- Corresponding Author:
- N. Sati Department of Pharmaceutical Sciences, H. N. B. Garhwal University, Srinagar Garhwal-246 174, India. E-mail: satinitin@yahoo.co.in
| Date of Submission | 2 May 2008 |
| Date of Revision | 28 July 2009 |
| Date of Acceptance | 24 September 2009 |
| Indian J Pharm Sci, 2009, 71 (5): 572-575 |
Abstract
Benzoyl chloride was added to the solution of anthranilic acid in pyridine to afford crude 2-phenyl-benzo[d][1, 3] oxazin-4-one (1). To the solution of compound 1 in dry toluene, various substituted anilines were added and the mixture refluxed for 8 h to afford 2-phenyl-3-(substituted phenyl)-3H-quinazolin-4-ones (2a-2f). All the compounds were obtained in solid state in yields varying between 40 to 70%. Spectral characterization included FTIR, 1 H NMR and Electrospray MS. The synthesized compounds were screened for 5-HT 2 antagonist activity. Some of the title compounds have been found to show significant 5-HT 2 antagonist activity. The compound 2b, 3-(2-chlorophenyl)-2-phenyl-3H-quinazolin-4-one was the most potent derivative in the series of compound synthesized.
Keywords
5-HT2 antagonists, aryl quinazolines, quinazolin-4-ones, serotonin antagonists
Serotonin receptors (5-HT2) receptors are of significant clinical interest because of their involvement in various disorders [1,2]. Centrally acting 5-HT2 antagonists have shown promising effects in animal models for anxiety [3], depression [4] and in certain drug abuse models [5-7]. In schizophrenic patients, improvement of negative symptoms [8] and extra pyramidal symptoms [9,10] has been demonstrated by 5-HT2 antagonists.
Quinazolines linked to aryl moiety are reported as anticonvulsant, antidepressant, tranquilizer and antagonist of 5-HT2 receptors [11-15]. In view of these observations, we herein report the synthesis of 2-phenyl-3-(substituted phenyl)-3H-quinazolin- 4-ones and evaluate them for 5-HT2 antagonist activity. Our synthetic efforts were directed to Þ nd the effect of substitution in the phenyl ring on the antagonistic activity at 5-HT2 receptor.
Melting points of the synthesized compounds were determined by open capillary method and are uncorrected. Thin layer chromatography of synthesized compounds was performed on precoated silica gel G254 plates and visualized in iodine or UV. The IR spectra of synthesized compounds were recorded on Perkin-Elmer FTIR in potassium bromide discs. The proton nuclear magnetic resonance (1H NMR) was recorded in NMR Varian Mercury 300 MHz. The solvents used were DMSO-d6 and acetone. Chemical shifts are reported in δ ppm, downÞ eld from tetramethylsilane (δ 0.00). Splitting patterns are designated as singlet (s), doublet (d) and multiplet (m). The electrospray mass spectra (ESMS) were recorded on a Micromass Quattro II triple quadrupole mass spectrometer. Elemental analysis was performed (C, H and N). All the target compounds and the reference olanzapine were orally administered (5-HTP was intraperitoneally administered). All protocols of animal experiments have been approved by Institutional Animal Ethics Committee. Antagonism of serotonin (5-HT2) receptor was studied by inhibition of L-5-hydroxytryptophan induced head twitches behaviour [16].
Group of six male Wistar rats (180-280 g) was used in this test procedure. Sixty minutes prior to scoring head twitch behaviour, L-5-hydroxy tryptophan (5- HTP) was administered intraperitoneally at dosage of 10 mg/kg. Thirty minutes following the injection, test compounds were administered orally. Control group were administered the appropriate vehicle. Sixty minutes post 5-HTP administration; each animal was individually placed in a clear plastic cage and observed for head twitch response [16]. The number of head twitches per animal was recorded over a 10 m interval and the total summed for each group. The percentage change from control for each group was then calculated. ED50 values were calculated by sigmoidal dose-response curve analysis using the program PRISM (Graph pad Software). P-value less than 0.05 (P<0.05) was considered statistically signiÞ cant.
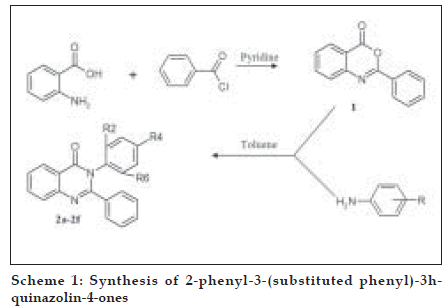
The title compounds were synthesized as per Scheme 1, the procedural details are as follows; Phenyl-benzo[d][1,3]-oxazin-4-one (1) benzoyl chloride (0.21 mol) was added to the solution of anthranilic acid (0.01 mol) in pyridine drop-wise at room temperature with stirring for 30 min followed with the addition of 5% NaHCO3. The separated solid was Þ ltered, washed with NaHCO3 and dried to afford crude 2-phenyl-benzo-[d][1,3]-oxazin-4- one (1), which was recrystallized in ethanol. Yield 63%, mp 120-121û, IR (KBr, cm-1) 3040 (-CH aromatic), 1764 (CO in lactone), 1315 (-CN in tert. amines), 1H NMR (δ ppm, DMSO-d6) 7.2-7.32 (m, 5H, phenyl at C2), 7.8-8.01 (m, 4H, C5, C6, C7 and C8 protons), ESMS: 224 (M+H) +, 146
General method for the preparation of 2-phenyl-3- (substituted phenyl)-3H-quinazolin-4-ones (2a-2f) is as follows. To a solution of compound 1 in dry toluene, substituted anilines were added and the mixture reß uxed for 8 h. The mixture was allowed to cool to room temperature and then poured in ice cold water. The precipitated solid was separated and dried to afford the target compounds 2-phenyl-3- (substituted phenyl)-3H-quinazolin-4-ones (2a-2f). All the compounds were recrystallized in methanol. The characterization and spectral data of the synthesized compounds is given in Tables 1 and 2, respectively.
| Compd | R2 | R4 | R6 | Mol. Formulaea | mpb | Yieldc,d | Rfe |
|---|---|---|---|---|---|---|---|
| 2a | H | H | H | C20H14N2O | 116-118 | 53 | 0.56 |
| 2b | Cl | H | H | C20H13ClN2O | 122-124 | 61 | 0.52 |
| 2c | H | F | H | C20H13FN2O | 135-137 | 43 | 0.53 |
| 2d | CH3 | H | CH3 | C22H18N2O | 159-161 | 51 | 0.63 |
| 2e | CH3 | CH3 | H | C22H18N2O | 145-147 | 66 | 0.57 |
| 2f | OCH3 | H | H | C21H16N2O2 | 142-144 | 69 | 0.60 |
aElemental analyses results were within ± 0.4% of the calculated values, bMelting points are expressed in ûC and are uncorrected, cSolvent for recrystallization is alcohol, dYields are not optimized, eMobile phase is 20 % EtOAc-benzene
Table 1: Characterization data of the title compounds
| Comp | IRa (cm-1) | 1H NMRb (δ ppm) | ES MS (m/z) |
|---|---|---|---|
| 3030 (-CH aromatic), 1665 (CO in δ lactam), 1312 | 6.95-7.05 (m, 5H, phenyl at C3), 7.13-7.18 (m, | ||
| 2a | 5H, phenyl at C2), 7.73-8.01 (m, 4H, C5, C6, C7 | 299, 221 | |
| (-CN in tert. amines) | |||
| and C8 protons) | |||
| 3029 (-CH aromatic), 1680 (CO in δ lactam), 1310 | 6.91-7.02 (m, 4H, phenyl at C3), 7.13-7.18 (m, | 333, 255 | |
| 2b | 5H, phenyl at C2), 7.75-8.02 (m, 4H, C5, C6, C7 | ||
| (-CN in tert. amines) | |||
| and C8 protons) | |||
| 3021 (-CH aromatic), 1675 (CO in δ lactam), 1325 | 6.93-7.05 (m, 4H, phenyl at C3), 7.15-7.19 (m, | ||
| 2c | 5H, phenyl at C2), 7.8-8.04 (m, 4H, C5, C6, C7 and | 317, 239 | |
| (-CN in tert. amines), 1050 (C-F) | |||
| C8 protons) | |||
| 3049 (-CH aromatic), 1668 (CO in δ lactam), 1305 | 2.34 (s, 3H, methyl at phenyl ring), 2.35 (s, 3H, | ||
| 2d | methyl at phenyl ring), 7.04-7.08 (m, 3H, phenyl | 327, 249 | |
| (-CN in tert. amines) | at C3), 7.11-7.15 (m, 5H, phenyl at C2), 7.74-8.1 | ||
| (m, 4H, C5, C6, C7 and C8 protons) | |||
| 3023 (-CH aromatic), 1673 (CO in δ lactam), 1319 | 2.33 (s, 3H, methyl at phenyl ring), 2.35 (s, 3H, | ||
| 2e | methyl at phenyl ring), 7.03-7.07 (m, 3H, phenyl | 327, 249 | |
| (-CN in tert. amines) | at C3), 7.12-7.19 (m, 5H, phenyl at C2), 7.7-8.04 | ||
| (m, 4H, C5, C6, C7 and C8 protons) | |||
| 3033 (-CH aromatic), 1667 (CO in δ lactam), 1310 | 3.83 (s, 3H, -OCH3 protons), 7.05-7.09 (m, 4H, | ||
| 2f | phenyl at C3), 7.10-7.19 (m, 5H, phenyl at C2), | 329, 251 | |
| (-CN in tert. amines) | |||
| 7.73-8.01 (m, 4H, C5, C6, C7 and C8 protons) | |||
aKBr pellet was used for determination, bSolvent used was dueterated acetone
Table 2: Spectral characterization data of the title compounds
Compound 1 showed IR absorption at 1764 cm-1 corresponding to carbonyl group stretching in lactones. 1H NMR spectral studies showed multiplets in the regions 7.2-7.3 δ and 7.8-8.01 δ, assigned respectively to protons of phenyl ring at C2 and the protons at C5, C6, C7 and C8. The ESMS of the compound 1 showed its (M+H) + peak at 224.
The IR spectra of the title compounds 2a-2f showed characteristic absorption peaks in the range 1665- 1680 cm-1, assigned to carbonyl stretching in δ lactams. The 1H NMR spectral studies showed multiplets in the range 6.91-7.09, 7.13-7.19 and 7.73-8.1δ, assigned respectively to phenyl at C3, phenyl at C2 and the protons at C5, C6, C7 and C8. The ES MS of title compounds showed their respective (M+H)+ peaks. All these observations confirmed the structures of the title compounds 2a-2f. Characterization data of the synthesized compounds is given in Table 1 .
The propensity of title compounds to antagonize 5-HTP induced head twitch was evaluated and their respective ED50 values are reported in the Table 3. Our synthetic efforts in the series of compounds synthesized were directed to find the effect of electronic character of the substituent in phenyl ring on the afÞ nity for 5-HT2 receptor. As is evident from Table 3, the efficacy of the compounds to antagonize the head twitch depended greatly on the nature of substituent. Of the synthesized compounds, not all were able to antagonize the head twitches induced by 5-HTP. The presence of electron withdrawing groups (Cl and F) at ortho- and para- positions lead to potent derivatives 2b and 2c. Substitution of the orthohydrogen by electron donating group (-OCH3) decreased the potency; however it was more potent than the unsubstituted compound 2a. Di-substitution with methyl at 2, 4 and 2, 6 positions yielded the least potent members of the series, i.e the compounds 2d and 2e. The most potent compound in the series was 3-(2-chlorophenyl)-2-phenyl-3Hquinazolin- 4-one (compound 2b). Thus, the series synthesized afforded compounds having varying degree of afÞ nity for the 5-HT2 receptor and offers interesting series of molecules to be worked upon for reÞ nement of biological activity.
| Compd. | ED50a | Compd. | ED50a |
|---|---|---|---|
| (mg / kg) | (mg / kg) | ||
| 2a | 28.63 | 2e | > 40 |
| 2b | 15.35 | 2f | 25.13 |
| 2c | 17.19 | Olanzapine | 4.36 |
| 2d | > 40 |
aED50 was calculated by sigmoidal dose response curve analysis using the program PRISM (Graphpad Software) after oral administration of drug. P-value less than 0.05 (P<0.05) was considered statistically signiÞ cant.
Table 3: Inhibition of head twitches after administration of test and reference compound
Acknowledgements
We thank Sophisticated Analytical Instrument Facility, Central Drug Research Institute, Lucknow for spectral analysis.
References
- Jeff LH, Abd I, Stacy PI, Tietler M, Richard AG. Ketanserin analogues: Structure-affinity relationships for 5-HT2 and 5-HTlc serotonin receptor binding. J Med Chem 1992;35:4903-10.
- Giuseppe C, Ferdinando F, Paolo G, Elisa P, Vincenzo S, Stefana A, et al. Synthesis and binding affinities for 5-HT1A, 5-HT2A and 5-HT2C receptors of a series of 1- and 2-(4-Arylpiperazinylalkyl)-4-(Benzoyl)-1,2,3-triazole derivatives. Eur J Med Chem 1999;39: 719-27.
- Colpaert FC, Meert TF, Neimegeers CJ, Janssen PAJ. Behavioural and 5-HT antagonist effect of Ritanserin: A pure and selective antagonist of LSD discrimination in rat. Psychopharmacology 1985;86:45-54.
- Marek GJ, Li AA, Seiden LS. Selective 5-hydroxytryptamine antagonists have antidepressant like effects on differential reinforcement of low rate 72 second schedule. J PharmacolExpTher 1989;250:52-9.
- Meert TF, Janssen PAJ. Ritanserin. A New Therapeutic approach for drug abuse: Part 1: Effect on alcohol. Drug Dev Res 1992;25:235-49.
- Meert TF, Janssen PA. Ritanserin: A new therapeutic approach for drug abuse: Part 2: Effect on Cocaine. Drug Dev Res 1992;25:39-53.
- Meert TF, Janssen PA. Ritanserin: A new therapeutic approach for drug abuse: Part 3: Effect on fentanyl and sucrose. Drug Dev Res 1992;25:55-66.
- Ceuleans DL, Hoppenbrouwers ML, Gelders YG, Reyntjens AJ. The influence of ritanserin, a 5-HT2 receptor antagonist, in anxiety disorders: A double blind placebo controlled study versus lorazepam. Pharmacopsychiatry 1985;18:303-5.
- Bersani G, Pozzi F, Marini S, Grispini A, Pasini A, Valducci M, et al. Neuroleptic induced extrapyramidal side effects: Clinicalperspectives with ritanserin, a new selective 5-HT2 receptor blocking agent. CurrTher Res 1986;40:492-9.
- Paiva T, Arriaga F, Wauquier A, Lara E, Largo R, Leitao JN. Effects of ritanserin on sleep disturbances of dysthmic patients. Psychopharmacology 1988;96:395-9.
- Jim A, Jens P, John A, Jorn BN, Mikael B. Selective, centrally acting serotonin 5-HT2 antagonist:2: Substituted (4-Flurophenyl)-1H-indoles. J Med Chem 1992;35:4823-31.
- Liu D, Wikstrom HV, Dijkstra D, Vries JB, Venhuis BJ. Extremely potent orally active Benzo[g]quinoline analogue of the dopaminergic prodrug: 6-(N,N-Di-n-propyl) amino-3,4,5,6,7,8-hexahydro-2H-naphthalen-1-one. J Med Chem 2006;49;1494-8.
- Hinschberger A, Butt S, Lelong V, Boulouard M, Dumuis A, Dauphin F, et al. New Benzo[h] [1,6]naphthyridine and Azepino[3,2-c]quinoline derivatives as selective antagonists of 5-HT4 receptors: Binding profile and pharmacological characterization. J Med Chem 2003;46:138-47.
- Hiroaki H, Yoshikazu M, Ichiro M, Shunji I, Nobuyuki Y, Akio I, etal. 5-HT3receptor antagonists: 1: New quinoline derivatives. J MedChem 1992;35:4893-902.
- Joseph GC, Richard LH, Mustafa I, Ranbir KB, John PL. Conformationally restricted congeners of dopamine derived from octahydrobenzo[g]quinoline and octahydrobenzo[f]quinoline. J Med Chem 1984;27:190-5.
- Joseph TS, Kenneth JB, Yulin C, Edward JG, Paul GC, Roy C, et al. 3-[[ (Aryloxy)alkyllpiperidinyl]-l,2-Benzisoxazoles as D2/5-HT2 antagonists with potential atypical antipsychotic activity: Antipsychotic profile of iloperidone. J Med Chem 1995;38:1119-31.