i.e. ratio of thiol/disulfide group was found to steadily decrease with increase in reaction pH (fig. 3).
Less than 3.45 μg/ml =0.019 μmol/g (linearity range 3.45-17.25 μg/ml) primary amino groups could be detected in the SA-cys and the control using the TNBS-reagent which also suggests conjugation of cysteine to sodium alginate and presence of insignificant amounts of unreacted cysteine in polymer.
Drug content of the tablets prepared from the lyophilized premixes of SA-cys and control with TH were 104.14±1.17% (mean±SD) and 105.77±1.54% (n=3; mean±SD) respectively. From the low values of standard deviation, the prepared tablets were found to be uniform in respect of the evaluated parameter.
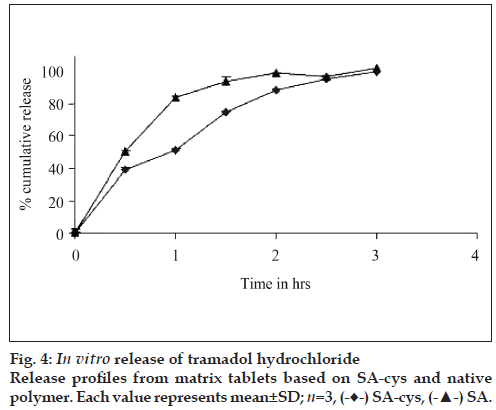
Release of TH from the tablets based on SA-cys and control is depicted in fig 4. Almost 90% of drug was released from the matrices based on the control polymers within 1 h, whereas matrices based on SAcys was capable of retarding the release of drug upto 3 h. It was also observed that after 3 h tablets based on control polymers had disintegrated completely whereas tablets based on SA-cys were present as a cohesive gelled mass.
Tensile studies represent the most widely employed in vitro test method for the assessment of the adhesive strength of mucoadhesives. In the present study, as shown in fig 5, an approximately 2-fold increase in the minimal detachment force (MDF) was observed for SA-cys as compared to control polymer, demonstrating the high affinity of the thiolated alginate for mucosal tissue. In a previous report by Bernkop et al. on thiolation of alginate, a 3.9-fold increase in total work of adhesion was observed for SA-cys conjugate containing comparable ratio of immobilized thiol groups [13].
Total thiol content of SA-cys after sterilization of its solution by autoclave was not found to be significantly (P<0.1) different from the content prior to sterilization with only a 0.94±0.19 % (mean±SD; n=2) decrease in thiol content.
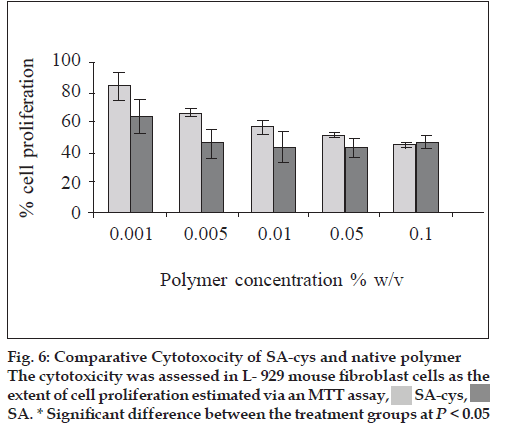
A concentration-dependent cytotoxicity profile was observed in all the tested compounds. At the lower concentrations of polymer (0.001 and 0.005% w/v) the thiolated derivative was significantly (P<0.05) less toxic than the native compound. As the polymer concentration increased from 0.001% to 0.1% w/v, for both samples, cell proliferation decreased (fig 6). One published study on in vitro cytotoxicity of thiolated polymers which were 4-thiobutylamidine conjugates of d-glucosamine and chitosan also reports similar dose dependent cytotoxicity [15].
Another observation was that the increase in toxicity with increasing concentration of polymer was more marked in case of the SA-cys treated cells.
Discussion
All features of thiolated polymers such as enhanced mucoadhesive strength, controlled drug release, permeation enhancement and enzyme inhibition depend on amount of sulfhydryl groups bound to the polymer structure. Therefore, SA-cys conjugate prepared was characterized for its thiol content expressed as μmol/gm of polymer.1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDAC), a water soluble coupling agent, is most commonly employed in polypeptide synthesis [16]. EDAC catalyzes the formation of amide bonds between carboxylic acids and amines by activating the carboxylate group to form an o-acylurea derivative. A 1:1 molar ratio of EDAC:-COOH group is essential for activation [5]. A suitable pH for this reaction is in the mildly acidic region and a pH of 6 was used in the present studies. In the next step of the reaction, nucleophilic attack by the nitrogen of the primary amino group of cysteine intermediate results in the formation of the amide bond between –COOH group of the sodium alginate and –NH2 group of the cysteine.
Rate of hydrolysis of intermediate formed during the coupling reaction [16] and extent of formation of disulfide bonds during the synthesis [17] are both pH-dependent and affect the amount of thiol groups bound to sodium alginate. Maintaining a pH of 4 during the first 2 h of the reaction resulted in the highest extent of thiolation. Hydrolysis rate of the o-acylurea intermediate formed in the reaction was possibly higher at pH values above 4 used in the experiment resulting in lesser extent of thiolation. Further, lower thiol content was obtained with a 20 min incubation period of sodium alginate with EDAC at pH 6. This could be the result of inadequate activation of carboxyl groups of sodium alginate, which results in the attachment of fewer cysteine molecules and consequently a lower thiol content.
The oxidation of thiol (-SH) to disulfide (-S-S-) during synthesis is inevitable. The extent of oxidation increases at pH values above 5 and on exposure to light or higher temperatures during reaction and isolation. Hence thiol content was estimated before and total bound thiol was estimated after the reduction of disulfide groups by sodium borohydride. In addition, free cysteine in the lyophilized polymer was also quantified to rule out the presence of unbound cysteine contributing to the thiol content. The bound and the unbound cysteine were quantified using Ellamn’s reagent complexing with the thiol group of cysteine and TNBS complexing with the amino group of cysteine, respectively.
The extent of oxidation of the immobilized thiol groups was found to be more at higher pH values. Thiol – disulfide interchange involves the nucleophilic attack of thiolate anion along the –S-S- bond axis of disulfide anion and as the pH is increased more thiolate anion is formed [17]. This results in greater extent of disulfide bond formation.
Free amino content of the SA-cys indicates the presence of unreacted cysteine not separated from the polymer during dialysis; the low value obtained indicating that free cysteine was nearly absent from the lyophilized polymer. Presence of negligible amount of remaining primary amino groups and numerous thiol moieties is a strong evidence of formation of amide bonds between alginate and cysteine.
Matrix tablets were prepared from lyophilized drug and polymer premixture. This was essential, since, due to the rubbery texture of the polymer, the drug could not be mixed homogenously with lyophilized product in case of both thiolated and control products. The polymer could neither be crushed nor sieved. Hence, in order to bring about homogenous mixing of the drug with polymer, aqueous solution of the drug was added to the polymer solution and the mixture was lyophilized. Tablets were compressed from this lyophilized product.
In vitro drug release studies were carried out at pH 6.8 because this pH is reported to favor the formation of inter/intra molecular disulfide bonds [17]. SA-cys revealed an improvement in release retarding potential in comparison to the control polymer. This ability to resist disintegration/erosion and prolong drug release from thiolated polymer based tablets is attributed to the formation of disulfide bonds within the three dimensional polymeric network [17]. This crosslinking leads to an increase in viscosity of the gel layer on the surface of the tablet and a corresponding lowering of the diffusivity of the drug molecules. Similarly, presence of the gelled mass of SA-cys tablets at the end of the three hour release study was due to the formation of intra-molecular disulfide bonds [13]. These findings are in agreement with previous literature reports on other thiolated polymers including thiolated derivatives of chitosan, carboxy methyl cellulose and polycarbophil [9,18].
Release studies were carried out for three hrs on tablets containing only 10 mg of TH due to the constraints in the scale up of synthesis of SA-cys. However, above findings can be used as a preliminary base for the further exploration of controlled release potential of SA-cys.
Mucins are large, extra cellular glycoproteins with molecular weights ranging from 0.5 to 20 MDa. 20% mass of the mucin molecule is made up of protein which is arranged into distinct regions. Regions located at both carboxyl and amino terminals have a high proportion of cysteine (>10%). These cysteine molecules present in the protein core of mucin are responsible for formation of disulfide bonds with –SH groups [19]. Therefore, enhanced mucoadhesive properties of thiolated alginate are due to the formation of covalent bonds between thiol groups of the polymer and cysteine-rich subdomains of glycoproteins in the mucus layer. The resulting disulfide bond is a bridging structure most commonly encountered in biological systems. In contrast, unmodified sodium alginate is only able to form noncovalent bonds via ionic interactions and hydrogen bonds within the mucus layer resulting in a less effective gluing of the dosage form to the mucosal surface. This increased mucoadhesive ability is one of the most striking features of the thiolated class of polymers.
Thiolation of sodium alginate improves various inherent properties of the polymer and makes it a potential carrier for drug delivery. Establishment of the toxicity profile of this newly synthesized excipient is important for its practical use in drug delivery systems. The USP recommends determination of biological reactivity of polymeric materials on L-929 mammalian fibroblast cell cultures. These cell lines have also been reported for evaluation of toxicity of newly synthesized polymers and for evaluation of biocompatibility of novel drug delivery systems like liposomes and nanoparticles [20].
In the present studies, a concentration dependent cytotoxicity profile of SA-cys was observed as assessed by MTT. The reason for the lower cytotoxicity of the SA-cys compared to sodium alginate at low concentrations could be attributed to the fact that the bridging amide groups and the newly introduced sulfhydryl group are commonly encountered functional groups in the body rendering the polymer better tolerated as compared to the unmodified native polymer. Cell proliferation decreased for cells in contact with higher concentrations of both polymers. At the highest concentration examined (0.1 % w/v) SA-cys showed toxicity equivalent to that of native polymer towards L-929 mouse fibroblast cells. The cross-linking of SA-cys due to the formation of disulfide bonds during incubation period would result in an increase in molecular weight and viscosity of the polymer which would exert a greater mechanical pressure on the underlying fibroblast cells. This phenomenon would be more prominent at higher concentrations of polymer. This could also explain the faster rate of decline in cell proliferation with increase in concentration of SA-cys as compared to control polymer. From the findings thiolation is seen to influence the biocompatibility characteristics of the native polymer in a positive manner and can only result in safer derivatives of polymers that are already considered safe for use in drug delivery. Also, in our studies, autoclaving was found not to significantly alter the polymer in respect of its thiol content. Further, Daigle et al. have demonstrated that depolymerization reaction of the sodium alginate during autoclaving was inhibited at pH 7-8 [21]. Since the pH of the alginate solutions was adjusted to neutral before sterilization, we conclude that the process of sterilization did not result in significant destruction of the test polymer.
In conclusion, thiolation offers a simple solution to improving properties of certain polymeric materials. The resulting polymer derivative showed improved adhesion to mucosal surfaces and greater ability to sustain release of incorporated drug molecules. These properties are due to disulfide bond formation: The former with mucin and the later within the thiol domains of the modified polymer molecules. Enhanced mucoadhesive and controlled release properties coupled with the improved biocompatibility make it a suitable excipient for buccal/oral/ocular/nasal drug delivery systems. However the physicochemical properties of the polymer need further improvement.
Acknowledgements
The authors acknowledge the University of Mumbai for a research grant, Naprod Life Sciences for help in freeze drying of samples, Unichem Laboratories for sample of tramadol hydrochloride and Signet Chemical Co. for sample of Protanal LF240 D.
References
- Bernkop-Schnürch A. Mucoadhesive drug delivery systems in oral drug delivery. Drug Discov Today 2005;2:83-7.
- Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev 2005;57:1666-91.
- Naisbett B, Woodley J. The potential use of tomato lectin for oral drug delivery. Lectin binding to rat small intestine in vitro. Int J Pharm 1994;107:223-30.
- Bernkop-Schnürch A, Gabor F, Szostak MP, Lubitz W. An adhesive drug delivery system based on K99-fimbriae. Eur J Pharm Sci 1995;3:293-9.
- Bernkop-Schnürch A. Thiomers: A new generation of mucoadhesive polymers. Adv Drug Deliv Rev 2005;57:1569-82.
- Leitner V. Walker GF. Bernkop-Schnürch A. Thiolated polymers: Evidence for the formation of disulphide bonds with mucus glycoproteins. Eur J Pharm Biopharm 2003;56:207-14.
- Bernkop-Schnürch A, Clausen AE, Hnatyszyn M. Thiolated polymers: Synthesis and in vitro evaluation of polymer–cysteamine conjugates. Int J Pharm 2001;226:185-94.
- Leitner V, Marschütz M, Bernkop-Schnürch A. Mucoadhesive and cohesive properties of poly (acrylic acid)-cysteine conjugates with regard to their molecular mass. Eur J Pharm Sci 2003;18:89-96.
- Kafedjiiskia K, Kraulandb AH, Hofferc MH, Bernkop-Schnürch A. Synthesis and in vitro evaluation of a novel thiolatedchitosan.Biomaterials 2005;26:819-26.
- Miyazaki S, Nakayama A, Oda M. Drug release from oral mucosal adhesive tablets of chitosan and sodium alginate. Int J Pharm 1995;118:257-63.
- Yi Li X, Kong XY, Xi SS. Zheng G, Guo YW, Qian Z. Préparation of Alginate Coated Chitosan Microparticles for Vaccine Delivery. BMC Biotechnology 2008;8:89.
- Gombotz WR, Wee SF. Protein release from alginate matrices. Adv Drug Del Rev 1998;31:267-85.
- Bernkop-Schnürch A, Kast CE, Richter MF. Improvement in the mucoadhesive properties of alginate by the covalent attachment of cysteine. J Control Release 2005;71:277-85.
- Zhang YN, Ping QN, Xiao B. Microencapsulation and characterization of tramadol–resin complexes. J Control Rel 2000;66:107-13.
- Guggi D, Langoth N, Hoffer MH, Wirth M, Bernkop-Schnürch A. Comparative evaluation of cytotoxicity of a glucosamine-TBA conjugateand a chitosan-TBA conjugate. Int J Pharm 2004;278:353-60.
- Vijay KI, Seghal D. A method for the high efficiency of water soluble carbodiimide- mediated amidation. Anal Biochem 1994;218:87-91.
- Guggi D, Marschütz MK, Bernkop-Schnürch A. Matrix tablets based on thiolated poly(acrylic acid): pH-dependent variation in disintegration and mucoadhesion. Int J Pharm 2004;274:97-105.
- Bernkop-Schnürch A, Scholler S, Biebel RG. Development of controlled drug release systems based on thiolated polymers. J Control Release 2000;66:39-48.
- Bansil R, Turner B. Mucin structure, aggregation, physiological function and biomedical application. CurrOpin Colloid Interface Sci 2006;11:164-70.
- Pan J, Feng S. Targeting and imaging cancer cells by folate-decorated, quantum dots (QDs)- loaded nanoparticles of biodegradable polymers. Biomaterials 2009;30:1176-83.
- Daigle DJ, Cotty PJ. The Effect of Sterilization, pH, Filler and Spore Inoculum Concentration on the Preparation of Alginate Pellets. BiocontrolSci Tech 1997;7:3-10.
 after Reduction
after Reduction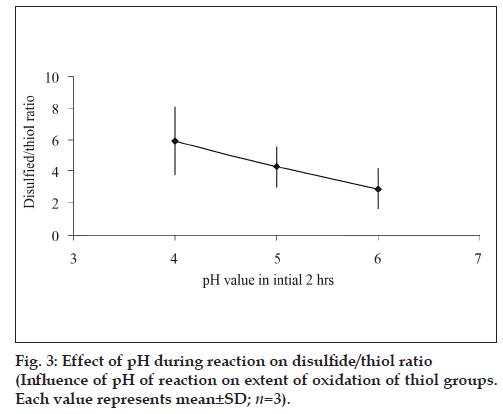
 SA-cys,
SA-cys, SA. * Significant difference between the treatment groups at P < 0.05
SA. * Significant difference between the treatment groups at P < 0.05




