- *Corresponding Author:
- K. K. Sivakumar
Department of Pharmaceutical Chemistry, Karpagam University, Coimbatore-641 021,India
E-mail: sivaveni2004@gmail.com
| Date of Submission | 29 November 2012 |
| Date of Revision | 03 June 2013 |
| Date of Acceptance | 10 June 2013 |
| Indian J Pharm Sci, 2013;75(4):463-475 |
Abstract
In the present investigation, a series of 12 Mannich bases (QP1-12) and 5 Schiff bases (QSP1-5) of pyrazol-5(4H)-one moiety containing 3-(hydrazinyl)-2-phenylquinazolin-4(3H)-one has been synthesized and characterized by physicochemical as well as spectral means. The synthesized Mannich and Schiff bases were screened for their preliminary antimicrobial activity against Gram-positive and Gram-negative bacterial as well as fungal strains by the determination of zone of inhibition. Mannich bases (QP1-12) were found to be more potent antibacterial agents against Gram-positive bacteria, whereas Schiff bases (QSP1-5) were more potent against Gram-negative bacteria and fungi. Minimum inhibitory concentration result demonstrated that Mannich base compound (QP7) having ortho -OH and para -COOH group showed some improvement in antibacterial activity (minimum inhibitory concentration of 48.88×10−3 μM/ml) among the tested Gram-positive organisms and it also exhibit minimum inhibitory concentration of value of 12.22×10−3 μM/ml for Klebsiella pneumoniae. The antitubercular activity of synthesized compounds against Mycobacterium tuberculosis (H 37 Rv) was determined using microplate alamar blue assay. Compound QP11 showed appreciable antitubercular activity (minimum inhibitory concentration of 6.49×10−3 μM/ml) which was more active than the standard drugs, ethambutol (minimum inhibitory concentration of 7.60×10−3 μM/ml) and ciprofloxacin (9.4×10−3 μM/ml). Compounds QP11, QP9, QSP1, QSP2, and QSP5 have good selective index and may be selected as a lead compound for the development of novel antitubercular agents.
Keywords
Antimicrobial, antimycobacterium, cytotoxicity, pyrazolone, quinazolinones
Mycobacterium tuberculosis, the causative agent of tuberculosis (TB), infects approximately onethird of the world’s population. Literature reports reveal that the estimates of global TB incidence is around 8-9 million new cases and 1.6 million human deaths annually [1,2]. The incidence of TB infection has steadily risen in the last decade. The first-line drugs used for the treatment of TB include isoniazid (INH), rifampicin (RMP), ethambutol (EMB), streptomycin (SM), and pyrazinamide (PZA); second-line drugs include ethionamide, prothionamide, clycloserine, capreomycin, p-aminosalicylic acid, and fluoroquinolones. Prolonged treatment regimens (9-12 months) are required in order to assure therapeutic effectiveness of these drugs in TB patients [3].
The emergence of multidrug resistant tuberculosis (the disease caused by the strains of M. tuberculosis resistant to two mainstay first line antiTB drugs, isoniazid and rifampicin) is increasing worldwide. Moreover, no new antitubercular drug has been registered during last four decades. Further, the lifethreatening infections caused by multidrug resistant Gram-positive and Gram-negative pathogenic bacterial and fungal strains also increased to an alarming level [4,5]. This initiated an urgent need to search for alternative new chemical entities for the treatment of tubercular and microbial infections.
Pyrazolones constitute an important class of heterocyclic compounds that are the key structures for the development of new chemical entities. Pyrazolones linked to other heterocyclic nucleus through azomethine –NHN=CH protons have attracted many researchers because of their wide range of biological activities such as antibacterial [6], antifungal [7], antioxidant [8], analgesic [9], antiinflammatory [10], anticancer [11], and antiTB [12] activities. Literature reports reveal that pyrazoles gained much attention as antimicrobial agents after the discovery of natural pyrazole C-glycoside pyrazofurin, a broad spectrum antimicrobial agent [13].
Like pyrazolones, quinazolines are also an important class of heterocyclic nucleus that has been widely explored for its biological potential viz. antimicrobial [14], antifungal [15], antihyperglycemic [16], analgesic [17], antiinflammatory [18], and antiviral [19] activities. There are two basic approaches to develop new drugs: (a) synthesis of analogs, modification or derivatives of existing compounds for shortening and improving treatment and (b) searching for novel structures that the microorganism/pathological condition has never been presented with before [20]. A combinational therapeutic drug with different mechanisms of action is one of the methods that are being adopted to treat disorders mentioned above. Besides the exploitation of new targets, there is another approach of merging two or more pharmacophores into a single molecule. Therefore, a single molecule containing more than one pharmacophore, each with different mode of action, could be beneficial for the treatment of the above mentioned disorders. These ‘‘merged’’ pharmacophores may be addressing the active site of different targets and offer the possibility to overcome drug resistance. In addition, this approach can also reduce unwanted side effects. The success of this hybridization approach has already been applied for the development of novel antibacterial and antimalarial agents to overcome drug resistance [21]. In pursuit of the above and in continuation of our studies toward the development of NCEs for the treatment of tuberculosis [22-24], in the present study, we have planned to couple the quinazolinone nucleus to pyrazolone nucleus through azomethine (–NHN=CH) linkage. With this objective in mind, we hereby report the synthesis, in vitro antimicrobial, antitubercular and cytotoxicity screening of Mannich and Schiff bases of pyrazol-5(4H)-one moiety containing 3-(hydrazinyl)-2- phenylquinazolin-4(3H)-one.
Materials and Methods
Starting materials were obtained from commercial sources and were used without further purification. Reaction progress was observed by thin layer chromatography using commercial silica gel plates (Merck Ltd., Mumbai). Melting points were determined in open capillary tubes on a Sonar melting point apparatus and are uncorrected. Proton nuclear magnetic resonance (1H NMR) spectra were determined by Bruker 300 MHz FT- NMR spectrometer in appropriate deuterated solvents and are expressed in parts per million (δ, ppm) downfield from tetramethylsilane (internal standard). NMR data are given as multiplicity (s, singlet; d, doublet; t, triplet; m, multiplet) and number of protons. Elemental analysis (C, H, and N) was undertaken with Elemental vario EL III Carlo Erba 1108 analyzer. The infrared (IR) spectra were run as KBr disk on Jasco FTIR 4100 spectrophotometer. Mass spectra of the synthesized compounds were recorded in MS (EI) Jeol GC mass spectrometer.
Synthesis of 2-phenyl-4H-3,1-benzoxazin-4-one (Q1) and 3-amino-2-phenylquinazolin-4(3H)-one (QA)
The compounds 2-phenyl-4H-3,1-benzoxazin-4-one (Q1) and 3-amino-2-phenylquinazolin-4(3H)-one (QA) were synthesized using the reported methods (Scheme 1) [19,25].
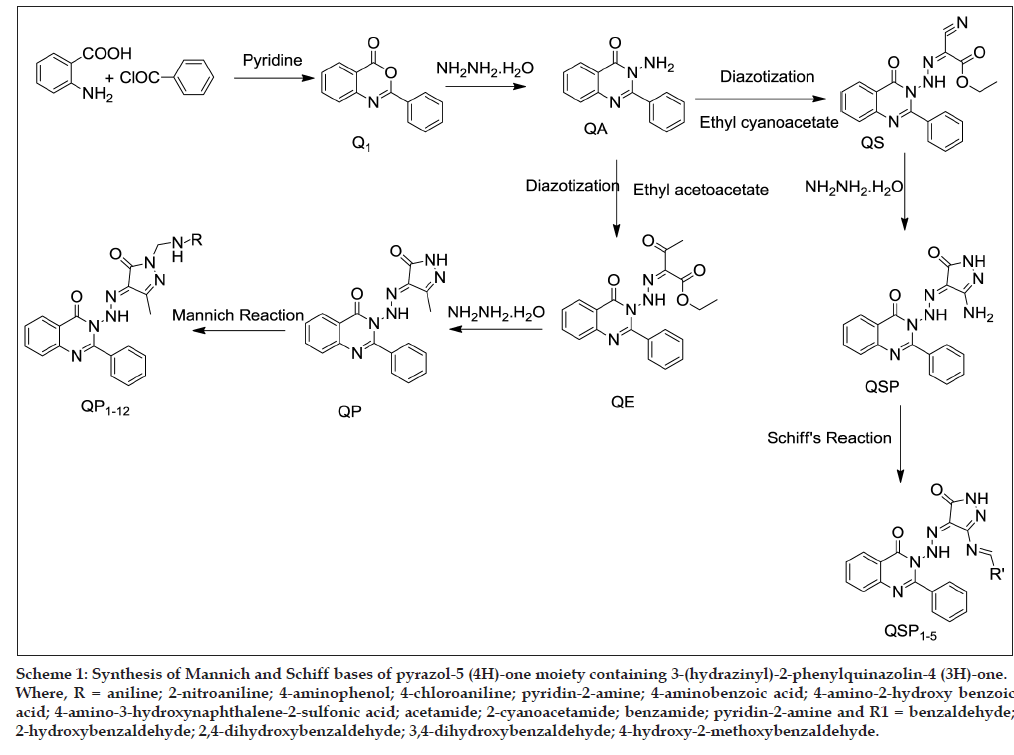
Scheme 1: Synthesis of Mannich and Schiff bases of pyrazol-5 (4H)-one moiety containing 3-(hydrazinyl)-2-phenylquinazolin-4 (3H)-one. Where, R = aniline; 2-nitroaniline; 4-aminophenol; 4-chloroaniline; pyridin-2-amine; 4-aminobenzoic acid; 4-amino-2-hydroxy benzoic acid; 4-amino-3-hydroxynaphthalene-2-sulfonic acid; acetamide; 2-cyanoacetamide; benzamide; pyridin-2-amine and R1 = benzaldehyde; 2-hydroxybenzaldehyde; 2,4-dihydroxybenzaldehyde; 3,4-dihydroxybenzaldehyde; 4-hydroxy-2-methoxybenzaldehyde.
General procedure for the synthesis of hydrazono derivatives (QE and QS)
3-Amino-2-phenyl quinazolin-4(3H)-one (QA, 2.37 g, 0.01 mol) was dissolved in concentrated HCl (6 ml) and cooled to 0-5º. The cold mixture was stirred vigorously and added to cold solution of sodium nitrite (1.5 g in 10 ml of water) in dropwise manner while maintaining the temperature at 0-5º. The mixture was stirred for another 30 min after the completion of addition. An ice-cold mixture of appropriate active methylene compound (0.01 mol) [ethyl acetoacetate (for QE), ethyl cyanoacetate (for QS)] and sodium acetate (4.10 g, 0.05 mol) in 50 ml of ethanol was added dropwise to the solution of diazonium salt with vigorous stirring. After the addition was complete, the stirring was continued for another 1 h and left for 2 h at room temperature. The solid product thus obtained was collected, dried, and recrystallized from ethanol [26].
Ethyl-3-oxo-2-[2-(4-oxo-2-phenyl-3,4-dihydroquinazolin- 3-yl)hydrazin-1-ylidene]butanoate (QE): Mp (º) 145-147; Yield: 55%; IR (KBr pellets) cm−1: 3332 (NH str., NNHN=R), 3242 (CH str., aromatic), 1718.67 and 1603.21 (C=O str., carboxylic ester and acetyl carbonyl group), 1541.14 (C=N str., hydrazone); 1H-NMR (DMSO-d6) δ ppm: 7.12 (s, 1H, NH of hydrazone –N=NH–), 7.20-7.94 (m, 9H, Ar-H), 2.52 (s, 3H, COCH3), 1.39 (t, 3H, - COOCH2CH3), 4.17 (m, 2H, -COOCH2CH3).
Ethyl cyano[2-(4-oxo-2-phenyl-3,4-dihydroquinazolin- 3-yl)hydrazin-1-ylidene]formate (QS): Mp (º) 132-134; Yield: 58%; IR (KBr pellets) cm−1: 3324 (NH str.), 3238 (CH str., aromatic), 2366 (CN str.), 2346 and 1726 (C=O str., carboxylic ester), 1536.14 (C=N str., hydrazone); 1HNMR (DMSO-d6) δ ppm: 7.09 (s, 1H, NH of hydrazone -N=NH-), 7.28-8.04 (m, 9H, Ar-H), 1.35 (t, 3H, -COOCH2CH3), 4.23 (m, 2H, -COOCH2CH3).
General procedure for the synthesis of pyrazolones (QP and QSP)
The corresponding hydrozone derivative (QE and QS, 0.001 mol) was added with hydrazine hydrate (5 ml, 0.1 mol) in 30 ml of ethanol and the reaction mixture was heated under reflux for 6 h. Then the reaction mixture was allowed to cool in ice and the precipitated pyrazolones (QP and QSP) was filtered, washed with water, dried and recrystallized from ethanol [26].
3-[2-(3-methyl-5-oxo-4,5-dihydro-1H-pyrazol-4- ylidene) hydrazin-1-yl]-2-phenyl-3,4-dihydoquinazolin- 4-one (QP): Mp (º) 136-138; Yield: 67%; IR (KBr pellets) cm–1: 3442.25 (NH str.), 3102.23, 2895 (Ar-H), 1653.42, (C=O str., pyrazolone ring), 1477.21 (C=N str.), 767.53 (CH=CH str., of aromatic); 1H-NMR (DMSO-d6) δ ppm: 7.11 (s, 1H, NH of hydrazone –N=NH–), 7.02 (s, 1H, NH of pyrazolone), 7.23-8.02 (m, 9H, Ar-H), 0.84 (s, 3H, CH3 of pyrazolone); Elemental Analysis (Anal.) calculated for C18H14N6O2: C, 62.42; H, 4.07; N, 24.27; Found: C, 62.26; H, 3.96; N, 23.96.
3-[2-(3-amino-5-oxo-4,5-dihydro-1H-pyrazol-4-ylidene) hydrazin-1-yl]-2-phenyl-3,4-dihydroquinazolin-4-one (QSP): Mp (º) 163-165; Yield: 59%; IR (KBr pellets) cm−1: 3423.26 (NH str.), 3347.42 (NH2 str.) 1670.23, 1640.56 (C=O str., pyrazolone and quinazolinone ring), 1597.21 (C=N str.), 787.53 (CH=CH str., aromatic); 1H-NMR (DMSOd6) δ ppm: 7.14 (s, 1H, NH of hydrazone –N=NH–), 7.04 (s, 1H, NH of pyrazolone), 7.14-8.05 (m, 9H, Ar-H), 2.14 (s, 2H, NH2); Anal. calculated for C17H13N7O2: C, 58.79; H, 3.77; N, 28.23; found: C, 57.45; H, 3.68; N, 27.96.
General procedure for synthesis of N-Mannich bases of 3-(2-(3-methyl-5-oxo-1H-pyrazol-4 (5H)- ylidene)hydrazinyl)-2-phenylquinazolin-4(3H)-one (QP1-12)
N-Mannich bases of 3-(2-(3-methyl-5-oxo-1H-pyrazol-4 (5H)-ylidene)hydrazinyl)-2-phenylquinazolin-4(3H)- one (QP1-12) were synthesized using the reported methods [27] with slight modifications as follows. A mixture of 3-(2-(3-methyl-5-oxo-1H-pyrazol-4(5H)- ylidene)-hydrazinyl)-2-phenylquinazolin-4(3H)-one (1.73 g, 0.005 mol) and 90% formaldehyde (6 ml) was refluxed with different amines and amides (0.1 mol) in ethanol (30 ml) and the reaction was monitored by TLC. After the completion of reaction, the resulting mixture was poured into crushed ice and the precipitated title compounds were filtered, dried and recrystallized from ethanol. The physical properties of QP1-12 are given in Table 1.
| Compound code | R and R1* | Molecular formula | Molecular weight | Melting point (°) | % yield | Rf value | Log P |
|---|---|---|---|---|---|---|---|
| QP1 | Aniline | C25H21N7O2 | 451.48 | 89‑91 | 68 | 0.87 | 4.49 |
| QP2 | 2‑nitroaniline | C25H20N8O4 | 496.48 | 81‑83 | 65 | 0.75 | 5.08 |
| QP3 | 4‑aminophenol | C25H21N7O3 | 467.48 | 115‑117 | 69 | 0.63 | 4.19 |
| QP4 | 4‑chloroaniline | C25H20ClN7O2 | 485.93 | 105‑107 | 68 | 0.72 | 5.1 |
| QP5 | Pyridin‑2‑amine | C24H20N8O2 | 452.47 | 133‑135 | 70 | 0.8 | 3.87 |
| QP6 | 4‑aminobenzoic acid | C26H21N7O4 | 495.49 | 215‑217 | 67 | 0.82 | 4.15 |
| QP7 | 4‑amino‑2‑hydroxy benzoic acid | C26H21N7O5 | 511.49 | 93‑95 | 69 | 0.75 | 4.5 |
| QP8 | 4‑amino‑3‑hydroxy naphthalene‑2‑sulfonic acid | C29H23N7O6S | 597.6 | 127‑129 | 68 | 0.74 | 5.01 |
| QP9 | Acetamide | C21H19N7O3 | 417.42 | 123‑125 | 71 | 0.84 | 2.24 |
| QP10 | 2‑cyanoacetamide | C22H18N8O3 | 442.43 | 134‑136 | 78 | 0.65 | 2.19 |
| QP11 | Benzamide | C26H21N7O3 | 479.49 | 93‑95 | 71 | 0.68 | 4.09 |
| QP12 | Pyridin‑2‑amine | C25H20N8O3 | 480.48 | 113‑115 | 78 | 0.75 | 3.26 |
| QSP1 | *Benzaldehyde | C24H17N7O2 | 435.44 | 108‑110 | 81 | 0.61 | 3.39 |
| QSP2 | *2‑hydroxy benzaldehyde | C24H17N7O3 | 451.44 | 162‑164 | 66 | 0.72 | 3.68 |
| QSP3 | *2,4‑dihydroxy benzaldehyde | C24H17N7O4 | 467.44 | 180‑182 | 82 | 0.76 | 3.38 |
| QSP4 | *3,4‑dihydroxy benzaldehyde | C24H17N7O4 | 467.44 | 172‑174 | 78 | 0.67 | 3.38 |
| QSP5 | *4‑hydroxy‑2‑methoxy benzaldehyde | C25H19N7O4 | 481.46 | 168‑170 | 76 | 0.82 | 3.53 |
Table 1: Physicochemical properties synthesized compounds (qp1-12 and qsp1-5).
3-{2-[-3-methyl-5-oxo-1-[(phenylamino)methyl]-4,5- dihydro-1H-pyrazol-4-ylidene]hydrazin-1-yl}-2-phenyl- 3,4-dihydroquinazolin-4-one (QP1): IR (KBr pellets) cm−1: 3295.89 (NH Str., secondary amine), 1661.49, 1619.91 (C=O str., 2-phenylquinazolin-4 (3H)-one, pyrazolone ring), 1557.64 (C=N str., pyrazolone), 826.28 (CH=CH str., Aromatic); 1H-NMR (DMSOd6) δ ppm: 7.23 (s, 1H, - NH-N= of hydrazone), 6.42-8.16 (m, 14 H, Ar-H), 4.54 (d, 2H, –NCH2 of pyrazolone), 0.84 (s, 3H, CH3 of pyrazolone), 3.82 (t, 1H, NH of C6H5NH); Exact mass: m/z: 451.48 [M]+; Anal. calculated for C25H21N7O2: C, 66.51; H, 4.69; N, 21.72; found: C, 66.32; H, 4.56; N, 21.43.
3-{2-[-3-methyl-1-{[(2-nitrophenyl) amino] methyl}- 5-oxo-4,5-dihydro-1H-pyrazol-4-ylidene]hydrazin- 1-yl}-2-phenyl-3,4-dihydroquinazolin-4-one (QP2): IR (KBr pellets) cm−1: 3362.89 (NH Str., secondary amine), 1653.72, 1619.91 (C=O str., 2-phenylquinazolin-4 (3H)-one, pyrazolone ring), 1574.59 (C=N str., pyrazolone), 1510.95 (C-NO2 str., aromatic), 830.20 (Ar-CH=CH); 1H-NMR (DMSO-d6) δ ppm: 7.14 (s, 1H, –NH-N= of hydrazone), 6.53-8.06 (m, 13 H, Ar-H), 4.65 (d, 2H, -NCH2 of pyrazolone), 0.88 (s, 3H, CH3 of pyrazolone), 3.89 (t, 1H, NH of C6H5NHNO2); Exact mass: m/z: 497.48 [M]+; Anal. calculated for C25H20N8O4: C, 60.48; H, 4.06; N, 22.57; found: C, 60.24; H, 4.00; N, 22.26.
3-{2-[-1-{[(4-hydroxyphenyl)amino]methyl}-3- methyl-5-oxo-4,5-dihydro-1H-pyrazol-4-ylidene] hydrazin-1-yl}-2-phenyl-3,4-dihydroquinazolin-4- one (QP3): IR (KBr pellets) cm−1: 3282.95 (NH Str., secondary amine), 1672.62, 1619.91 (C=O str., 2-phenylquinazolin-4(3H)-one, pyrazolone ring), 1552.93 (C=N str., pyrazolone), 1315.65 (C-OH str., aromatic), 790.80 (Ar-CH=CH); 1H-NMR (DMSO-d6) δ ppm: 7.18 (s, 1H, –NH-N= of hydrazone), 6.33- 8.12 (m, 13 H, Ar-H), 4.72 (d, 2H, -NCH2 of pyrazolone), 0.79 (s, 3H, CH3 of pyrazolone), 3.92 (t, 1H, NH of C6H5NHOH), 4.77 (s, 1H, C6H5OH); Exact mass: m/z: 467.48 [M]+; Anal. calculated for C25H21N7O3: C, 64.23; H, 4.53; N, 20.97; found: C, 63.93; H, 4.32; N, 20.58.
3-{2-[-1-{[(4-chlorophenyl)amino]methyl}-3-methyl- 5-oxo-4,5-dihydro-1H-pyrazol-4-ylidene] hydrazin- 1-yl}-2-phenyl-3,4-dihydroquinazolin-4-one (QP4): IR (KBr pellets) cm−1: 3227.01 (NH str., secondary amine), 1670.05, 1623 (C=O str., 2-phenylquinazolin- 4(3H)-one, pyrazolone ring), 1597.73 (C=N str., pyrazolone), 810.92 (Ar-CH=CH), 701.96 (C-Cl str., aromatic); 1H-NMR (DMSO-d6) δ ppm: 7.11 (s, 1H, -NH-N= of hydrazone), 6.23-7.86 (m, 13 H, Ar-H), 4.75 (d, 2H, -NCH2 of pyrazolone), 0.93 (s, 3H, CH3 of pyrazolone), 3.95 (t, 1H, NH of C6H5NHCl); Exact mass: m/z: [M]+2 and [M]+ peaks at 487.12 (19.38%) and 485.14 (78.00%), respectively; Anal. calculated for C25H20N8O4: C, 60.48; H, 4.06; N, 22.57; found: C, 60.38; H, 4.02; N, 22.27.
3-{2-[-3-methyl-5-oxo-1-{[(pyridin-2-yl)amino] methyl}-4,5-dihydro-1H-pyrazol-4-ylidene] hydrazin-1-yl}-2-phenyl-3,4-dihydroquinazolin-4- one (QP5): IR (KBr pellets) cm−1: 3982.69 (NH str., secondary amine), 1682.48, 1619.91 (C=O str., 2-phenylquinazolin-4(3H)-one, pyrazolone ring), 1534.59 (C=N str., pyrazolone), 827.22 (Ar-CH=CH); 1H-NMR (DMSO-d6) δ ppm: 7.14 (s, 1H, -NH-N=of hydrazone), 6.53-8.23 (m, 13 H, Ar-H), 4.83 (d, 2H, –NCH2 of pyrazolone), 0.87 (s, 3H, CH3 of pyrazolone), 3.97 (t, 1H, NH attached to pyridine); Exact mass: m/z: 452.47 [M]+; Anal. calculated for C24H20N8O2: C, 63.71; H, 4.46; N, 24.76; found: C, 63.32; H, 4.04; N, 24.36.
4-({[-3-methyl-5-oxo-4-[2-(4-oxo-2-phenyl-3,4- dihydroquinazolin-3-yl)hydrazin-1-ylidene]-4,5- dihydro-1H-pyrazol-1-yl]methyl}amino)benzoic acid (QP6): IR (KBr pellets) cm−1: 3372.89 (NH Str., secondary amine), 31510.95 (COOH str., aromatic), 1672.95, 1605 (C=O str., of 2-phenylquinazolin-4(3H)- one pyrazolone ring), 1526.59 (C=N str., pyrazolone), 837.91 (Ar-CH=CH); 1H NMR (DMSO-d6) δ ppm: 7.19 (s, 1H, -NH-N= of hydrazone), 11.84 (s, 1H, COOH), 6.70-8.22 (m, 13H, Ar-H), 4.68 (d, 2H, -NCH2 of pyrazolone), 0.81 (s, 3H, CH3 of pyrazolone), 3.91 (t, 1H, NH of C6H5NHCOOH); Exact mass: m/z: 495.17 [M]+; Anal. calculated for C26H21N7O4: C, 63.02; H, 4.27; N, 19.79; found: C, 62.94; H, 4.16; N, 19.59.
2-hydroxy-4-({-3-methyl-5-oxo-4-[2-(4-oxo-2-phenyl- 3,4-dihydroquinazolin-3-yl)hydrazin-1-ylidene]- 4,5-dihydro-1H-pyrazolyl]methyl}amino)benzoic acid (QP7): IR (KBr pellets) cm−1: 3272.89 (NH Str., secondary amine), 2825.21 (COOH str., aromatic), 1669.09 (C=O str., of 2-phenylquinazolin-4(3H)-one ring), 1491.67 (C=N str., pyrazolone), 762.90 (Ar- CH=CH); 1H NMR (DMSO-d6) δ ppm: 7.09 (s, 1H, –NH-N= of hydrazone), 11.34 (s, 1H, COOH), 6.47-8.02 (m, 12H, Ar-H), 4.71 (d, 2H, -NCH2 of pyrazolone), 0.91 (s, 3H, CH3 of pyrazolone), 3.99 (t, 1H, NH of C6H5NHOHCOOH), 4.84 (s, 1H, C6H5OH); Exact mass: m/z: 511.16 [M]+; Anal. calculated for C26H21N7O5: C, 61.05; H, 4.14; N, 19.17; found: C, 60.96; H, 4.09; N, 19.05.
3-hydroxy-4-({[-3-methyl-5-oxo-4-[2-(4-oxo- 2-phenyl-3,4-dihydroquinazolin-3-yl)hydrazin- 1-ylidene]-4,5-dihydro-1H-pyrazol-1-yl]methyl} amino)naphthalene-2-sulfonic acid (QP8): IR (KBr pellets) cm−1: 3372.89 (NH Str., secondary amine), 3126.01 (Ar-OH str., aromatic), 1669.09 (C=O str., of 2-phenylquinazolin-4(3H)-one ring), 1524.45 (C=N str., pyrazolone), 1046.14 (S=O, sulfoxide), 780.20 (Ar-CH=CH). 1H NMR (DMSO-d6) δ ppm: 12.60 (s, 1H, –NH-N= of hydrazone), 7.47-8.19 (m, 14H, 9H-Ar of 2-phenylquinazolin-4(3H)-one and 5 H naphthyl), 3.30 (s, 2H, CH2 attached to pyrazolone at N1 position), 2.51 (s, 3H, CH3 of pyrazolone); Exact mass: m/z: 597 [M]+; Anal. calculated for C29H23N7O6S1: C, 58.28; H, 3.88; N, 16.41; O, 16.06; S, 5.37; found: C, 58.19; H, 3.79; N, 16.36.
N-{[-3-methyl-5-oxo-4-[2-(4-oxo-2-phenyl-3,4- dihydroquinazolin-3-yl)hydrazin-1-ylidene]-4,5- dihydro-1H-pyrazol-1-yl]methyl}acetamide (QP9): IR (KBr pellets) cm−1: 3372.89 (NH Str., secondary amine), 1671 (C=O str., of 2-phenylquinazolin- 4(3H)-one ring), 1590.09 (C=N str., pyrazolone), 1604.00 (-CONH- out of plane bending), 830.20 (Ar-CH=CH); 1H NMR (DMSO-d6) δ ppm: 7.03 (s, 1H, –NH-N= of hydrazone), 7.19-7.77 (m, 9 H, Ar-H), 4.81 (d, 2H, –NCH2 of pyrazolone), 0.90 (s, 3H, CH3 of pyrazolone), 7.93 (t, 1H, NH of NHCOCH3), 2.12 (s, 3H, NHCOCH3); Exact mass: m/z: 417.15[M]+; Anal. calculated for C21H19N7O3: C, 60.42; H, 4.59; N, 23.49; found: C, 60.24; H, 4.48; N, 23.38.
C-[-3-methyl-5-oxo-4-[2-(4-oxo-2-phenyl-3,4- dihydroquinazolin-3-yl)hydrazin-1-ylidene]-4,5- dihydro-1H-pyrazol-1-yl]carbamoyl cyanide (QP10): IR (KBr pellets) cm−1: 3292.45 (NH Str., secondary amine), 1658.83, 1614.61 (C=O str., of 2-phenylquinazolin-4(3H)-one, pyrazolone ring), 1544.89 (C=N str., pyrazolone), 1706.12 (CN str.), 830.20 (Ar-CH=CH); 1H NMR (DMSO-d6) δ ppm: 7.11 (s, 1H, –NH-N= of hydrazone), 7.24-7.95 (m, 9 H, Ar-H), 4.86 (d, 2H, –NCH2 of pyrazolone), 0.89 (s, 3H, CH3 of pyrazolone), 7.91 (t, 1H, NH of NHCOCH2CN), 3.22 (s, 3H, NHCOCH2CN); Exact mass: m/z: 442.43 [M]+; Anal. calculated for C22H18N8O3: C, 59.72; H, 4.10; N, 25.33; found: C, 59.46; H, 3.96; N, 25.10.
N-{[-3-methyl-5-oxo-4-[2-(4-oxo-2-phenyl-3,4- dihydroquinazolin-3-yl)hydrazin-1-ylidene]- 4,5-dihydro-1H-pyrazol-1-yl]methyl}benzamide (QP11): IR (KBr pellets) cm−1: 3296.65 (NH Str., secondary amine), 1680.91, 1625 (C=O str., 2-phenylquinazolin-4 (3H)-one, pyrazolone ring), 1604.00 (–CONH- out of plane bending), 1560.59 (C=N str., pyrazolone), 823.45 (Ar-CH=CH); 1H NMR (DMSO-d6) δ ppm: 7.08 (s, 1H, –NH-N= of hydrazone), 7.24-8.07 (m, 14 H, Ar-H), 4.80 (d, 2H, –NCH2 of pyrazolone), 0.86 (s, 3H, CH3 of pyrazolone), 7.95 (t, 1H, NH of NHCOC6H5); Exact mass: m/z: 479.17 [M]+; Anal. calculated for C26H21N7O3: C, 65.13; H, 4.41; N, 20.45; found: C, 65.06; H, 4.32; N, 20.23.
N-{[-3-methyl-5-oxo-4-[2-(4-oxo-2-phenyl-3,4- dihydroquinazolin-3-yl)hydrazin-1-ylidene]-4,5- dihydro-1H-pyrazol-1-yl]methyl}pyridine-2- carboxamide (QP12): IR (KBr pellets) cm−1: 3286.65 (NH str., secondary amine), 1690.62, 1616.12 (C=O str, of 2-phenylquinazolin-4(3H)-one, pyrazolone ring), 1602.66 (–CONH- out of plane bending), 1580.18 (C=N), 823.45 (Ar-CH=CH); 1H-NMR (DMSO-d6) δ ppm: 7.04 (s, 1H, -NHN= of hydrazone), 7.26-8.93 (m, 13 H, Ar-H), 4.71 (d, 2H, –NCH2 of pyrazolone), 0.83 (s, 3H, CH3 of pyrazolone), 7.97 (t, 1H, NH attached to carboxypyridine); Exact mass: m/z: 480.17[M]+; Anal. calculated for C25H20N8O3: C, 62.49; H, 4.20; N, 23.32; O, 9.99; C, 65.13; H, 4.41; N, 20.45; found: C, 62.36; H, 4.12; N, 23.24.
General procedure for synthesis of Schiff bases of 3-[2-(3-amino-5-oxo-4,5-dihydro-1Hpyrazol- 4-ylidene) hydrazin-1-yl]-2-phenyl-3,4- dihydroquinazolin-4-one (QSP1-5)
The mixture of QSP (0.005 mol) and corresponding aldehydes (0.005 mol) in 20 ml of ethanol was heated under reflux for 2-3 h in the presence of glacial acetic acid. After completion of reaction, the resulting mixture was poured into crushed ice and the precipitated title compound was filtered, dried and recrystallized from ethanol [28]. The physical properties of compounds QSP1-5 are given in Table 1.
3-{2-[-5-oxo-3-[-(phenylmethylidene)amino]-4,5- dihydro-1H-pyrazol-4-ylidene]hydrazin-1-yl}-2-phenyl- 3,4-dihydroquinazolin-4-one (QSP1): IR (KBr pellets) cm−1: 3256.65 (-NH str., secondary amine), 1650.23, 1616.12, (C=O str., 2-phenylquinazolin-4(3H)-one, pyrazolone ring), 1602.66 (-CONH- out of plane bending), 1516.18 (C=N), 823.45 (Ar-CH=CH); 1H NMR (DMSO-d6) δ ppm: 7.06 (s, 1H, –NH-N= of hydrazone), 8.12 (s, H, N=CH-), 7.11-8.15 (m, 15H, Ar-H); Exact mass: m/z: 435.44 [M]+; Anal. calculated for C24H17N7O2: C, 66.20; H, 3.94; N, 22.52; found: C, 65.98; H, 3.56; N, 22.32.
3-{2-[-3-[-[(2-hydroxyphenyl) methylidene] amino]-5-oxo-4,5-dihydro-1H-pyrazol-4-ylidene] hydrazin-1-yl}-2-phenyl-3,4-dihydroquinazolin-4- one (QSP2): IR (KBr pellets) cm−1: 3266.65 (-NH str., secondary amine), 1667.45, 1623.23, (C=O str., 2-phenylquinazolin-4(3H)-one, pyrazolone ring), 1609.75 (–CONH- out of plane bending), 1513.21 (C=N), 1357.63 (Ar-OH), 831.56 (Ar- CH=CH); 1H NMR (DMSO-d6) δ ppm: 7.03 (s, 1H, -NH-N= of hydrazone), 8.16 (s, H, N=CH-), 6.74-8.15 (m, 14H, Ar-H), 4.93 (s, 1H, C6H5OH); Exact mass: m/z: 451.44 [M]+; Anal. calculated for C24H17N7O3: C, 63.85; H, 3.80; N, 21.72; found: C, 63.29; H, 3.65; N, 21.45.
3-{2-[-3-[-[(2,4-dihydroxyphenyl) methylidene] amino]-5-oxo-4,5-dihydro-1H-pyrazol-4-ylidene] hydrazin-1-yl}-2-phenyl-3,4-dihydroquinazolin-4- one (QSP3): IR (KBr pellets) cm−1: 3274.25 (-NH str., secondary amine), 1637.23, 1612.69, (C=O str., 2-phenylquinazolin-4(3H)-one, pyrazolone ring), 1611.45 (–CONH- out of plane bending), 1510.19 (C=N str.), 1335.32 (Ar-OH), 824.58 (Ar- CH=CH); 1H NMR (DMSO-d6) δ ppm: 7.05 (s, 1H, -NH-N= of hydrazone), 8.13 (s, H, N=CH-), 6.85-8.05 (m, 13 H, Ar-H), 4.96 [(s, 2H, C6H5 (OH)2]; Exact mass: m/z: 451.44 [M]+; Anal. calculated for C24H17N7O4: C, 63.85; H, 3.80; N, 21.72; found: C, 63.73; H, 3.45; N, 21.48.
3-{2-[-3-[-[(3,4-dihydroxyphenyl)methylidene]amino]-5- oxo-4,5-dihydro-1H-pyrazol-4-ylidene] hydrazin-1-yl}- 2-phenyl-3,4-dihydroquinazolin-4-one (QSP4): IR (KBr pellets) cm−1: 3284.28 (-NH str., secondary amine), 1657.83, 1634.25, (C=O str., 2-phenylquinazolin- 4(3H)-one, pyrazolone ring), 1609.85 (-CONH- out of plane bending), 1509.49 (C=N), 1326.49 (Ar-OH), 804.68 (Ar-CH=CH); 1H NMR (DMSO-d6) δ ppm: 7.01 (s, 1H, –NH-N= of hydrazone), 8.10 (s, H, N=CH–), 6.74-7.95 (m, 13 H, Ar-H), 4.91 [(s, 2H, C6H5 (OH)2]; Exact Mass: m/z: 451.44 [M]+; Anal. calculated for C24H17N7O4: C, 63.85; H, 3.80; N, 21.72; found: C, 63.73; H, 3.45; N, 21.48.
3-{2-[(-3-[(4-hydroxy-2-methoxyphenyl)methylidene] amino]-5-oxo-4,5-dihydro-1H-pyrazol-4-ylidene] hydrazin-1-yl}-2-phenyl-3,4-dihydroquinazolin-4- one (QSP5): IR (KBr pellets) cm−1: 3278.65 (-NH str., secondary amine), 1668.23, 1621.66 (C=O str., 2-phenylquinazolin-4(3H)-one, pyrazolone ring), 1613.76 (-CONH- out of plane bending), 1511.69 (C=N), 1313.76 (Ar-OH), 812.58 (Ar- CH=CH); 1H NMR (DMSO-d6) δ ppm: 7.04 (s, 1H, -NH-N= of hydrazone), 8.12 (s, H, N=CH-), 6.24-7.97 (m, 13 H, Ar-H), 4.93 (s, 2H, C6H5 OH), 3.70 (s, 3H, OCH3); Exact Mass: m/z: 451.44 [M]+; Exact Mass: m/z: 465.46 [M]+; Anal. calculated for C25H19N7O4: C, 62.37; H, 3.98; N, 20.36; found: C, 62.31; H, 3.95; N, 20.39.
Determination of zone of inhibition
All the synthesized compounds were screened for preliminary antibacterial activity against six Grampositive strains (Micrococcus luteus, Staphylococcus aureus, Bacillus subtilis, Coryne bacterium, Bacillus lentus, Staphylococcus albus) six Gramnegative strains (Escherichia coli, Pseudomonas aeruginosa, Rhodospirillum rubrum, Vibrio cholerae, Salmonella paratyphi, Klebsiella pneumoniae) and six fungal strains (Candida albicans, Monascus purpureus, Aspergillus niger, Trichophyton rubrum, Aspergillus fumigatus, Aspergillus parasiticus) by disk diffusion method [29]. Ciprofloxacin (5 μg/disk) and clotrimazole (5 μg/disk) were used as standard drugs for antibacterial and antifungal activity, respectively. Activity was determined by measuring the diameter of the zone showing complete inhibition. The results of the antibacterial and antifungal studies are listed in Tables 2, 3 and 4, respectively.
| Compound code | M. luteus | S. aureus | B. substills | C. bacterium | B. lentus | S. albus | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZI in mm | % of I | MIC× 10−3 | ZI in mm | % of I | MIC× 10−3 | ZI in mm | % of I | MIC× 10−3 | ZI in mm | % of I | MIC× 10−3 | ZI in mm | % of I | MIC× 10−3 | ZI in mm | % of I | MIC× 10−3 | |
| QP1 | ‑ | ‑ | NT | 12 | 50 | 221.49 | ‑ | ‑ | NT | 12 | 60 | 100 | 11 | 55 | 100 | ‑ | ‑ | NT |
| QP2 | 21 | 87.5 | 50.35 | 20 | 83.33 | 50.35 | 18 | 100 | 50.35 | 17 | 85 | 100.71 | 15 | 75 | 50.35 | 10 | 37.03 | 50.35 |
| QP3 | 10 | 41.66 | 53.47 | 20 | 83.33 | 53.47 | ‑ | ‑ | NT | ‑ | ‑ | NT | ‑ | ‑ | NT | 10 | 37.03 | 53.47 |
| QP4 | 22 | 91.66 | 102.9 | 15 | 62.5 | 51.45 | 16 | 88.88 | 102.9 | 18 | 90 | 102.9 | 15 | 75 | 102.9 | 12 | 44.44 | 51.45 |
| QP5 | 10 | 41.66 | 110.5 | ‑ | ‑ | NT | 12 | 66.66 | 55.25 | ‑ | ‑ | NT | 10 | 50 | 55.25 | 9 | 33.33 | 55.25 |
| QP6 | 12 | 50 | 50.46 | 15 | 62.5 | 50.46 | 12 | 66.66 | 50.46 | 18 | 90 | 100.91 | 12 | 60 | 50.46 | 15 | 55.55 | 50.46 |
| QP7 | 13 | 54.16 | 48.88 | 10 | 41.66 | 48.88 | 15 | 83.33 | 48.88 | 16 | 80 | 48.88 | 12 | 60 | 48.88 | 10 | 37.03 | 48.88 |
| QP8 | 13 | 54.16 | 83.67 | 12 | 50 | 83.67 | ‑ | ‑ | NT | 14 | 70 | 83.67 | 11 | 55 | 83.67 | 13 | 48.14 | 83.67 |
| QP9 | 19 | 79.16 | 59.89 | 10 | 41.66 | 59.89 | 17 | 94.44 | 59.89 | 19 | 85 | 59.89 | 19 | 95 | 59.89 | 10 | 37.03 | 119.78 |
| QP10 | 15 | 62.5 | 56.51 | ‑ | ‑ | NT | 16 | 88.88 | 113.01 | ‑ | ‑ | NT | ‑ | ‑ | NT | 10 | 37.03 | 56.51 |
| QP11 | 20 | 83.33 | 51.92 | 15 | 62.5 | 51.92 | 17 | 94.44 | 51.92 | 16 | 80 | 103.84 | 19 | 95 | 51.92 | 21 | 77.77 | 51.92 |
| QP12 | 20 | 83.33 | 52.03 | 12 | 50 | 52.03 | 15 | 83.33 | 104.06 | 14 | 70 | 104.06 | 11 | 55 | 52.03 | 19 | 70.37 | 52.03 |
| QSP1 | ‑ | ‑ | NT | 8 | 33.33 | 57.15 | ‑ | ‑ | NT | ‑ | ‑ | NT | ‑ | ‑ | NT | 17 | 62.96 | 114.3 |
| QSP2 | 15 | 62.5 | 55.38 | 12 | 50 | 110.76 | ‑ | ‑ | NT | 12 | 60 | 110.76 | ‑ | ‑ | NT | 20 | 74.07 | 110.76 |
| QSP3 | 18 | 75 | 53.48 | 18 | 75 | 106.97 | ‑ | ‑ | NT | 15 | 75 | 106.97 | ‑ | ‑ | NT | 17 | 62.96 | 53.48 |
| QSP4 | 19 | 79.16 | 53.48 | 20 | 83.33 | 106.97 | ‑ | ‑ | NT | 15 | 75 | 106.97 | ‑ | ‑ | NT | 17 | 62.96 | 106.97 |
| QSP5 | 19 | 79.16 | 53.71 | 19 | 79.16 | 107.42 | 9 | 50 | 214.84 | 14 | 70 | 53.71 | 11 | 55 | 214.84 | 18 | 66.66 | 214.84 |
| Standard | 24 | 100 | 0.57 | 24 | 100 | 9.43 | 18 | 100 | 18.86 | 20 | 100 | 75.45 | 20 | 100 | 2.38 | 27 | 100 | 75.45 |
| CFN | ||||||||||||||||||
| Solvent | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ |
| DMSO | ||||||||||||||||||
ZI=zone of inhibition, % of I= percent inhibition, MIC=minimum inhibitory concentration, CFN=ciprofloxacin, DMSO=dimethyl sulphoxide, -=indicate no inhibition,NT=not tested.
Table 2: IN VITRO Antibacterial activity data aganist gram‑positive bacteria.
| Compound code | E. coli | P. aeruginosa | R. ruberum | V. cholerae | S. paratyphyi | K. pneumoniae | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZI in mm | % of I | MIC× 10−3 | ZI in mm | % of I | MIC× 10−3 | ZI in mm | % of I | MIC× 10−3 | ZI in mm | % of I | MIC× 10−3 | ZI in mm | % of I | MIC× 10−3 | ZI in mm | % of I | MIC× 10−3 | |
| QP1 | ‑ | ‑ | NT | 10 | 62.5 | 110.75 | ‑ | ‑ | NT | ‑ | ‑ | NT | 10 | 45.45 | 221.49 | ‑ | ‑ | NT |
| QP2 | 17 | 100 | 50.35 | 14 | 87.5 | 50.35 | 15 | 78.94 | 50.35 | 16 | 72.72 | 50.35 | 11 | 50 | 50.35 | 10 | 62.5 | 50.35 |
| QP3 | 10 | 58.82 | 106.96 | ‑ | ‑ | NT | ‑ | ‑ | NT | 11 | 50 | 106.96 | 11 | 50 | 106.96 | ‑ | NT | |
| QP4 | 18 | 5.88 | 51.45 | 11 | 68.75 | 51.45 | 13 | 68.42 | 51.45 | 16 | 72.72 | 102.9 | 10 | 45.45 | 102.9 | 11 | 68.75 | 51.45 |
| QP5 | 11 | 64.7 | NT | ‑ | ‑ | NT | ‑ | ‑ | NT | 9 | 40.9 | 55.25 | 9 | 40.9 | 55.25 | ‑ | NT | |
| QP6 | 25 | 47.05 | 50.46 | 15 | 93.75 | 50.46 | 13 | 68.42 | 100.91 | 10 | 45.45 | 50.46 | 10 | 45.45 | 50.46 | 12 | 75 | 50.46 |
| QP7 | 17 | 100 | 24.44 | 11 | 68.75 | 24.44 | 10 | 52.63 | 48.88 | 12 | 54.54 | 48.88 | 10 | 45.45 | 48.88 | 12 | 75 | 12.22 |
| QP8 | 10 | 58.82 | 41.83 | ‑ | 43.75 | 41.83 | ‑ | ‑ | NT | 11 | 50 | 83.67 | 10 | 45.45 | 83.67 | 11 | 68.75 | 83.67 |
| QP9 | 20 | 17.64 | 59.89 | 11 | 68.75 | 59.89 | 12 | 63.15 | 59.89 | 10 | 45.45 | 59.89 | 19 | 86.36 | 59.89 | 11 | 68.75 | 59.89 |
| QP10 | 9 | 52.94 | 56.51 | 9 | 56.25 | 113.01 | ‑ | ‑ | NT | ‑ | ‑ | NT | 10 | 45.45 | 113.01 | ‑ | NT | |
| QP11 | 9 | 52.94 | 51.92 | 12 | 75 | 51.92 | 12 | 63.15 | 51.92 | 9 | 40.9 | 51.92 | 17 | 77.27 | 51.92 | 10 | 62.5 | 51.92 |
| QP12 | 10 | 58.82 | 52.03 | 13 | 81.25 | 52.03 | 12 | 63.15 | 52.03 | 11 | 50 | 52.03 | 11 | 50 | 104.06 | 9 | 56.25 | 52.03 |
| QSP1 | ‑ | ‑ | NT | 9 | 56.25 | 114.3 | 8 | 42.1 | 114.3 | 11 | 50 | 114.3 | 11 | 50 | 114.3 | 9 | 56.25 | 114.3 |
| QSP2 | 10 | 58.82 | 55.38 | 10 | 62.5 | 110.76 | 11 | 57.89 | 110.76 | 14 | 63.63 | 55.38 | 12 | 54.54 | 55.38 | 10 | 62.5 | 55.38 |
| QSP3 | 13 | 76.47 | 53.48 | 12 | 75 | 53.48 | 13 | 68.42 | 53.48 | 16 | 72.72 | 53.48 | 14 | 63.63 | 53.48 | 12 | 75 | 53.48 |
| QSP4 | 13 | 76.47 | 53.48 | 11 | 68.75 | 53.48 | 12 | 63.15 | 26.74 | 16 | 72.72 | 26.74 | 14 | 63.63 | 53.48 | 14 | 87.5 | 53.48 |
| QSP5 | 18 | 5.88 | 53.71 | 16 | 100 | 26.86 | 15 | 78.94 | 53.71 | 17 | 77.27 | 53.71 | 13 | 59.09 | 53.71 | 14 | 87.5 | 53.71 |
| Standard | 17 | 100 | 4.72 | 16 | 100 | 9.43 | 19 | 100 | ‑ | 22 | 100 | 18.86 | 22 | 100 | ‑ | 16 | 100 | 0.57 |
| CFN | ||||||||||||||||||
| Solvent | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ |
| DMSO | ||||||||||||||||||
ZI=zone of inhibition, % of I= percent inhibition, MIC=minimum inhibitory concentration, CFN=ciprofloxacin, DMSO=dimethyl sulphoxide, -=indicate no inhibition,NT=not tested.
Table 3: IN VITRO Antibacterial activity data aganist gram‑negative bacteria
| Compound code | C. albicans | M. purpureus | A. niger | T. rubrum | A. fumigatus | S. albus | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZI in mm | % of I | MIC× 10−3 | ZI in mm | % of I | MIC× 10−3 | ZI in mm | % of I | MIC× 10−3 | ZI in mm | % of I | MIC× 10−3 | ZI in mm | % of I | MIC× 10−3 | ZI in mm | % of I | MIC× 10−3 | |
| QP1 | ‑ | ‑ | NT | ‑ | ‑ | NT | ‑ | ‑ | NT | ‑ | ‑ | NT | ‑ | ‑ | NT | ‑ | ‑ | NT |
| QP2 | 13 | 61.9 | 50.35 | ‑ | ‑ | NT | 11 | 61.11 | 50.35 | ‑ | ‑ | NT | 10 | 52.63 | 50.35 | ‑ | ‑ | NT |
| QP3 | ‑ | ‑ | NT | ‑ | ‑ | NT | 9 | 50 | 106.96 | ‑ | ‑ | NT | 9 | 47.36 | 53.47 | ‑ | ‑ | NT |
| QP4 | 10 | 47.61 | 51.45 | ‑ | ‑ | NT | 10 | 55.55 | 51.45 | ‑ | ‑ | NT | 9 | 47.36 | 51.45 | 8 | 50 | 51.45 |
| QP5 | ‑ | ‑ | NT | ‑ | ‑ | NT | 11 | 61.11 | NT | ‑ | ‑ | NT | ‑ | ‑ | NT | ‑ | ‑ | NT |
| QP6 | 12 | 57.14 | 50.46 | 11 | 68.75 | 100.91 | 11 | 61.11 | 50.46 | 10 | 50 | 100.91 | 9 | 47.36 | 50.46 | 11 | 68.75 | 50.46 |
| QP7 | 12 | 57.14 | 97.75 | 10 | 62.5 | 195.5 | 12 | 66.66 | 195.5 | 9 | 45 | 195.5 | ‑ | ‑ | NT | 10 | 62.5 | 195.5 |
| QP8 | 9 | 42.85 | 41.83 | 8 | 50 | 41.83 | ‑ | ‑ | NT | ‑ | ‑ | NT | ‑ | ‑ | NT | ‑ | ‑ | NT |
| QP9 | 8 | 38.09 | 119.78 | ‑ | ‑ | NT | ‑ | ‑ | NT | 11 | 55 | 119.78 | ‑ | ‑ | NT | 9 | 56.25 | 119.78 |
| QP10 | ‑ | ‑ | NT | ‑ | ‑ | NT | ‑ | ‑ | NT | ‑ | ‑ | NT | ‑ | ‑ | NT | ‑ | ‑ | NT |
| QP11 | 11 | 52.38 | 103.84 | 9 | 56.25 | 103.84 | 9 | 50 | 103.84 | 11 | 55 | 103.84 | 9 | 47.36 | 103.84 | 12 | 75 | 103.84 |
| QP12 | 10 | 47.61 | 104.06 | 9 | 56.25 | 104.06 | 9 | 50 | 104.06 | 12 | 60 | 104.06 | 10 | 52.63 | 104.06 | 10 | 62.5 | 104.06 |
| QSP1 | ‑ | ‑ | NT | ‑ | ‑ | NT | 9 | 50 | 57.15 | ‑ | ‑ | NT | ‑ | ‑ | NT | ‑ | ‑ | NT |
| QSP2 | 15 | 71.42 | 110.76 | 10 | 62.5 | 110.76 | ‑ | ‑ | NT | ‑ | ‑ | NT | ‑ | ‑ | NT | 12 | 75 | 110.76 |
| QSP3 | 13 | 61.9 | 53.48 | 12 | 75 | 53.48 | 10 | 55.55 | 106.97 | 13 | 65 | 53.48 | 14 | 73.68 | 53.48 | 16 | 100 | 53.48 |
| QSP4 | 13 | 61.9 | 26.74 | 10 | 62.5 | 53.48 | 10 | 55.55 | 106.97 | 14 | 70 | 106.97 | 17 | 89.47 | 53.48 | 14 | 87.5 | 53.48 |
| QSP5 | 14 | 66.66 | 26.86 | 10 | 62.5 | 53.71 | 14 | 77.77 | 53.71 | 15 | 75 | 53.71 | 16 | 84.21 | 53.71 | 14 | 87.5 | 53.71 |
| Standard | 21 | 100 | 12.5 | 16 | 100 | 6.25 | 18 | 100 | 25.5 | 20 | 100 | 0.39 | 19 | 100 | 0.39 | 16 | 100 | 12.5 |
| CFN | ||||||||||||||||||
| Solvent | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ |
| DMSO | ||||||||||||||||||
ZI=zone of inhibition, % of I= percent inhibition, MIC=minimum inhibitory concentration, CFN=ciprofloxacin, DMSO=dimethyl sulphoxide, -=indicate no inhibition,NT=not tested.
Table 4: IN VITRO Antibacterial activity data aganist gram‑positive bacteria
Determination of minimum inhibitory concentration
The minimum inhibitory concentration (MIC) of μg/ml values of the titled compounds were carried out by two-fold serial dilution method [30]. The MICs are recorded through visual observations after 24 h (for bacteria) and 72-96 h (for fungi) of incubation. Ciprofloxacin was used as standard for bacterial studies and clotrimazole was used as standard for fungal studies. The lowest concentration at which there was no visible growth was taken as MIC. The results of the MIC study were listed in Tables 2, 3 and 4, respectively.
Evaluation of antimycobacterial activity
All the synthesized compounds were screened at 100- 1.56 μg/ml concentration in a broth micro dilution assay with alamar blue, also known as microplate alamar blue assay [31] (MABA) against M. tuberculosis H37Rv (MTB) to determine MIC. These results in comparison with isoniazid, ethambutol, and ciprofloxacin as the reference drugs and the results are listed in Table 5.
| Compound code | TB Activity MIC×10–3 (MTB37RV) | Cytotoxicity activity IC50 µM | Selective Index (SI) (SI=IC50/MIC) |
|---|---|---|---|
| QP1 | – | NT | – |
| QP2 | – | NT | – |
| QP3 | 53.47 | 62.08 | 1.16 |
| QP4 | – | NT | – |
| QP5 | 110.5 | 87 | 0.79 |
| QP6 | – | NT | – |
| QP7 | 48.88 | 42.21 | 0.86 |
| QP8 | 20.92 | 206.4 | 10.92 |
| QP9 | 14.98 | 272 | 15.16 |
| QP10 | – | NT | – |
| QP11 | 6.49 | 171 | 26.35 |
| QP12 | – | NT | – |
| QSP1 | 14.29 | 156.1 | 10.92 |
| QSP2 | 13.84 | 225.9 | 16.39 |
| QSP3 | 26.74 | 223.4 | 8.35 |
| QSP4 | – | NT | – |
| QSP5 | 13.34 | 160.1 | 12 |
| Isoniazid | 0.8 | – | – |
| Ethambutol | 7.6 | – | – |
| Ciprofloxacin | 9.4 | – | – |
In vitro minimum inhibition concentration against multi drug resistant Mycobacterium tuberculosis (H37Rv), cytotoxicity activity against mouse embryonic fibroblasts line (NIH 3T3) and selective index (SI) (SI=IC50/MIC) for Compounds (QP1-12 and QSP1-5). -indicates no inhibition, NT=not tested.
Table 5: IN VITRO Antitubercular, cytotoxicity activity and selective index for synthesized compounds
Evaluation of in vitro cytotoxicity activity
Synthesized compounds were evaluated as cytotoxicity against the normal cell line (mouse embryonic fibroblasts line-NIH 3T3) by MTT assay [32,33]. Briefly, 15 ml of MTT (5 mg/ml) in phosphate buffered saline (PBS) was added to each well and incubated at 37º for 4 h. The medium with MTT was then flicked off and the formed formazan crystals were solubilized in 100 ml of DMSO and then measured the absorbance at 570 nm using micro plate reader. The % cell inhibition was determined using the following formula.
% cell Inhibition=[100-(Asample/Acontrol)]×100. Nonlinear regression graph was plotted between % cell inhibition and Log10 concentration and IC50 was determined using Graph Pad Prism software. The results of the cytotoxicity study were listed in Table 5.
Selectivity index
Selectivity index [34] (SI) was calculated for all the title compounds taking into account the MIC against M. tuberculosis H37Rv and the IC50 on mouse embryonic fibroblasts cell line (NIH 3T3) (SI=IC50/MIC) by the MTT assay.
Results and Discussion
The synthetic procedures adopted to obtain Mannich (QP1-12) and Schiff bases (QSP1-5) of Pyrazol- 5(4H)-one moiety containing 3-(hydrazinyl)- 2-phenylquinazolin-4(3H)-one are depicted in Scheme 1 and the physicochemical properties of the synthesized compounds are given in Table 1. Compound 2-phenyl-4H-3,1-benzoxazinone (Q1) and 3-amino-2-phenylquinazolin-4(3H)-one (QA) were prepared according to the previously reported procedure. For the purpose, the diazonium salt solution of (QA) was coupled with ethyl acetoacetate/ ethyl cyanoacetate in ethanol (50 ml) in the presence of sodium acetate (0.05 mol) at 0-5° to form key intermediates ethyl 3-oxo-2-(2-(4-oxo-2-phenyl quinazolin-3(4H)yl) hydrazono)butanoate (QE) and ethyl cyano[2-(4-oxo-2-phenyl-3,4-dihydroquinazolin- 3-yl)hydrazin-1-lidene]formate (QS), respectively. The fair percentage yield (50-55%) of intermediate (QE and QS) may be attributed to any one or more of the following reasons (a) the reaction may be reversible and the position of equilibrium unfavorable to the product; (b) the incursion of side reactions leading the formation of byproducts; (c) the premature work up of the reaction before its completion; (d) the volatilization of products during reaction or during work up; (e) the loss of product due to incomplete extraction, inefficient crystallization; (f) the presence of contaminants in the reactants or reagents leading to a less efficient reaction [35]. The key intermediates (QE and QS) were cyclized by refluxing with hydrazine hydrate in the presence of ethanol to yield 3-(2-(3-methyl-5-oxo-1H-pyrazol-4(5H)-ylidene) hydrazinyl)-2-phenylquinazolin-4(3H)-one (QP) and 3-[2-(3-amino-5-oxo-4,5-dihydro-1H-pyrazol-4-ylidene) hydrazin-1-yl]-2-phenyl-3,4-dihydroquinazolin-4-one (QSP), respectively. If a compound contains active hydrogen attached to nitrogen atom, it undergoes condensation readily, furnishing N-Mannich base in good yield. The compound (QP) pyrazolone may be considered as a cyclic amide and hydrogen atom attached to nitrogen atom should be appreciably labile to participate in the Mannich condensation. Therefore, the condensation of pyrazolone (QP) with formaldehyde (90%) and various amines/amides resulted in the formation the corresponding Mannich base derivatives (QP1-12).
The compounds (QSP) containing amine group at 3rd position in the pyrazolone ring is appreciably labile to participate in the Schiff reaction. These primary amines in pyrazolone moiety were subjected to Schiff reaction with various aldehydes to yield compounds QSP1-5. The structures of compounds (QP1-12 and QSP1-5) were assigned by IR, 1H-NMR, mass spectral and elemental analysis data, which were consistent with the proposed molecular structures. Structures of key intermediates (QP and QSP) were confirmed by IR spectra which showed that the disappearance of the characteristic bands of the carboxylic acid ester, acetyl carbonyl/cyano group at 1764 cm–1. IR stretching band at 1712 cm–1 attributed to NH group stretching and the bands of pyrazolone and quinazolinone ring C=O groups appeared at 1605-1638 and 14640-1690 cm–1, respectively. The IR spectra of the Mannich bases (QP 1-12) and Schiff base (QSP1-5) confirmed the presence of –NH-N=C, –NC=O and fused ring system present in the synthesized compounds by the presence of IR stretching bands at 3330, 1685, 1560 cm–1, respectively. Singlet signals derived from –NH-N=C protons at 7.01-7.23 δ ppm indicates the hydrazone formation. The aromatic protons appeared as multiplets ranging from 6.23-8.93 δ ppm and a singlet appeared at δ 0.79-0.93 corresponding to C3 –CH3 protons of pyrazolone except compound (QP8) which derived from 4-amino- 3-hydroxynaphthalene-2-sulfonic acid, due to the bulk substituted at N1 position. So that methyl protons signals are appearing in 2.51 ppm. In the mass spectra of compound (QP4) containing chlorine atom showed fragments corresponding to the typical chlorine isotope patterns. Thus, the mass spectrum of (QP4) shows its M+2 and M+ peaks at m/z 487.12 (19.38%) and 485.14 (78%), respectively.
Formation of Mannich bases (QP1-12) was confirmed by doublet signals in the range 4.54-4.86 δ ppm. In case of Schiff bases (QSP1-5) singlet signals appeared within the range 8.10-8.16 δ ppm due to –N=CH- proton which confirmed the formation of Schiff bases. Further, the formation of title compounds was confirmed by recording mass spectra, which confirmed the molecular weights and results of elemental analysis were ±0.4% of the theoretical values.
In vitro antibacterial activity results (Tables 2 and 3) revealed that compound QP4 was found to be most potent against Micrococcus luteus with 91.66% inhibition. Against Staphylococcus aureus, compounds QP2 and QSP4 displayed most significant antibacterial activity (83.33% inhibition). In case of Bacillus substilis, compound QP2 displayed antibacterial activity equipotent to standard drug ciprofloxacin, i.e., 100% inhibition. Compounds QP4 and QP6 were found to be the most potent against Coryne bacterium with 90% inhibition, whereas compounds QP9 and QP11 exhibited most potent antibacterial activity against Bacillus lentus with 95% inhibition and compound QP11 showed most significant antibacterial activity against Staphylococcus albus (77.77% inhibition).
In case of E. Coli, compound QP6 (100 μg/disk) was found to be most potent (147% inhibition) and which exhibited 1.5 times antibacterial activity than standard drug ciprofloxacin. Against P. aeruginosa, QSP5 displayed antibacterial activity equipotent to standard drug ciprofloxacin, i.e., 100% inhibition. In case of Salmonella paratyphi, compound QP9 was found to be most potent with 86.36% inhibition. Against Klebsiella pneumoniae, compounds QSP4 and QSP5 exhibited maximum 87.50% growth inhibition.
The antifungal activity results (Table 2) indicated that compound QSP2 was found most potent antifungal agent against Candida albicans having 71.42% growth inhibition. QSP5 was found to be most potent antifungal agent against Aspergillus niger and Trichophyton rubrum causing 77.77 and 75.00% growth inhibition, respectively. Against Aspergillus fumigatus, compound QSP4 exhibited maximum (89.47%) inhibition. QSP3 exhibited antifungal activity comparable to standard, i.e., 100% growth inhibition against Aspergillus parasiticus. In general, Schiff bases were found to be more potent antifungal agents than Mannich bases. In vitro minimum inhibition concentration result demonstrates that Mannich base QP7 (having Ortho –OH and para –COOH group) showed some improvement in anti bacterial activity (MIC=48.88×10–3 μM/ml) among the tested Gram-positive bacterial strains. On the other hand, compound QP7 exhibit that MIC value 12.22×10–3 μM/ml for K. pneumoniae and 24.44×10–3 μM/ml for E. Coli, and P. Aeruginosa. Among the Schiff base, compound QSP4 demonstrate sound activity against R. Ruberum, V. Cholerae and C. Albicans (MIC=26.74×10–3 μM/ml) and also compound QSP5 shows fair activity against P. aeruginosa and C. albicans (MIC=26.86×10–3 μM/ml). Overall antibacterial and antifungal MIC result review that none of the synthesized compounds are equal or more potent aganist tested microorganism compared with standard drugs ciprofloxacin and clotrimazole. In general, Mannich bases were found more potent against Gram +ve bacteria and Schiff bases were found more potent against Gram –ve bacteria and The in vitro antitubercular potential of synthesized Mannich and Schiff bases was determined against M. tuberculosis (H37Rv). Results of antitubercular activity (Table 5) indicated that varying degree of antitubercular activity was observed among the synthesized compounds. Mannich base having unsubstituted phenyl nucleus (QP1) was not active against M. tuberculosis. Addition of electron withdrawing nitro, chloro, and carboxylic acid substituents to phenyl nucleus (QP2, QP4, and QP6) did not improve antitubercular potential of the synthesized compounds. Addition of electron releasing substituent (–OH, QP3) to the phenyl nucleus and replacement of phenyl nucleus by pyridine nucleus (QP5) also produced compounds with weak antitubercular potential. Compound QP9 (having acyl group) showed some improvement in antitubercular activity (MIC=14.29×10–3 μM/ml). So, finally by varying the acyl substituent we got most potent antitubercular agent QP11 (MIC=6.49×10–3 μM/ml). Replacement of phenyl nucleus of QP11 by pyridine nucleus (QP12) resulted in the loss of activity. The synthesized Schiff bases QSP1-5 showed good activity but all of them were less active than QP11. In general, Schiff bases were found to be less potent antitubercular agents than Mannich bases and all the synthesized compounds were less active than the standard drugs and except compound QP11. It showed appreciable antitubercular activity (MIC=6.49×10–3 μM/ml) which is more active than the standard drugs, ethambutol (MIC=7.60×10–3 μM/ml) and ciprofloxacin (9.4×10–3 μM/ml). Compound QP11 has the potential to be selected as a lead compound for the development of antitubercular agents.
Mouse embryonic fibroblasts cell line (NIH 3T3) were used for the in vitro toxicity evaluation of the some selected synthesized compounds QP3, QP5, QP7, QP8 QP9, QP11, QSP1, QSP2, QSP3, and QSP5 by MTT assay. Among the screened compounds, the Schiff bases QSP2 and QSP3 showed highest IC50 at 225.90 and 223.40 μM, respectively and compounds QSP1 and QSP5 showed IC50 at 156.10 and 160.10 μM, respectively. The Mannich bases (MIC=6.49×10-3 μM/ml) QP8, QP9 and QP11 showed IC50 of 206.40, 272 and 171 μM, respectively. Other Mannich base compounds QP3, QP5, and QP7 demonstrate IC50 of 62.08, 87 and 48.21, respectively. Thus, suggesting that their antimicrobial and reported antiTB activity was not due to general cytotoxicity. The selective index (SI) was calculated by dividing IC50 by the MIC of TB activity values. SI is defined as the ratio of the measured IC50 in mouse embryonic fibroblasts cell line to the MIC of TB activity described above. Furthermore, compounds QP11, QP9, QP8, QP3, QP7, and QP95 showed excellent SI of 26.34, 18.16, 10.92, 01.16, 00.86, and 00.79. On the other hand, the Schiff base compounds QSP2, QSP5, QSP1, and QSP3 showed better SI of 16.32, 12.00, 10.92, and 08.35. If the SI is ≥10, the compound is then investigated further. Compounds QP11, QP9, QSP1, QSP2, and QSP5 have the potential to be selected as a lead compound for the development of antitubercular agents.
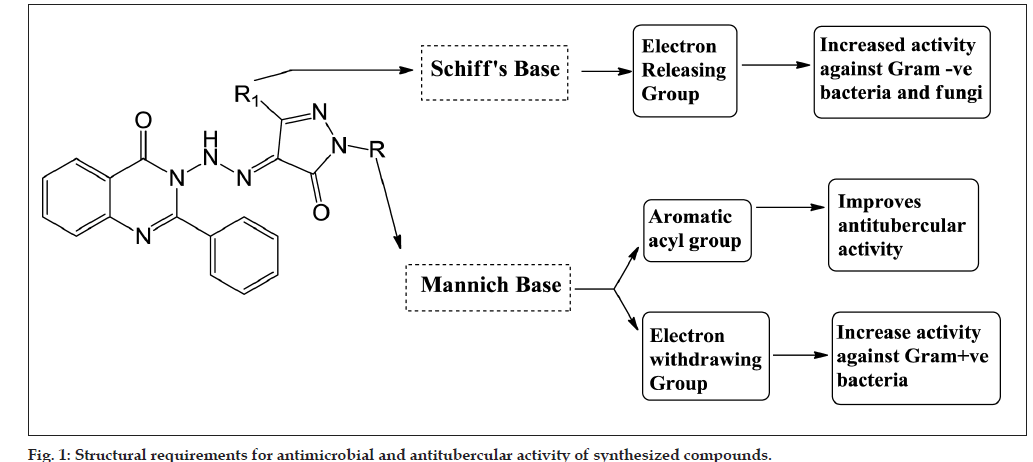
From the results of the in vitro antitubercular and antimicrobial activity of the synthesized substituted pyrazolone derivatives, the following structure activity relationship (SAR) was derived, which is presented in fig. 1 and is summarized as follows: (1) results of antimicrobial study indicated that Mannich bases were more potent against Gram-positive bacteria whereas Schiff bases more potent against Gramnegative bacteria and fungal species. (2) Electron withdrawing groups in Mannich bases showed high antibacterial activity against Gram-positive bacteria whereas Schiff bases with electron releasing groups showed high activity against Gram-negative bacteria and fungal species. This indicated the fact that different structural requirements are needed for the binding of drug to different microorganisms. This fact is supported by the studies of Sortino et al. [36]. (3) The results of antitubercular study indicated that Schiff bases (QSP1-5) were found to be more potent antitubercular agents than Mannich bases (QP1-12) except compound QP11 having aromatic acyl (benzoyl) group which drastically improved the antitubercular activity of compound QP11. This is evidenced by the antitubercular activity of compound QP1 having phenyl substitution lacks the antitubercular activity. (4) Introduction of electron releasing groups to phenyl nucleus of Schiff bases did not improve the antitubercular potential of the synthesized compounds significantly. (5) Addition of electron withdrawing/releasing groups to phenyl nucleus of Mannich bases as well as replacement of phenyl nucleus by pyridine nucleus did not resulted in any significant improvement in the antitubercular potential of the synthesized compounds.
Acknowledgements
The authors are thankful to Dr. Thavamani D. Palaniswami, Managing Trustee, Kovai Medical Research and Education Trust, Coimbatore and Karpagam University, Coimbatore for providing facilities to carry out this research work. The authors are grateful to the Indian Institute of Science, Bengaluru and the Indian Institute of Technology, Chennai for providing NMR and Mass spectral data.
References
- Mathema B, Kurepina NE, Bifani PJ, Kreiswirth BN. Molecular epidemiology of tuberculosis: Current insights. ClinMicrobiol Rev 2006;19:658-85.
- WHO report. Global tuberculosis control: Surveillance, planning and financing. Geneva: World Health Organization, 2007. (WHO/HTM/ TB/2007-376).
- Smith I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. ClinMicrobiol Rev 2003;16:463-96.
- Melagraki G, Afantitis A, Markopoulou OI, Detsi A, Koufaki M, Kontogiorgis C, et al. Synthesis and evaluation of the antioxidant and antiinflammatory activity of novel coumarin-3-aminoamides and their alpha-lipoic acid adducts. Eur J Med Chem 2009;44:3020-6.
- Moreau F, Desroy N, Genevard JM, Vongsouthi V, Gerusz V, Le Fralliec G, et al. Discovery of new Gram-negative antivirulence drugs: Structure and properties of novel E. coli Waa C inhibitors. Bioorg Med ChemLett 2008;18:4022-6.
- Desai AR, Desai KR. Niementowski reaction: Microwave induced and conventional synthesis of quinazolinones and 3-methyl-1H-5-pyrazolones and their antimicrobial activity 2006. ARKIVOC 2005;13:98-108.
- Bondock S, Rabie R, Etman HA, Fadda AA. Synthesis and antimicrobial activity of some new heterocycles incorporating antipyrine moiety.Eur J Med Chem 2008;43:2122-9.
- Manojkumar P, Ravi TK, Gopalakrishnan S. Antioxidant and antibacterial studies of arylazopyrazoles aryl hydrazonopyrazolones containing coumarin moiety. Eur J Med Chem 2009;44:4690-4.
- Amir M, Kumar H, Khan SA. Synthesis and pharmacological evaluation of pyrazoline derivatives as new antiinflammatory and analgesic agents. Bioorg Med ChemLett 2008;18:918-22.
- Adnan A, Bekhit A, Fahmy HT, Rostom SA, Baraka AM. Design and synthesis of some substituted 1H-pyrazolyl-thiazolo [4,5-d] pyrimidines as antiinflammatory/antimicrobial agents. Eur J Med Chem 2003;38:27-36.
- Tripathy R, Ghose A, Singh J, Bacon ER, Angeles TS, Yang SX, et al. 1,2,3-Thiadiazole substituted pyrazolones as potent KDR/VEGFR-2 kinase inhibitors. Bioorg Med Chem 2007;17:1793-8.
- Kucukguzel GS, Rollas S. Synthesis, characterization of novelcoupling products and 4-arylhydrazono-2-pyrazoline-5-ones as potential antitubercular agents. Farmaco 2002;57:583-7.
- Bekhit AA, Ashour HM, Ghany YS, Bekhit AE, Baraka A. Synthesis and biological evaluation of some thiazolyl and thiadiazolyl derivatives of 1H- pyrazole as antiinflammatory and antimicrobial agents. Eur J Med Chem2008;43:456-63.
- Patel JA, Mistry BD, Desai KR. Synthesis and antimicrobial activity of newequinazolinones. J Org Chem 2006;11:97-102.
- Ouyang G, Zhang P, Gangfang X, Baoan S, Song Y, Linhong J, et al. Synthesis and antifungal bioactivities of 3-alkyl quinazolin-4-one derivatives. Molecules 2006;11:383-92.
- Bishnoi A, Awasthi R, Pandey VK, Tiwari AK, Awasthi NK, Srivastava K, et al. Drug design based on the principle of conjunction: Synthesis, characterization and anti hyperglycemic activity study of 3-[2-(3-aroyl-2,4-diphenyl-3,4-dihydro-2H [1,3] oxazino [5,6-H] quinolin-6-yl) ethyl] 2-phenyl-quinazolin-4 (3H)-ones. Int J Drug Discov 2009;2:52-5.
- Alagarsamy V, Muthukumar V, Pavalarani N, Vasanthanathan P, Revathi R. Synthesis, analgesic and antiinflammatory activities of some novel 2,3-disubstituted quinazolin-4 (3H)-ones. Biol Pharm Bull 2003;26:557-9.
- Al-Deeb AO, AlafeefyAM. Synthesis of some new 3H-quinazolin-4-one derivatives as potential antitubercular agents. World ApplSci J 2008;5:94-9.
- Gao X, Cai X, Kai Yan K, Song B, Gao L, Chen Z. Synthesis and antiviral bioactivities of 2-aryl- or 2-methyl-3-(substituted-benzalamino)-4 (3H)-quinazolinone derivatives. Molecules 2007;12:2621-42.
- Sriram D, Yogeswari P, Devakaram RV. Synthesis, in vitro and in vivo antitubercular activities of diclofenac acid hydrazones and amides.Bioorg Med Chem 2006;14:3113-8.
- Solomon VR, Hua C, Lee H. Design and synthesis of anti-breast cancer agents from 4-piperazinylquinoline: A hybrid pharmacophore approach. Bioorg Med Chem 2010;18:1563-72.
- Kumar P, Narasimhan B, Yogeeswari P, Sriram D. Synthesis and antitubercular activities of substituted benzoic acid N’-(substituted benzylidene/furan-2-ylmethylene)-N-(pyridine-3-arbonyl)-hydrazides. Eur J Med Chem 2010;45:6085-9.
- Narasimhan B, Sharma D, Kumar P, Yogeeswari P, Sriram D. Synthesis, antimicrobial and antitubercular evaluation of [2-(substituted phenyl)-imidazol-1-yl]-pyridin-3-ylmethanones. J Enzyme Inhib Med Chem 2011;26:720-7.
- Narang R, Narasimhan B, Sharma S, Sriram D, Yogeeswari P, Clercq ED, et al. Synthesis, antitubercular, antiviral, antimicrobial activities, and QSAR studies of nicotinic acid benzylidenehydrazide derivatives. Med Chem Res 2012;12:1557-76.
- Tiwari AK, Singh VK, Bajpai A, Shukla G, Singh S, Mishra AK. Synthesis and biological properties of 4-(3H)-quinazolone derivatives.Eur J Med Chem 2007;42:1234-8.
- Saleh MA, Abdel-Megeed MF, Abdo MA, Shokr AB. Synthesis of novel 3H-quinazolin-4-ones containing pyrazolinone, pyrazole and pyrimidinone moieties. Molecules 2003;8:363-73.
- Pandeya SN, Sriram D, Nath G, De Clercq E. Synthesis, antibacterial, antifungal and antiHIV evaluation of Schiff and Mannich bases of isatin derivatives with 3-amino-2-methylmercapto quinazolin-4 (3H)-one. Pharm ActaHelv 1999;74:11-7.
- Alam MS, Choi JH, Lee DU. Synthesis of novel Schiff base analogues 4-amino-1,5-dimethyl-2-phenylpyrazol-3-one and their evaluation for antioxidant and antiinflammatory activity. Bio Med Chem2012;20:4103-8.
- John DT, James HJ. Antimicrobial Susceptibility testing: General Considerations. Manual of Clinical Microbiology. 7th ed. Washington DC: American Society for Microbiology; 1999. p. 1469-73.
- Andrews JM. Determination of minimum inhibitory concentrations. J AntimicrobChemother 2001;48:5-16.
- Collins LA, Franzblau SG. MicroplateAlamar Blue Assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother 1997;41:1004-9.
- Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, et al. Feasibility of high flux anticancer drug screen using a diverse panel of cultured human tumour cell lines. J Natl Cancer Inst 1991;83:757-66.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55-63.
- Orme I, Secrist J, Anathan S, Kwong C, Maddry J, Reynolds R, et al. Search for new drugs for treatment of tuberculosis. Antimicrob Agents Chemother 2001;45:1943-6.
- Furniss BS, Hannaford AJ, Smith PW, Tatchell AR. Vogel’s Text Book of Practical Organic Chemistry. 5th ed. Singapore: Longman Singapore Publishers Pvt. Ltd,; 1994. p. 33-4.
- Sortino M, Delgado P, Jaurez S, Quiroga J, Abonia R, Insuasey B, et al. Synthesis and antifungal activity of (Z)-5-arylidenerhodanines. Bioorg Med ChemLett 2007;15:484-94.