- *Corresponding Author:
- S. Khan
Department of Chemistry, University of Management and Technology, Lahore-54000, Pakistan
E-mail: shakilahmad56@gmail.com
| Date of Submission | 24 January 2017 |
| Date of Revision | 11 August 2017 |
| Date of Acceptance | 27 March 2018 |
| Indian J Pharm Sci 2018;80(3):480-488 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Ni(II), Cu(II) and Zn(II) complexes of diphenyldithiocarbamate have been synthesized with the aim of developing antioxidants, potential antibacterial and antifungal agents. Synthesized metal complexes were characterized by magnetic susceptibility, elemental analysis; conductivity measurements, mass spectra, infrared, ultra violet/visible and nuclear magnetic resonance verified the chemical composition of products. Magnetic susceptibility and spectroscopic studies suggested the predicted geometry of the diphenyldithiocarbamate complexes as octahedral geometry of Ni(II) complex distortion square-planer geometry distorted towards tetrahedral for Cu(II) and Zn(II) complexes. Synthesized metal complexes exhibited remarkable antioxidant potential as compared to standard. Zn(II) complex of diphenyldithiocarbamate exhibited IC50 of 31.45±0.31 μM while the standard, butylated hydroxytoluene exhibited IC50 of 44.67±0.45 μM antioxidant activity. Synthesized metal complexes showed very good antibacterial and antifungal potential against Rhodococcuss, Actinomyces viscosus, Bacillus subtilis, Escherichia coli and the fungi, Aspergillus niger, Aspergillus flavus, Candida and Acetomyceta. It’s concluded that synthesized metal complexes showed better antioxidant, antibacterial and antifungal potential as compared to diphenyldithiocarbamate as well as the standard and might turn out to be a valuable treatment of numerous kinds of diseases.
Keywords
Diphenyldithiocarbamate (DPDTC), spectroscopic studies, antioxidant, antibacterial, antifungal activities
Dithiocarbamates (DTCs) are the synthetic compounds that involved in the forming of complexes with most of the stabilize transition metals in a various oxidation states. DTCs draw much attention in recent years and a large number of DTCs complexes have been synthesized on a large scale in the last 40-50 y [1-4]. A new synthetic strategy has been reported to prepare the DTCs [5]. They also have a variety of applications in agriculture, like pesticides and fungicides, in therapy, against alcoholism as arrestors of human immune deficiency virus infections such as AIDS in fungal diseases as well as in the rubber industries because they have strong metal binding capacity [6-10]. Coordination compounds of DTCs have been widely studied because metal complexes of this have been reported as an antitumor, antibacterial and antifungal agent [11-13]. Previously reported studies on the preparation of DTCs obtained from amino acids and their coordination compounds with several transition metal cations have been reported [14-17]. Some metal complexes of DTCs have been also reported to show de-toxicant and immune pharmacological properties [18]. The DTC stoichiometry has much external consideration in recent years due to its ability to act as a bidentate ligand [1-3]. DTCs such as diethyl DTC and pyrrolidine DTC (PDTC) display cytotoxic properties and they have been used to treat metal poisoning [4-6]. The complexes are thermodynamically stable, so they are used as chelating agent for the extraction of trace metals [5,7]. Metal complexes of DTC present a wide range of applications in agriculture, medicine, industry, and analytical and organic chemistry [7,8]. The metal complexes of DTC with transition metals i.e. Zn, Ni, Cu, Mn, Pt show enhanced biological activities such as antibacterial, antimalarial, and fungicidal activities compared to free ligands due to drop in polarity of metals after complexation [9,10]. DTC metal complexes of Zn, Ni, and Cu, also showed remarkable antitumor properties [11]. Particularly, Ni(II) and palladium (II) play an important role in both fundamental research and application of their physical properties. Nickel and palladium complex with PDTC has been studied extensively. DTC ligands have been reported in the dithiocarboxy group as the ligator group and behave like a bidentate ligand in most of complexes. DTCs act as uni-negative bidentate ligands and they can also found in both hexa and tetra coordination geometry of the many transition metal complexes [19-25]. The present research work involved the synthesis of the ligand diphenyl DTC (DPDTC) and its respective metal complexes with Zn(II), Ni(II) and Cu(II) to enhance the antibacterial, antifungal and antioxidant activities of DPDTC by complexation. Further, all of complexes were characterized using different spectroscopic techniques. Antioxidant and antimicrobial activities of the complexes were also studied.
Materials and Methods
Carbon disulfide (CS2), diphenylamine, sodium hydroxide, ZnCl2.2H2O, CuCl2.2H2O and NiCl2.6H2O and other organic solvents were purchased from Merck and used without further purification. Elemental analyses of all compounds were performed on a Fisons elemental analyser. Conductance measurements of the synthesized complexes measured on conductivity meter, model WD-35604-00 at room temperature. Infrared (IR) spectra were recorded in Fourier-transform infrared spectroscopy (FTIR) spectrophotometer, Nicolet IS50, within the range of 400-4000 cm-1. Electronic spectra were recorded for solution of the ligand and their metal complexes in dimethylformamide as on Jasco UV/Vis spectrophotometer model V-770 UV/ Vis. Magnetic susceptibility of all the synthesized complexes was determined by using the Holmarc’s magnetic susceptibility with model HO-ED-EM-07 at room temperature. 1H-nuclear magnetic resonance (NMR) spectra and 13C-NMR were recorded in dimethyl sulfoxide (DMSO)-d6 at room temperature using tetramethylsilane as internal standard on a Bruker 400 MHz and 101 MHz spectrophotometer. Mass spectra of all the complexes were performed on Finnigan MAT LCQTM mass spectrometer.
Synthesis of ligand Na-DPDTC
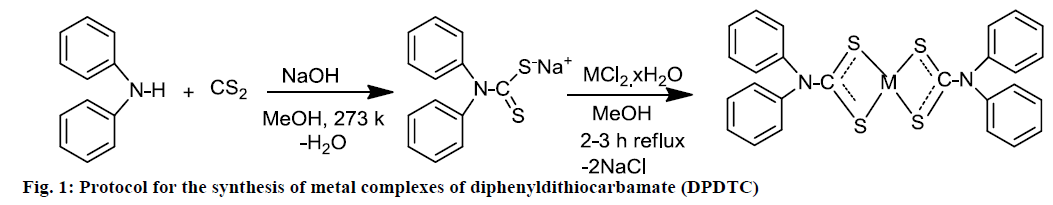
Na-DPDTC was synthesized using the previously reported methods with some modification [26]. DTCs ligand was prepared by mixing methanolic solution of diphenylamine (0.0845 g, 0.5 mmol), 5 ml cold 10 N solution of sodium hydroxide and 0.030 ml (0.5 mmol) CS2 added drop-wise. The resulting mixture was kept at constant stirring for 3-4 h at temperature 273 k. The temperature maintained to avoid any possible decomposition by using a mixture of sodium salts, melting ice and water. Off-white precipitate of DTC ligand was filtered, washed with ethanol and dried over silica gel. Yield: 90 %, colour: off-white, melting point (m.p.); 189-192, anal. calc. for C13H10NS2Na (molecular weight, MW: 267.34 g/mol): C, 58.40; H, 3.77; N, 5.24; S, 23.99; Na, 8.60 % and found: C, 58.36; H, 3.72; N, 5.28; S, 23.95; Na, 8.64 %. Selected FTIR (KBr, v, cm-1): (NCS/CN): 1465; (CS2)sy: 994; (CS2)asy: 1156; (S-M): 423. Electronic spectra (λmax in nm): 267, 287.
Synthesis of metal complexes of DPDTC
Metal complexes of DPDTC were prepared by taking the ligand to metal molar ratio of 2:1. An aqueous solution of ZnCl2.2H2O (0.25 mmol, 0.043 g), CuCl2.2H2O (0.25 mmol, 0.042 g) and NiCl2.6H2O (0.25 mmol, 0.059 g) was mixed separately with 0.5 mmol (0.134 g) DTC ligand dissolved in methanol. The resulting mixture was reflux for 2 h and the colored precipitates of DPDTC metal complexes were obtained for each case and the solid precipitated was filtered, washed with diethyl ether-methanol (1:1 ratio) and dried over silica gel.
Zn [S2CN(C6H5)2] complex
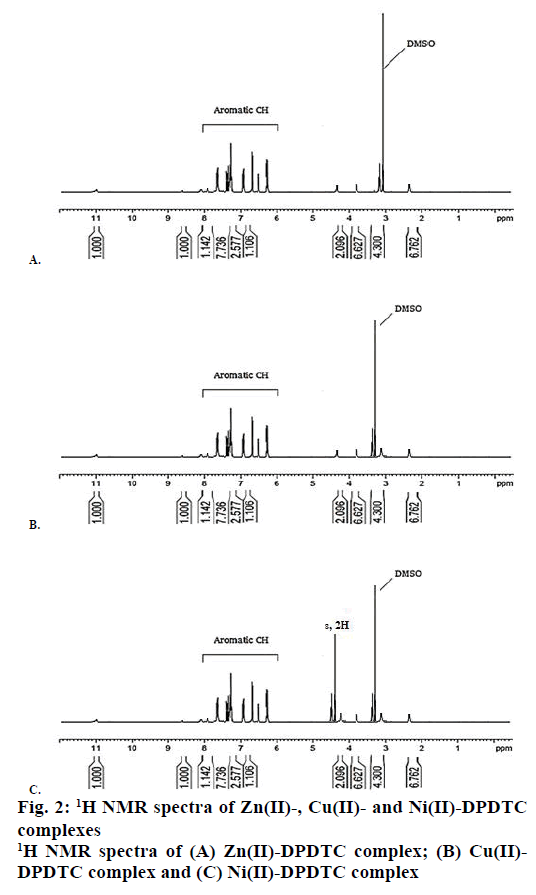
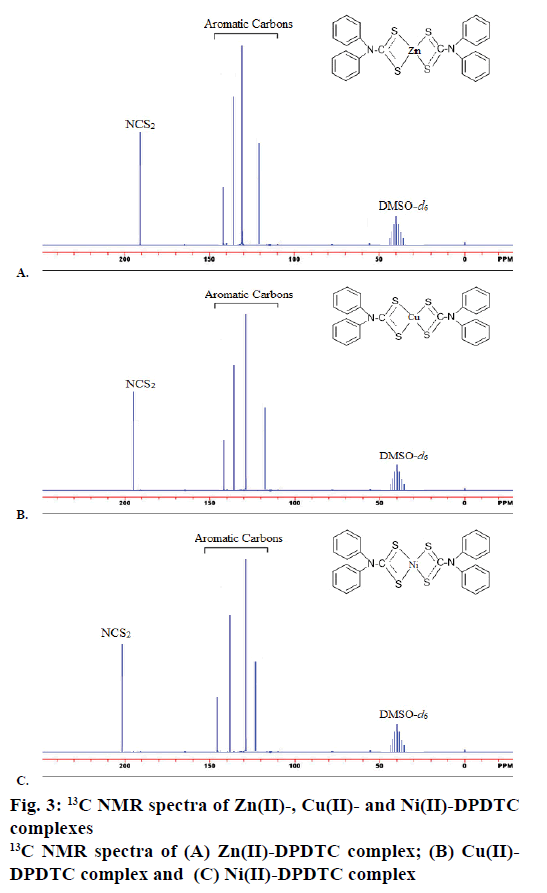
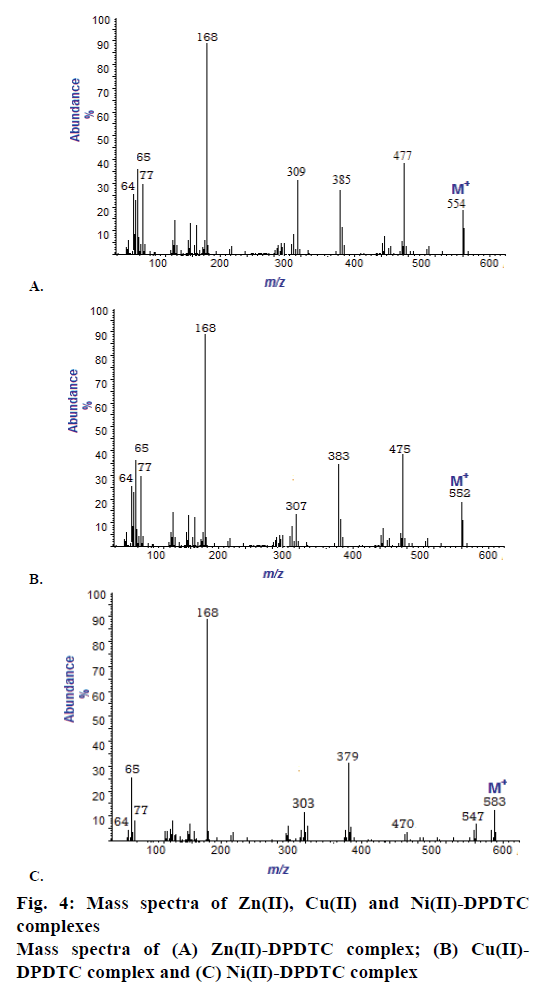
Yield: 88 %, colour: purple, m.p.; 239-241, Λm: 342 Ohm-1 cm2 mol-1, μ eff: 3.67. Analysis: calculated for C26H20N2S4Zn (MW: 554.10 g/mol): C, 56.36; H, 3.64; N, 5.06; S, 23.23; Zn, 11.72 % and found: C, 56.31; H, 3.60; N, 5.09; S, 23.27; Zn, 11.68 %. Selected FTIR (KBr, v, cm-1): (NCS/CN): 1481; (CS2)sy: 987; (CS2)asy: 1128; (S-M): 431. Electronic spectra (λmax in nm): 259, 298, 345. 1H-NMR (400 MHz, DMSO-d6) δ= 6.29- 7.56 (m, 20H, -C6H6). 13C-NMR (DMSO-d6) δ= 189.81 (-CS2), 141.92, 129.67, 127.55, 121.90 (-C6H5).
Cu(II) [S2CN(C6H5)2] complex
Yield: 83 %, colour: radish-brown, m.p.; 273-275, Λm: 653 Ohm-1cm2mol-1, μ eff: 1.81. Analysis: calculated for C26H20N2S4Cu (MW: 552.256 g/mol): C, 56.55; H, 3.65; N, 5.07; S, 23.22; Cu, 11.51 % and found: C, 56.60; H, 3.69; N, 5.02; S, 23.27; Cu, 11.54 %. Selected FTIR (KBr, v, cm-1): (NCS/CN): 1474; (CS2)sy: 978; (CS2)asy: 1131; (S-M): 427. Electronic spectra (λmax in nm): 379, 434, 547, 467. 1H-NMR (400 MHz, DMSO-d6) δ= 6.17-7.60 (m, 20H, -C6H6). 13C-NMR (DMSO-d6) δ= 196.74 (-CS2), 142.75, 129.69, 127.25, 119.62 (-C6H5).
Ni(II) [S2CN(C6H5)2(H2O)2] complex
Yield: 79 %, colour: green, m.p.; 263-265, Λm: 557 Ohm-1cm2mol-1, μeff: 3.67. Analysis: calculated for C26H24N2S4O2Ni (MW: 583.43 g/mol): C, 53.52; H, 4.15; N, 4.80; S, 21.98; Ni, 10.26 % and found: C, 53.48; H, 4.19; N, 4.79; S, 21.99; Ni, 10.23 %. Selected FTIR (KBr, v, cm-1): (NCS/CN): 1477; (CS2)sy: 996; (CS2)asy: 1143; (S-M): 429; (H2O): 2600-3600; (OH): 1648. Electronic spectra (λmax in nm): 367, 412, 670, 559. 1H-NMR (DMSO-d6) δ= 6.21-7.52 (m, 20H, -C6H6), 4.41 (s, 2H, H2O). 13C-NMR (400 MHz, DMSO-d6) δ= 201.42 (-CS2), 134.97, 123.53, 127.05, 124.94 (-C6H5).
DPPH free radical scavenging activity
The DPPH free radical controlling effect was concluded by using method proposed by Stasko et al. [27] To examine the activity of the Na-DPDTC and their metal complexes at various concentrations 1000, 500, 250, 150 μg/ml and 60 μg/ml and butylated hydroxytoluene (BHT) was used as a reference drug [28]. Then 3 ml solution of DPPH in methanol (0.01 g/250 ml methanol) was mixed with sample solution and mixed energetically and permitted to place in room temperature towards 1 h. The absorbance was measured at 517 nm against volatile flammable liquid alcohol as a void in spectrophotometer. All measurements were taken triplicate and lesser absorbance indicates high level of free radical controlling capacity [29,30]. Free radical scavenger effects of DPPH are estimated using the formula, DPPH inhibition = Abscontrol–Abssample/ Abcontrol×100, where Abcontrol is the absorbance of DPPH radical+DMSO and Absample is the absorbance of DPPH radical+sample/standard.
Antimicrobial activity
Antimicrobial activity of Na-DPDTC and the synthesized metal complexes were tested using the agar-well diffusion method [31,32] against diverse bacteria, Rhodococcuss, P. aeruginosa, B. subtilis and E. coli and fungi, A. niger, A. flavus, Candida and Acetomyceta. The culture media were prepared by dissolving around 12 g nutrient agar in 600 ml distilled water for bacteria and 38 g of the potato dextrose ager in 1 l of distilled water was poured in it for the preparation of solution. The culture media were buffered at a pH of 7.4 and sterilized in autoclave at 121° for 15 min to purify the media [33,34]. All the bacterial and fungal strains were adjusted to 0.5 McFarland standard, which was prepared by adding 0.05 ml of 1 % w/v BaCl2·2H2O in phosphate buffered saline (PBS) to 9.95 ml of 1 % v/v H2SO4 in PBS, which is visually comparable to a microbial suspension containing approximately 1.5×108 CFU/ml. The stock solutions of synthesized complexes were aseptically filled (100 μl in each dish) into Petri dishes and then allowed to attain room temperature. After keeping the petri dishes in the flat position for one hour, the incubation period was allowed to proceed for 24 h at 37° for the bacterial cultures and for the fungal species 48 h at 37° [35], the development was checked with the assistance of zone reader in millimeter by measuring the zone of hindrance around the specimen well. Standard antifungal drug fluconazole and antibacterial drug ampicillin (100 μg/ml) were used for comparison under similar conditions. These studies were performed in triplicate, and the average was taken as the final reading.
Results and Discussion
Numerous methods for synthesis of DTCs were reported using the amine with CS2. In this investigation, synthesis of Na-DPDTC by the reaction of CS2 with a diphenylamine in the presence of NaOH at 0° was reported. The temperature maintained to avoid any possible decomposition. The metal complexes of DPDTC were synthesized by reaction of ligand with metals salts and analytical data indicated the ligand to metals ratio 2:1 of all the synthesized complexes. The molecular formulae of the ligands and their metal complexes were proposed according to the data from spectral, elemental analysis, conductivity and magnetic susceptibility of metal complexes. The proposed mechanism for the synthesis of the compounds is shown in Figure 1.
Magnetic susceptibility of the Ni(II), Zn(II) and Cu(II) complexes of DPDTC have the magnetic moment in the range of 1.73-3.67 B.M. The magnetic moment of Ni(II) complex of DPDTC is 3.67 B.M, which agrees with the formation of octahedral complex [25]. The Zn(II) and Cu(II) complexes of DPDTC usually have a distorted square planer configuration with magnetic moment values, 1.73 B.M and 1.81 B.M, respectively [35,36].
Functional groups can be simply recognized in IR spectroscopy. Consequently, IR spectroscopy considered as valuable basis for attaining binding mode of the Na-DPDTC to metal ions in the complexes formation. The three main regions are of interest of the DPDTC and their metal complexes, which give the valuable information about the coordination behaviour of ligand to metals and have a strong agreement about structure of the complexes as in the region of 1456- 1488 cm-1 was assigned the stretching vibrations of v(C-N) for NCS2 – with the shift of 9-16 cm–1 in higher wave number in all the complexes, indicating its involvement in coordination with metal ions [37]. IR spectra of all the synthesized complexes give the strong peak of asymmetric absorption frequency of v(CS2)asy at 1128-1156 cm–1 with the shift of 13-28 cm–1 to the lower wave number and symmetric absorption frequency of v(CS2)sy at 978-996 cm–1 and which indicated that the bidentate ligand to the central metals [38]. The sharp stretching vibration bands found in the spectra of the Ni(II) complex of DPDTC appear in medium peak at 3469 cm–1 and weak peak at 1648, which are not observed in the spectra of the ligand and other complexes [39]. New absorption bonds of M-S stretching mode of vibration are assigned within the range of 423-431 cm–1 of all the synthesized complexes [40]. These values are close agreement with the metal-sulphur donor ligands.
The UV/Vis spectra of the Na-DPDTC and its metal complexes have been recorded in DMSO at room temperature. UV spectra show the high absorption intensity due to presence of NCS2 chromosphere. In the spectrum of Na-DPDTC ligand two absorption bands were recorded at 287 and 267 nm, which are attributed to n→π* and π→π* transitions. The copper complexes of DPDTC exhibited two absorption bands in the visible region at 547 and 467 nm due to 2B1g→ 2A2g, 2B1g→ 4Eg transition, respectively [41]. A sharp band appeared at 556 nm is due to Jahn-Teller distortion, which indicate that the Cu(II) complex of DPDTC has the square-planar geometry. The nickel complex also exhibited the two absorption bands as 3A2g→3T1g (P) and 3A2g→3T1g (F) transitions in visible region at 670 and 559 nm, suggesting the octahedral geometry of the nickel complex [42]. The electronic spectra of the Zn(II) complex of DPDTC exhibited one broad peak at 345 nm, which assigned charge transfer of metal to ligand and d-d electronic transition was not exhibited due to the completely filled d-orbital. In general, Zn(II) complexes of DPDTC would have square-planar geometry due to four coordination bonds [43]. Strong bands appeared at 367 and 379 nm are assigned to transfer of charge from metal to ligand (M→LCT) and d-d bond for Ni(II) and Cu(II) complexes, respectively. Sulphur to metal charge transfer (Lπ→MCT) and d-d bond is assignable by the very intense bands at 412 and 434 nm for Ni(II) and Cu(II) complexes, respectively [44].
The 1H NMR spectra of synthesized metal complexes were recorded in DMSO-d6. These synthesized complexes appeared the multiples signals were in the region at δ=7.60-6.29 ppm, which assigned to proton of phenyl group [45]. The 1H NMR spectra of Zn(II), Cu(II) and Ni(II) complexes are shown in Figure 2. 13C NMR spectra of the complexes were observed the week signal of NCS2 carbon atoms moieties at the region 189.81-201.42 ppm [46]. The signals of carbon due to aryl group were observed at the region 141.92- 121.90, 142.75-119.62 and 144.97-123.94 ppm in the Zn(II), Cu(II) and Ni(II) complexes, respectively. The 13C NMR spectra of Zn(II), Cu(II) and Ni(II) complexes are shown in Figure 3.
The mass spectra of the Na-DPDTC and all synthesized complexes have been recorded at 300° and 70 eV. All the spectra exhibit parent peaks due to molecular ions and proposed molecular formula of these complexes was confirmed by comparing their molecular formula weights with m/z values. The mass spectra of ligand show molecular ion peak (M+) at m/z 267 resembles to the total molecular weight of the ligand. In the spectra of Zn(II), Cu(II) and Ni(II) shows the molecular ion peaks at m/z 554, m/z 552 and 583, respectively, supporting the composition of synthesized complexes. Mass spectra of Zn(II), Cu(II) and Ni(II) complexes are shown in Figure 4.
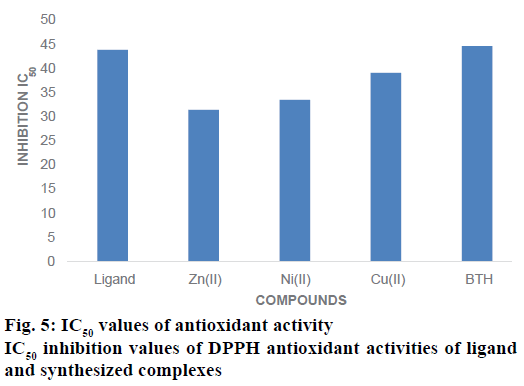
DPPH assay is the most extensively used method for evaluating the oxidative potential of different metal complexes. Free radicals are generated by enzymatic and non-enzymatic processes and if the colours of these free radicals disappear, it is understood that the compounds have the ability to scavenge free radicals [47,48]. The antioxidant activity of ligand and synthesized complexes have been evaluated for DPPH radical scavenging assay against the BHT was used as a reference drug at various concentrations. All the complexes and ligand showed the good antioxidant activity. The inhibitory concentration (IC50) of Zn(II) was lower 31.45± 0.31 μM, it also showed highest antioxidant activity than other compounds. The Ni(II) complex also exhibited highest antioxidant activity by showing lowest IC50 value of 33.55±0.35 μM. But IC50 value of Cu(II) of was lower 39.12±0.41 μM it also showed highest antioxidant activities than ligand as well as reference standard (BHT) [49]. The order of DPPH assay of the compounds is as Zn(II)>Ni(II)>Cu(II)>BHT>ligand. The oxidizing potential effects of the compounds are associated with in the presence of ligand and their complexes are exerting the actions by donation of hydrogen. IC50 inhibition value of DPPH antioxidant activities of Na-DPDTC and synthesized complexes are presented in Figure 5.
The antibacterial activity of Na-DPDTC and their metal complexes were investigated against four bacterial strains i.e. two Gram-negative bacterial strains as E. coli and P. aeruginosa and two Gram-positive bacterial strains as B. subtilis and Rhodococcus sp. All of the tested compounds showed the good biological activity against diverse bacteria. Results were demonstrated that Zn(II) complex exhibited the highest zones of inhibition i.e. 29±0.4, 26±1.2, 29±0.9, and 28±0.7 mm against Rhodococcus, E. coli, B. subtilis and P. aeruginosa, respectively as compared to ligand, standard drug and other complexes. It was observed from the results that least antibacterial activity was exhibited by the nickel complex of DPDTC with zones of inhibition 23±0.9, 23±1.6, 21±1.2 and 14±1.4 mm against Rhodococcus, E. coli, B. subtilis and P. aeruginosa, respectively in contrast to zinc and copper complexes [50]. On the other hand, these nickel complexes of DPDTC showed better inhibitory effect on all tested bacterial strains in contrast to ligand as well as the standard drug as shown in Table 1. While Cu(II) complex was found to be more active than the ligand against Rhodococcus and E. coli with zones of inhibition 20±1.6 and 26±0.7 mm, respectively [51,52], it also exhibited significant and better antibacterial activity against all tested bacterial strains when compared to the standard drug ampicillin. All results of antibacterial testing revealed that synthesized metal complexes (Zn, Cu and Ni) exhibited greater zones of inhibition against all tested bacterial pathogens when to compared to that produced by the standard drug. It was also manifested from the results of antibacterial activity that complexation of ligand with Zn, Cu and Ni could also be responsible for the enhanced antibacterial activity as the results in Table 1 has shown. Many literature reports also revealed the metal complexation of liganddependent antibacterial effect and the findings of the current investigation were in agreement with the reported results [53,54]. The order of antibacterial activity of the compounds was Zn(II)>Cu(II)>Ni(II)>ligand>ampicillin as shown in Table 1.
| Compounds | Inhibition zone of diameter (mm) | |||
|---|---|---|---|---|
| B. subtilus | P. aeruginosa | E. coli | Rhodococcus | |
| Ligand | 21±0.6 | 19±0.4 | 16±0.9 | 14±0.5 |
| Zn(II) | 29 ±0.9 | 28±0.7 | 26±1.2 | 29±0.4 |
| Cu(II) | 23±1.5 | 19±0.8 | 26±0.7 | 20±1.6 |
| Ni(II) | 21±1.2 | 14±1.4 | 23±1.6 | 23±0.9 |
| *Ampicillin | 11± 0.4 | 10±0.6 | 11±0.3 | 12±0.4 |
*Standard drug; antibacterial species. Zone of Inhibition (mm) include the agar wall diffusion assay hole diameter (4 mm), which carried 100 µg/ml from ligand and their metal complexes solution. The diameter of inhibition zones are means triplicate±SD and p<0.05 when compared with negative control i.e. blank/solvent (p<0.05 is taken as significant)
Table 1: Zones of inhibitions of ligand and synthesized metal complexes against different bacterial strains
Antifungal activity was tested against A. niger, A. flavus, C. albican and Acetomyceta. It was observed that highest antifungal activity was exhibited by the synthesized Cu(II) complex of DPDTC while lowest antifungal activity was observed with the ligand as shown in Table 2. The synthesized Cu(II) complexes of DPDTC produced larger zone of inhibition of 27±0.8, 21±0.2, 26±0.3 and 29±0.5 mm against A. niger, A. flavus, C. albican and Acetomyceta, respectively in comparison to ligand, fluconazole and other metal complexes (Zn and Ni) tested. The Zn(II) complexes of DPDTC exhibited higher antifungal activity compared to the ligand, the standard drug and Ni complex. It was also observed from the results that among all synthesized metal complexes least inhibitory effect on the growth of A. niger (19±1.5 mm), A. flavus (18±0.3 mm), C. albicans (21±0.3 mm) and Acetomyceta (23± 1.3 mm) was produced by the Ni complex of DPDTC, however this inhibitory effect was still greater than that of the ligand and the standard drug as shown in Table 2. The results of antifungal activity of Zn, Cu, Ni complexes of the ligand indicated that the enhancement in antifungal activity is due to complexation with heavy metals [52]. Results also demonstrated that the synthesized metal complexes showed highest antifungal activity against pathogenic fungal strains due also to the presence of DTC group in the complexes [53]. Results of the current study appear to be in agreement with those reported previously [55,56]. Many literature reports also revealed the metal complexation of ligand and DTC group-dependent antifungal effect [57]. The order of antifungal activity of the compounds was Cu(II)>Zn(II)>Ni(II)>ligand>fluconazole. The effect of inhibition of the ligand and synthesized complexes were represented in Table 2.
| Compounds | Inhibition zone of diameter (mm) | |||
|---|---|---|---|---|
| A. niger | A. flavus | C. albicans | Acetomyceta | |
| Ligand | 17± 0.4 | 16±0.7 | 16±0.7 | 18±0.8 |
| Zn(II) | 24±0.9 | 19±1.1 | 28±0.4 | 22±0.6 |
| Cu(II) | 27±0.8 | 21±0.2 | 26±0.3 | 29±0.5 |
| Ni(II) | 19±1.5 | 18±0.3 | 21±0.3 | 23±1.3 |
| *Fluconazole | 12±0.6 | 15±0.2 | 13±0.1 | 13±0.3 |
*Standard drug; antifungal species. Zone of Inhibition (mm) include the agar wall diffusion assay hole diameter (4 mm), which carried 100 µg/ml from ligand and their metal complexes solution. The diameter of inhibition zones are means triplicate±SD and p<0.05 when compared with negative control i.e. blank/solvent (p<0.05 is taken as significant)
Table 2: Zones of inhibitions of ligand and synthesized metal complexes against different fungal strains
Zn(II), Cu(II) and Ni(II) complexes of DPDTC have been synthesized by the diphenylamine and CS2 in the presence of NaOH and characterized by spectroscopic analysis. The characterization techniques confirmed that DPDTC ligands were bidentate coordination to the metal atom via sulphur atom of all the synthesized complexes. Conductance measurements indicated the complexes were of ionic nature. The IC50 of Zn(II) was lower 31.45± 0.31 μM it also showed highest antioxidant activity than ligand and other complexes as well as reference standard (BHT). The order of DPPH scavenging activity of these compounds was Zn(II)>Ni(II)>Cu(II)>BHT>ligand. These metal complexes exhibited antibacterial and antifungal activity against the tested microbial species. The antibacterial activity of the DPDTC ligand and Zn(II) complex indicated the highest antibacterial activity than the other complexes against the tested species in the order of Zn(II)>Cu(II)>DPDTC>Ni(II)>ampicillin. The antifungal activity of Cu(II) complex of DPDTC was found to be the highest against fungal strain as compared to Zn(II) and Ni(II) complexes of DPDTC and the rank order activity was, Cu(II)>Zn(II)>Ni(II)>Na- DPDTC>fluconazole. The antimicrobial activity might be explained by the interaction of the Cu, Ni, Zn-based complexes with the cytoplasmatic membrane of the fungi as well as bacteria. Such interactions with other rich electronic donor centres, amino acids, proteins, nucleosides, carbohydrates and steroids can increase lipid-solubility assisting the complexes to cross the cell membrane. The use of metal-based compounds might represent an alternative therapeutic route to overcome resistance to most used drugs such as fluconazole, amphotericin, among others. In any case, further research is necessary to fully understand and exploit the potential beneficial effects of these complexes.
Acknowledgements
This work was supported by the Department of Chemistry, School of Science, University of Management and Technology, Lahore, Pakistan.
Financial support and sponsorship
Nil.
Conflict of interest
The authors declare that this paper content has no conflict of interests.
References
- Siqqidi KS, Nami SAA, Chebude L, Chebudi Y. Template synthesis of symmetrical transition metal dithiocarbamates. J Braz Chem Soc 2006;17:107-12.
- Shahvelayati AS, Yavari I, Adhami F, Shoar ST. An efficient synthesis of dithiocarbamates from primary amines, CS2 and maleic anhydride. Iran J Org Chem 2009;4:244-7.
- Geetha N, Thirumaran S. Characterization studies and cyclic voltammetry on nickel(II) amino acid dithiocarbamates with triphenylphosphine in the coordination sphere. J Serb Chem Soc 2008;73:169-77.
- Damian C, Onwudiwe, Peter AA. Synthesis, characterization and thermal studies of Zn(II), Cd(II) and Hg(II) complexes of N-methyl-N-phenyldithiocarbamate: The single crystal structure of [(C6H5)(CH3)NCS2]4Hg2. Int J Mol Sci 2011;12:1964-78.
- Prakasam BA, Ramalingam K, Baskaran R, Bocelli G, Cantoni A. Synthesis, NMR spectral and single crystal X-ray structural studies on Ni(II) dithiocarbamates with NiS2PN, NiS2PC, NiS2P2 chromophores: Crystal structures of (4-methylpiperazinecarbodithioato)(thiocyanato-N) (triphenylphosphine)nickel(II) and bis(triphenylphosphine)(4-methylpiperazinecarbodithioato)nickel(II)perchlorate monohydrate. Polyhedron 2007; 26:1133-38.
- Malathy D, Vijayanthimala R. Synthesis, characterization, antimicrobial and cytotoxic studies on transition metal complexes of pentamethylene dithiocarbamate and diamines.IOSR-JAC 2015;8:52-9.
- Hassan FA. Synthesis, characterization and biological studies of some new mixed nickel(II) complexes containing dithiocarbamate and 1,10-phenanthroline ligands. Chem Mater Res 2015;7:9-15.
- Bond AM, Wallace GG. Preparation of metal dithiocarbamate complexes for chromatographic separation and multi-element determinations. Anal Chim Acta 1984;164:223-32.
- Scharfe RR, Sastri VS, Chakrabarti CL. Stability of metal dithiocarbamate complexes. Anal Chem1973;45:413-15.
- Jamaluddin NA, Baba I, Ibrahim N. Synthesis, structural and antibacterial studies of new dithiocarbamate complexes of Sb(III) and Bi(III). MJAS 2014;18:251-9.
- Jayaraju A, Ahamad MM, Rao RM, Sreeramulu J. Synthesis, characterization and biological evaluation of novel dithiocarbamate metal complexes. Der Pharma Chemica 2012;4:1191-94.
- Nabipour H. Synthesis of a new dithiocarbamate cobalt complex and its nanoparticles with the study of their biological properties. Int J Nano Dimens 2011;1:225-32.
- Mohamed GG, Ibrahim NA, Attia HAE. Synthesis and anti-fungicidal activity of some transition metal complexes with benzimidazole dithiocarbamate ligand. Spectrochim Acta A 2009;72:610-15.
- Nath J, Jamir L, Patel KB. Improved procedure for the preparation of isothiocyanates via iodine-mediated desulfurization of dithiocarbamic acid salts. Green Chem Lett Rev 2011;4:1-34.
- Venugopal K, Sreeramulu J. Synthesis, spectro chemical behavior of novel heterocyclic dithiocarbamate metal complexes-biological Activity.IOSR-JAC 2014;12:24-31.
- Kalia SB, Kaushal G, Kumar M, Cameotra SS, Sharma A, Verma ML, et al. Antimicrobial and toxicological studies of some metal complexes of 4-methylpiperazine-1-carbodithioate and phenanthroline mixed ligands. Braz J Microbiol 2009;40:916-22.
- Ahmad S, Sarwar M, Ahmad S, Ali S, Khurram. Mixed ligand Copper(II) complexes of Pyrrolidinedithiocarbamate and Diamines. J Chem Soc Pak 2010;32:443-48.
- Rogach AL, Koktysh DS, Harrsion M, Kotov NA. Layer-by-Layer assembled films of HgTe nanocrystals with strong infrared emission. Chem Mater 2000;12:1526-28.
- Nami SAA, Ullah I, Alam M, Lee DU, Sarikavakli N. Synthesis, characterization, molecular docking and biological studies of self-assembled transition metal dithiocarbamates of substituted pyrrole-2-carboxaldehyde. J Photochem Photobio B Bio 2016;160:392-99.
- Althahr LJN, AlTayy MAM. Synthesis and characterization of some metal(II) complexes of dithiocarbamate. Tikrit J Pure Sci 2013;18:115-21.
- Koivukorpi J, Kolehmainen E. Synthesis of both ionic species of ammonium dithiocarbamate derived cholic acid moieties. Molecules 2011;16:6306-12.
- Khan SA, Shahid S, Jabin S, Zaman S, Sarwar MN. Synthesis and characterization of un-doped and copper-doped zinc oxide nanoparticles for their optical and antibacterial studies. Dig J Nanomater Biostruct 2018;13(1):285-97.
- Shahid S, Khan SA, Ahmad W, Fatima U, Knawal S. Size-dependent Bacterial Growth Inhibition and Antibacterial Activity of Ag-doped ZnO Nanoparticles under Different Atmospheric Conditions. Indian J Pharm Sci 2018;80(1):173-80.
- Nami SA, Ullah I, Alam M, Lee DU, Sarikavakli N. Synthesis, characterization, molecular docking and biological studies of self-assembled transition metal dithiocarbamates of substituted pyrrole-2-carboxaldehyde. J Photochem Photobiol B 2016;160:392-99.
- Ghosh D, Sen K, Das AK. Structure and coordination in mono and dinuclear Zn(II)-pyrrolidine dithiocarbamate complexes. Struct Chem 2012;23:227-35.
- Shaheen F, Zia-ur-Rehman, Ali S, Meetsma A. Structural properties and antibacterial potency of new supramolecular organotin(IV) dithiocarboxylates. Polyhedron 2012;31:697-03.
- Stasko A, Brezova V, Biskupic S, Misik V. The potential pitfalls of using 1,1-diphenyl-2-picrylhydrazyl to characterize antioxidants in mixed water solvents. Free Radic Res 2007;41:379-90.
- Pellerito C, Nagy L, Pellerito L, Szorcsik A. Biological activity studies on organotin(IV)n+ complexes and parent compounds. J Organomet Chem 2006;691:1733-47.
- Dufour P, Lang JM, Giron C, Duclos B, Haehnel P, Jaeck D, et al. Sodium dithiocarb as adjuvant immunotherapy for high risk breast cancer: a randomized study. Biotherapy 1993;6:9-12.
- Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 1992;40:945-48.
- Khan SA, Shahid S, Sajid MR, Noreen F, Kanwal S. Biogenic synthesis of CuO nanoparticles and their biomedical applications: A current review. Int J Adv Res 2017;5(6):925-46.
- Khan SA, Shahid S, Ahmad W, Ullah S. Pharmacological Importance of Clerodendrum Genus: A Current Review. Int J Pharm Sci Res 2017;2(2):22-30.
- Khan SA, Jameel M, Kanwal S, Shahid S. Medicinal Importance of Allium Species: A current review. Int J Pharm Sci Res 2017;2(3):29-39.
- Khan SA, Shahid S, Bashir W, Kanwal S, Iqbal A. Synthesis, characterization and evaluation of biological activities of manganese-doped zinc oxide nanoparticles. Trop J Pharm Res 2017;16 (10):2331-39.
- Khan SA, Shahid S, Kanwal, S, Hussain, G. Synthesis characterization and antibacterial activity of Cr (III), Co (III), Fe (II), Cu (II), Ni (III) complexes of 4-(2-(((2-hydroxy-5-nitrophenyl) diazenyl) (phenyl) methylene) hydrazinyl) benzene sulfonic acid based formazan dyes and their applications on leather. Dyes Pigm 2017;148(C):31-43.
- Khan SA, Noreen F, Kanwal S, Iqbal A, Hussain G. Green synthesis of ZnO and Cu-doped ZnO nanoparticles from leaf extracts of Abutilon indicum, Clerodendrum infortunatum, Clerodendrum inerme and investigation of their biological and photocatalytic activities. Mater Sci Eng C Mater Biol Appl 2017; 82(C):46-59.
- Khan SA, Noreen F, Kanwal S, Hussain G. Comparative synthesis, characterization of Cu-doped ZnO nanoparticles and their antioxidant, antibacterial, antifungal and photocatalytic dye degradation activities. Dig J Nanomater Biostruct 2017;12(3):877-89.
- Qamar MA, Shahid S, Khan SA, Zaman S, Sarwar MN. Synthesis Characterization, Optical and Antibacterial Studies of Co-doped SnO2 Nanoparticles. Dig J Nanomater Biostruct 2017;12(4):1127-35.
- Rek JH, Pastorek R, Malo M, Sindalar Z, Pavlicek M. Nickel(II) cyclohexylethyldithiocarbamate complexes with monodentate P-donor ligands in the coordination sphere. J Serb Chem Soc 2004;69:1053-61.
- Shah FU, Glavatskih S, Antzutkin ON. Novel alkylborate-dithicarbamate lubricant additive: synthesis and tribophysical characterization. Tribol Lett 2012;45:67-78.
- Granifo J, Ferraudi G, Rillema DP. Photochemistry of copper(II) macrocyclic complexes: the charge transfer photochemistry of 2,12-dimethyl-3,7,11,17-tetraazabicyclo [11,3,1]heptadeca-1(17),2,11,13,15-pentaenecopper(II). J Photochem 1983;23:51-60.
- Jeliazova BG, Doicheva MA. Charge-transfer photochemistry of copper(II) dithiocarbamate mixed-ligand complexes. Polyhedron 1996;15:1277-82.
- Thanaa J, Al-Hasani, Mustafa K, Al-Taie. Synthesis, structural and antibacterial study of some metal ion dithiocarbamate-azo complexes. J Al-Nahrain Univ 2015;18:1-12.
- Gopal KV, Jyothi PS, Raju PAG, Rameshbabu K, Sreeramulu J. Synthesis and characterization of 2-amino pyridine dithiocarbamate ligand and its Cu(II), Co(II) metal complexes. J Chem Pharma Res 2013;5:50-9.
- Shaikh AR,Giridhar R, Megraud F, Yadav MR. Metalloantibiotics: synthesis, characterization and antimicrobial evaluation of bismuth-fluoroquinolone complexes against Helicobacter pylori. Acta Pharm 2009;59:259-71.
- Venugopal K, Rameshbabu K, Sreeramulu J. Synthesis and characterization of (novel heterocyclic) 3-amino-9-ethyl carbazole dithiocarbamate [AECZDTC] ligand and its metal complexes. Der Pharma Chemica 2015;7:252-60.
- Khan SA, Rasool N, Riaz M, Nadeem R, Rashid U, Rizwan K, et al. Evaluation of antioxidant and cytotoxicity studies of Clerodendrum inerme. Asian J Chem 2013;25(13):7457-62.
- Khan SA, Shahid S, Khan ZA, Iqbal A. Assessment of Stabilization of Canola Oil, Free Radical Scavenging and Cytotoxic Potential of Peucedanum graveolens (roots). Int J Sci Res 2016;6(3):529-35.
- Sravani T, Paarakh PM. Antioxident activity of Hedychium spicatum Buch-Ham. Rhizomes. IJNPR 2012;3:354-58.
- Ikotun AA, Ojo Y, Obafemi CA, Egharevba GO. Synthesis and antibacterial activity of metal complexes of barbituric acid. Afr J Pure Appl Chem 2011;5:97-03.
- Ijaz F, Sammia S, Khan SA, Ahmad W, Zaman S. Green synthesis of copper oxide nanoparticles using Abutilon indicum Leaf extract and its antimicrobial, antioxidant, photocatalytic dye degradation activities. Trop J Pharm Res 2017;16(4):743-53.
- Kalia SB, Kaushal G, Kumar M, Cameotra SS, Sharma A, Verma ML, et al. Antimicrobial and toxicological studies of some metal complexes of 4-methylpiperazine-1-carbodithioate and phenanthroline mixed ligands. Braz J Microbiol 2009;40:916-22.
- Khan SA, Kanwal S, Iqbal A, Ahmad W. Cu and Mn complexes of nicotinic acid and imidazole: a current review. Int J Adv Res 2017;5(4):1350-68.
- Ahmad W, Khan, SA, Munawar KS, Khalid A, Kawanl S. Synthesis, characterization and pharmacological evaluation of mixed ligand-metal complexes containing omeprazole and 8-hydroxyquinoline. Trop J Pharm Res 2017;16(5):1137-46.
- Rehman W, Hassan Z, Rashid U, Rahim F, Abid OUR, Waseem M. Synthesis, characterization antibacterial and antifungal activity of some transition metal complexes. Med Chem Res 2014;23:2207-11.
- Khan SA, Shahid S, Jameel M, Ahmad A. In vitro Antibacterial, Antifungal and GC-MS Analysis of seeds of Mustard Brown. Int J Pharm Chem 2016;6(4):107-15.
- Hussain G, Khan SA, Ahmad W, Athar M, Saleem R. A Kinetic Study Of Rubazoic Acid Formation Derived From 4-Amino-1(4-Sulphophenyl) 3-Methyl-2-Pyrazolin-5-One. Int J Adv Res 2017;5(4):234-41.