- *Corresponding Author:
- Divya Suares
Shobhaben Pratapbhai Patel School of Pharmacy and Technology Management, SVKM’s NMIMS, Vile Parle (W), Mumbai–400 056, India
E-mail: divyasuares@gmail.com
| Date of Submission | 10 March 2014 |
| Date of Revision | 31 January 2015 |
| Date of Acceptance | 13 September 2015 |
| Indian J Pharm Sci 2015;77(5):550-555 |
This is an open access article distributed under the terms of the Creative Commons Attribution‑NonCommercial‑ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The objective of this research work was to mask the intense bitter taste of fexofenadine hydrochloride using weak cation exchange resins and to formulate orodispersible tablet of taste masked drug-resin complex. Five resins indion 204, indion 234, indion 414, kyron T-114 and kyron T-314 were used. Depending on maximum drug loading capacity of resins indion 234 and kyron T-314 were finalized for further study. Drug-resin complex was optimized by considering parameters such as drug to resin ratio, soaking time of resins, stirring time, temperature and pH on maximum drug loading. The drug-resin complex was characterized by Fourier transform infrared spectroscopy. The drug-resin complex was also subjected to various evaluation studies such as taste mask evaluation by panel method, drug content and in vitro drug release at salivary and gastric pH. The orodispersible tablets of taste masked drug-resin complex for indion 234 and kyron T-314 were prepared by direct compression method. Formulated orodispersible tablets were subjected to various evaluation parameters such as diameter and thickness measurement, hardness test, weight variation test, in vitro United States Pharmacopoeia disintegration test, wetting time, test for content uniformity, assay, friability test and in vitro dissolution studies. The results indicate that orodispersible tablets of fexofenadine hydrochloride containing indion 234 and kyron T-314 are palatable and provide quick disintegration and fast drug release without addition of superdisintegrants.
Keywords
Fexofenadine hydrochloride, Indion 204, Indion 234, Indion 414, Kyron T-114, Kyron T-314
Organoleptic characteristics such as taste, appearance and odor are of prime importance while designing pharmaceutical products. There are several formulations that contain an active pharmaceutical ingredient that are bitter in taste or nauseous. This restricts the formulator from developing an oral formulation of bitter tasting actives as they may lead to lack of patient compliance [1].
Masking the bitter taste of actives using ion exchange resins is one among the economical methods reported. While designing tablet dosage forms, the tongue encounters bitter taste when the active ingredient is released in the mouth. Several studies have shown that the complexation of a bitter tasting active with ion exchange resin prevents the release of active from the complex in the saliva. Instead, the decomplexation takes place in the gastric fluid and releases the active ingredient in the stomach. Thus the intrinsic bioavailability of the active is unaffected. Resins are inert and not absorbed by the body. They are high molecular weight water insoluble polymers [2,3].
Fexofenadine hydrochloride is highly unpleasant and has strong bitter taste and after taste. It is prescribed for seasonal urticaria especially for adults and children 2 years or older [4,5]. Pediatrics and geriatrics experience difficulties in swallowing intact tablets, even with liquids. In addition, it is desired that the oral formulations for pediatrics and geriatrics should be pleasant in taste. In order to overcome this problem, formulation of an orodispersible tablet using ion exchange resin seems to be a better option [6].
Fexofenadine is highly active while administered through oral route. Though, there are several pharmaceutical formulations of fexofenadine available in the market, there still arises a need to develop a commercially acceptable cost-effective formulation with good patient compliance, especially for pediatrics and geriatrics [6].
Materials and Methods
Fexofenadine hydrochloride was obtained as a gift sample from Sun Pharmaceutical Industries Ltd., Mumbai. Resins such as indion 204, 234 and 414 and kyron T-114 and T-314 were obtained as gift samples from Ion Exchange India Ltd., Mumbai and Corel Pharma Chem., Baroda, respectively.
Selection of resin
Resins were selected on the basis of nature of the drug and requirement of formulation. Ideally, cation and anion exchange resins are used depending on the acidic and basic nature of the drug. Since fexofenadine hydrochloride contains a tertiary amino group, which is responsible for the bitter taste, weak cation exchange resins such as indion 204, indion 234 [7], indion 414, kyron T-114 and kyron T-314 were selected for the study.
Preparation of drug resin complex (DRC)
For preliminary study, drug:resin was taken in 1:1 ratio. An accurately weighed quantity of resin was taken in a 100 ml beaker containing 25 ml of deionised water. Resin was allowed to swell for 30 min. Appropriate amount of drug (as per 1:1 ratio) was added into the same beaker and pH of solution was recorded. The beaker was placed on a magnetic stirrer for 30 min at 30°. The solution was filtered using whatman filter paper. The filtrate was analyzed using appropriate dilution for determination of unbound drug at 220 nm using UV spectrophotometer. The residue on filter paper was dried in a hot air oven at 40°. Percentage of drug bound to resin was calculated from amount of unbound drug. This procedure was performed for all resins separately.
Optimizing drug loading capacity of resins
The optimization of drug loading capacity of resin was performed by determining the effect of various factors on drug loading. Effect of drug:resin ratio on drug loading was determined. Accurately weighed quantity of fexofenadine hydrochloride was taken and added to the resins (that were selected based on their drug loading capacity) as per drug:resin ratio of 1:1, 1:2 and 1:3. The resins were previously soaked in 25 ml of deionised water for 30 min. The solutions were stirred for 30 min at 30°. The mixtures were filtered and unbound drug in the filtrate was estimated at 220 nm.
Effect of soaking time of resin on drug loading was determined. Selected resins were soaked in 25 ml of deionised water for 10, 30, 40, 60, 90 and 120 min. Accurately weighed quantity of fexofenadine hydrochloride (as per 1:2 ratio) was added to previously soaked resins. The solutions were stirred for 30 min at 30°. The mixtures were filtered and unbound drug in the filtrate was estimated at 220 nm.
Effect of stirring time of resin on drug loading was determined. Selected resins were soaked in 25 ml of deionised water for 30 min. Accurately weighed quantity of fexofenadine hydrochloride (as per 1:2 ratio) was added to previously soaked resins. The solutions were stirred for 5, 10, 30, 60, 90 and 120 min at 30°. The mixtures were filtered and unbound drug in the filtrate was estimated at 220 nm.
Effect of temperature on drug loading was determined. Selected resins were soaked in 25 ml of deionised water for 30 min. Accurately weighed quantity of fexofenadine hydrochloride (as per 1:2 ratio) was added to previously soaked resins. The solutions were stirred for 30 min at 10, 20, 30, 40 and 50°. The mixtures were filtered and unbound drug in the filtrate was estimated at 220 nm.
Effect of pH on drug loading was determined. Accurately weighed quantity of fexofenadine hydrochloride (as per 1:2 ratio) was added to selected resins that were soaked in 25 ml of solution of pH 1.2, 2, 3, 4, 5, 6, and 7 (prepared from standard solutions of hydrochloric acid and sodium hydroxide solutions) for 30 min. The solutions were stirred for 30 min at 30°. The mixtures were filtered and unbound drug in the filtrate was estimated at 220 nm.
Characterization and evaluation of DRC
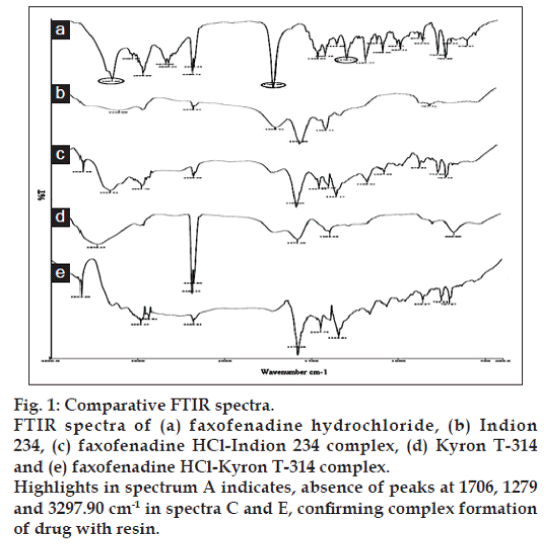
Pure drug, pure resin and DRC were subjected to Fourier transform infrared spectroscopy (FTIR) studies using KBr pellet method. The spectra of DRC were compared with spectra of pure drug (fexofenadine hydrochloride) and pure resin (indion 234 and kyron T-314), to confirm drug resin complex formation. The comparative spectra are depicted in fig. 1.
Volunteers in the age group of 21 to 24 years were selected for the taste evaluation of DRC. The study protocol was explained and a written consent was obtained from the volunteers. DRC equivalent to 30 mg of fexofenadine hydrochloride was held in the mouth for 20 seconds by each volunteer. Bitterness level was recorded against pure drug using a numerical scale given below: 0=tasteless; 1=acceptable bitterness; 2=slight bitterness; 3=moderate bitterness; 4=strong bitterness.
The drug content in the DRC was determined. An accurately weighed quantity of DRC of fexofenadine hydrochloride was transferred to a 50 ml volumetric flask containing 1N hydrochloric acid solution to break the complex. The volumetric flask was sonicated for 30 min. The solution was filtered through whatman filter paper and suitably diluted. The absorbance was measured at 220 nm. The drug content in DRC was calculated from calibration curve of fexofenadine hydrochloride in 1N hydrochloric acid solution.
The release of the drug from DRC was studied at salivary pH (pH 6.8 phosphate buffer) to determine the amount of drug that would be released in the mouth during the administration of formulation. This test was performed, as the bitterness of drug is directly related to the amount of drug released at salivary pH (i.e. in the mouth).
An accurately weighed quantity of DRC equivalent to 30 mg of fexofenadine hydrochloride was transferred to 10 ml of pH 6.8 phosphate buffer solution placed in a test tube. The mixture was filtered after shaking for 60 seconds. The filtrate was assayed for drug content at 220 nm. The drug content was calculated from the calibration curve of fexofenadine hydrochloride in pH 6.8 phosphate buffer.
The release of the drug from DRC was studied at gastric pH (0.001N HCl). The parameters for conducting drug release studies for fexofenadine hydrochloride mentioned in the dissolution guideline by CDER are as follows: Volume: 500 ml, Speed: 50 rpm, Apparatus: USP type II (Paddle type), Time points: 5, 10, 15, 30 and 45 min, Temperature: 37±0.5°.
An accurately weighed quantity of DRC equivalent to 30 mg of fexofenadine hydrochloride was subjected to release rate study using USP dissolution apparatus type II. Aliquots of 5 ml were withdrawn at time intervals of 5, 10, 15, 30 and 45 min and replaced with 5 ml of fresh dissolution medium maintained under the same conditions. Withdrawn samples were filtered through whatman filter paper and diluted suitably using dissolution medium. The amount of the drug released at each time interval was determined spectrophotometrically at 220 nm from the calibration curve of fexofenadine hydrochloride in 0.001N HCl.
Formulation and evaluation of orodispersible tablet of taste masked DRC
The powder blend for preparation of orodispersible tablets (ODT) of taste masked DRC containing indion 234 and kyron T-314 separately, included the following excipients: Mannitol as diluent, aspartame as artificial sweetener, aerosil 200 as permeabilizing agent, microcrystalline cellulose (PH 102) as diluent and swelling agent, purified talc as glidant and magnesium stearate as lubricant. The composition of orodispersible tablet is shown in Table 1.
| Ingredients | Weight (in %) |
|---|---|
| Drug‑resin complex | Equivalent to 30 mg of fexofenadine HCl |
| Mannitol | 1 |
| Aspartame | 1 |
| Aerosil 200 | 0.5 |
| Microcrystalline cellulose PH 102 | 30 |
| Purified talc | 1 |
| Magnesium stearate | 1 |
ODT: Orodispersible tablets, HCl: hydrochloride
Table 1: Composition of odt
The specified amount of DRC, mannitol, aspartame, aerosil 200 and microcrystalline cellulose were accurately weighed and sifted through sieve no. 44 and mixed thoroughly in a polybag using tumbling method. The blend was evaluated for its flow properties.
After determination of flow properties, purified talc and magnesium stearate were accurately weighed and sifted through sieve no. 44 and mixed thoroughly with the powder blend for 2 min. The final blend prepared was also subjected for evaluation of flow properties such as compressibility index, Hausner’s ratio and angle of repose. Flow characteristics were determined in order to decide the manufacturing method to be used to prepare the orodispersible tablets.
Tablets were prepared by direct compression method, at a fixed compression force using Karnavati rotary compression machine equipped with a 7 mm flat faced punch and die set. The tablets were evaluated for diameter and thickness measurement, hardness test, weight variation test, in vitro USP disintegration test, wetting time, test for content uniformity, assay, friability test and in vitro drug release studies.
Results and Discussion
While studying the drug loading capacity of resins with indion 204, 234, 414 and Kyron T-114, T-314, it was found that percentage of drug bound to indion 234 and kyron T-314 was more when compared to the percentage of drug bound to indion 204, indion 414 and kyron T-114, as shown in Table 2.
| Resin | Percentage of drug bound to resin |
|---|---|
| Indion 204 | 25.13 |
| Indion 234 | 65.87 |
| Indion 414 | 57.53 |
| Kyron T‑114 | 30.79 |
| Kyron T‑314 | 69.89 |
Table 2: Drug loading capacity of resins
For optimizing drug loading capacity of resin, drug: resin were taken in the ratios of 1:1, 1:2 and 1:3. It was found that, the drug loading efficiency of resin increases as the resin concentration increases, as shown in Table 3. Percentage of drug bound to resin was found to be more when drug:resin was taken in the ratio of 1:3. Since a marginal increase in percentage of drug bound to resin was observed from 1:2 to 1:3 ratio; the 1:2 ratio was selected for further study.
| Resin | Drug:resin | Percentage of drug bound to resin |
|---|---|---|
| Indion 234 | 1:1 | 65.87 |
| 1:2 | 87.72 | |
| 1:3 | 89.5 | |
| Kyron T‑314 | 1:1 | 69.89 |
| 1:2 | 89.25 | |
| 1:3 | 90.18 |
Table 3: Effect of drug:resin ratio on drug loading
Effect of soaking time of resin on drug loading showed that, the percentage of drug bound to resin was found to increase as the soaking time for resin increased, as shown in Table 4. A marginal increase in percentage of drug bound to resin was observed from 30 to 120 min. Hence, soaking time of 30 min was selected for further study.
| Drug:resin | Soaking time (min) | Percentage of drug bound to resin | |
|---|---|---|---|
| Indion 234 | Kyron T‑314 | ||
| 1:2 | 10 | 78.16 | 81 |
| 30 | 87.12 | 89.18 | |
| 40 | 87.2 | 89.32 | |
| 60 | 88.56 | 90.27 | |
| 90 | 88.88 | 90.96 | |
Table 4: Effect of soaking time on drug loading
Effect of stirring time on drug loading showed that, the percentage of drug bound to resin was found to increase as the stirring time increased, as shown in Table 5. A marginal increase in percentage of drug bound to resin was observed from 30 to 120 min. Hence, stirring time of 30 min was selected for further study. Effect of temperature on drug loading was not very significant, as shown in Table 6. Hence, the operational temperature was selected for further study. Effect of pH on drug loading showed that, the percentage of drug bound to resin decreased as the pH decreased, as shown in Table 7. Maximum loading was obtained between pH 4–5.
| Drug: resin | Soaking time (min) | Stirring time (min) | Percentage of drug bound to resin | |
|---|---|---|---|---|
| Indion 234 | Kyron T‑314 | |||
| 1:2 | 30 | 5 | 78.12 | 80.63 |
| 10 | 82 | 83.43 | ||
| 30 | 88.34 | 90.87 | ||
| 60 | 89.11 | 91 | ||
| 90 | 91.52 | 94.04 | ||
Table 5: Effect of stirring time on drug loading
| Drug: resin | Soaking time(min) | Stirring time(min) | Temperature(°) | Percentage of drug bound to resin | |
|---|---|---|---|---|---|
| Indion 234 | Kyron T‑314 | ||||
| 1:2 | 30 | 30 | 10 | 91.12 | 94.06 |
| 20 | 91.44 | 94.19 | |||
| 30 | 91.39 | 94.12 | |||
| 40 | 91.63 | 94.25 | |||
| 50 | 91.27 | 94.65 | |||
Table 6: Effect of temperature on drug loading
| Drug: resin | Soakingmtime(min) | Stirringmtime(min) | Temperature (°) | pH | Percentage of drug bound to resin | |
|---|---|---|---|---|---|---|
| Indion 234 | Kyron T‑314 | |||||
| 1:2 | 30 | 30 | 30 | 1.2 | 71.06 | 73.14 |
| 2 | 73.99 | 77.57 | ||||
| 3 | 81.44 | 86.89 | ||||
| 4 | 91.13 | 94.46 | ||||
| 5 | 91.65 | 94.21 | ||||
| 6 | 89.02 | 93.5 | ||||
| 7 | 88.78 | 93.05 | ||||
Table 7: Effect of ph on drug loading
The comparative FTIR spectra of fexofenadine hydrochloride, indion 234, kyron T-314, DRC of indion 234 and kyron T-314 are shown in fig. 1. FTIR spectra of fexofenadine hydrochloride shows major peaks at 1706 cm-1 representing –C–O stretching, a peak at 1279 cm-1 representing C–N stretching of tertiary amine and a peak at 3297.90 cm-1 representing –OH stretching. The absence of peaks at 1706 cm-1 and 1279 cm-1 in DRC confirms the complex formation of drug with resin. The peak at 3297.90 cm-1 in DRC corresponding to –OH stretching is also absent, which signifies that during DRC formation there is an interaction of the amino group of drug with the carboxylic group of the resin.
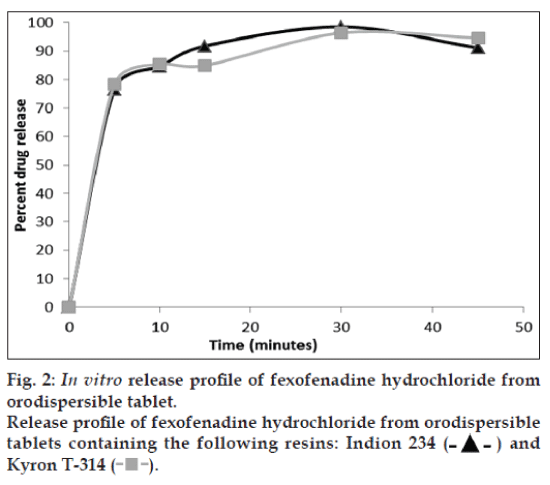
The taste of DRC containing Indion 234 (F1) and Kyron T-314 (F2) was acceptable bitterness and slight bitterness respectively, when compared with pure drug, as shown in Table 8. The amount of drug released at salivary pH was found to be very less. Thus, the amount of drug released from the DRC at salivary pH indicates its insufficiency in imparting bitter taste in the mouth during administration. The drug release from DRC at gastric pH was found to be more than 80% after 10 min. Thus, the amount of drug released from DRC at gastric pH indicates decomplexation of DRC in presence of gastric fluids and availability of drug for absorption. The release profile of drug from DRC is shown in fig. 2.
| Volunteer | Pure drug | DRC F1 (10 s) | DRC F1 (20 s) | DRC F2 (10 s) | DRC F2 (20 s) |
|---|---|---|---|---|---|
| 1 | 4 | 1 | 1 | 1 | 2 |
| 2 | 4 | 1 | 1 | 2 | 2 |
| 3 | 4 | 1 | 1 | 1 | 2 |
Table 8: Results of taste evaluation
The results obtained from compressibility index, Hausner’s ratio and angle of repose are shown in Table 9. The results show that the powder blend has excellent flow properties. It also indicates that the direct compression method can be used for the preparation of orodispersible tablets. The results of various evaluation parameters conducted for orodispersible tablets are shown in Table 10.
| Physical properties | For Indion 234 | For Kyron T‑314 |
|---|---|---|
| Bulk density (g/cm3) | 0.4735 | 0.3928 |
| Tapped density (g/cm3) | 0.5282 | 0.423 |
| Compressibility index (%) | 10.35 | 7.13 |
| Hausner’s ratio | 1.11 | 1.07 |
| Angle of repose (°) | 19.31 | 15.5 |
Table 9: Results of evaluation of blend
| Physical properties | For Indion 234 | For Kyron T‑314 |
|---|---|---|
| Diameter (mm) | 7.02 | 7.02 |
| Thickness (mm) | 3.22 | 3.18 |
| Hardness (kg/cm2) | 3 | 3 |
| Weight variation | 150±6.5 | 150±6.5 |
| In vitro disintegration time (s) | 19±2 | 15±1 |
| Wetting time (s) | 38 | 42 |
| Content uniformity (%) | 98.12 | 99.25 |
| Assay (%) | 99.36 | 101.09 |
ODT: Orodispersible tablets
Table 10: Results of evaluation of odt
Effective taste masking of fexofenadine hydrochloride was achieved with selected ion exchange resin (i.e. indion 234 and kyron T-314). The resins used have an added advantage of fast-disintegrating property and direct compressible quality. Hence it can be concluded that, preparation of orodispersible tablets of taste masked fexofenadine hydrochloride using direct compression technique, without the addition of superdisintegrants is a successful cost-effective method. They would be effective in providing fast onset of action without the need of water for swallowing.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Borodkin S. Ion exchange resins and sustained release. In: Swarbrick J, Boylan JC, editors. Encyclopedia of Pharmaceutical Technology, Vol. 8. New York: Marcel Dekker Inc.; 1993, p. 203-16
- Devi V, Krishna M. Ion-exchange resinates as controlled drug delivery system. In: Jain NK, editor. Advances in Controlled and Novel Drug Delivery. 1st ed. New Delhi: CBS Publishers and Distributors; 2001. p. 290-306.
- Faham A, Marechal D, Chenevier P. Orodispersible Tablets Containing Fexofenadine, US Patent 6723348 B2; 2004.
- Redkar MR, Gore SP, Devarajan PV. D-Zolv taste masked mouth dissolving tablets. Indian J Pharm Sci 2002;64:291-2.
- Nanda A, Kandarpu R, Garg S. An update on taste masking technologies for oral pharmaceuticals. Indian J Pharm Sci 2002;64:10-7.
- Moffat A, David M, Widdop B. Clarke’s Analysis of Drug and Poisons. 3rd ed. USA: Pharmaceutical Press; 2000. p. 1033-4.
- Pandya SJ, Pasha TY, Bhandari A, Patel JK, Trivedi N, Trivedi U. Design and optimization of taste masked fexofenadine hydrochloride resinate by ion exchange resin. Int J Drug Formul Res 2011;2:134-47