- Corresponding Author:
- Sushama Talegaonkar
Department of Pharmaceutics, Faculty of Pharmacy, Jamia Hamdard, New-Delhi-110062, India.
E-mail: stalegaonkar@hotmail.com
| Date of Submission | 1 April 2004 |
| Date of Revision | 12 December 2005 |
| Date of Acceptance | 17 February 2006 |
| Indian J Pharm Sci, 2006, 68 (2): 141-153 |
Abstract
There has been keen interest in the development of a novel drug delivery system. Novel drug delivery system aims to deliver the drug at a rate directed by the needs of the body during the period of treatment, and channel the active entity to the site of action. At present, no available drug delivery system behaves ideally achieving all the lofty goals, but sincere attempts have been made to achieve them through novel approaches in drug delivery. A number of novel drug delivery systems have emerged encompassing various routes of administration, to achieve controlled and targeted drug delivery. Encapsulation of the drug in vesicular structures is one such system, which can be predicted to prolong the existence of the drug in systemic circulation, and reduce the toxicity, if selective uptake can be achieved. Consequently a number of vesicular drug delivery systems such as liposomes, niosomes, transfersomes, and pharmacosomes were developed. Advances have since been made in the area of vesicular drug delivery, leading to the development of systems that allow drug targeting, and the sustained or controlled release of conventional medicines. The focus of this review is to bring out the application, advantages, and drawbacks of vesicular systems.
The quest never ends. From the very beginning of the human race; the quest is going on for newer and better alternatives, and in case of drugs it will continue; continue till we find a drug with maximum efficacy and no side effects. Many drugs, particularly chemotherapeutic agents, have narrow therapeutic window, and their clinical use is limited and compromised by dose limiting toxic effect. Thus, the therapeutic effectiveness of the existing drugs is improved by formulating them in an advantageous way.
In the past few decades, considerable attention has been focused on the development of new drug delivery system (NDDS). The NDDS should ideally fulfill two prerequisites. Firstly, it should deliver the drug at a rate directed by the needs of the body, over the period of treatment. Secondly, it should channel the active entity to the site of action. Conventional dosage forms including prolonged release dosage forms, are unable to meet none of these. At present, no available drug delivery system behaves ideally, but sincere attempts have been made to achieve them through various novel approaches in drug delivery [1].
Approaches are being adapted to achieve this goal, by paying considerable attention either to control the distribution of drug by incorporating it in a carrier system, or by altering the structure of the drug at the molecular level, or to control the input of the drug into the bioenvironment to ensure an appropriate profile of distribution.
Novel drug delivery system aims at providing some control, whether this is of temporal or spatial nature, or both, of drug release in the body. Novel drug delivery attempts to either sustain drug action at a predetermined rate, or by maintaining a relatively constant, effective drug level in the body with concomitant minimization of undesirable side effects. It can also localize drug action by spatial placement of controlled release systems adjacent to, or in the diseased tissue or organ; or target drug action by using carriers or chemical derivatization to deliver drug to particular target cell type.
Different types of pharmaceutical carriers are present. They are – particulate, polymeric, macromolecular, and cellular carrier. Particulate type carrier also known as a colloidal carrier system, includes lipid particles (low and high density lipoprotein-LDL and HDL, respectively), microspheres, nanoparticles, polymeric micelles and vesicular like liposomes, niosomes pharmacosomes, virosomes, etc [2-5]. The vesicular systems are highly ordered assemblies of one or several concentric lipid bilayers formed, when certain amphiphilic building blocks are confronted with water. Vesicles can be formed from a diverse range of amphiphilic building blocks. The terms such as synthetic bilayers allude to the non-biological origin of such vesiculogenes. Biologic origin of these vesicles was first reported in 1965 by Bingham [6], and was given the name Bingham bodies. Much water has flown since then.
In this article, an attempt has been made to touch upon different aspects related to the vesicular system, including method of preparation, stabilization, drawbacks, and applications. Various types of vesicular systems such as liposomes, niosomes, transfersomes, and pharmacosomes, have been discussed in detail, while other emerging systems have been discussed briefly.
Vesicular Systems
In recent years, vesicles have become the vehicle of choice in drug delivery. Lipid vesicles were found to be of value in immunology, membrane biology, diagnostic techniques, and most recently, genetic engineering [7-9]. Vesicles can play a major role in modeling biological membranes, and in the transport and targeting of active agents.
Biological membranes form the ubiquitous delimiting structures that surround and compartmentalize all cells and organelles. The bilayer arrangement of lipids is perhaps the only organizational feature that is common to all biological membranes. Numerous theoretical models of membrane structure have appeared since the publication of the cell theory by Schleiden and Sehwann in 1839. Experimental models provide insight into the motional dynamics and static structures of some isolated compartments of biological membranes. Lipid vesicles are just one type of the many experimental models of biomembranes. Although developed for basic research, many technological innovations have arisen from the applications of these models. Lipid vesicles have evolved successfully, as vehicles for controlled delivery..
Conventional chemotherapy for the treatment of intracellular infections is not effective, due to limited permeation of drugs into cells. This can be overcome by use of vesicular drug delivery systems. Encapsulation of a drug in vesicular structures can be predicted to prolong the existence of the drug in systemic circulation, and perhaps, reduces the toxicity if selective uptake can be achieved [10]. The phagocytic uptake of the systemic delivery of the drug-loaded vesicular delivery system provides an efficient method for delivery of drug directly to the site of infection, leading to reduction of drug toxicity with no adverse effects. Vesicular drug delivery reduces the cost of therapy by improved bioavailability of medication, especially in case of poorly soluble drugs. They can incorporate both hydrophilic and lipophilic drugs. Vesicular drug delivery systems delay drug elimination of rapidly metabolizable drugs, and function as sustained release systems. This system solves the problems of drug insolubility, instability, and rapid degradation. Consequently, a number of vesicular delivery systems such as liposomes, niosomes, pharmacosomes etc, were developed.
Liposomes
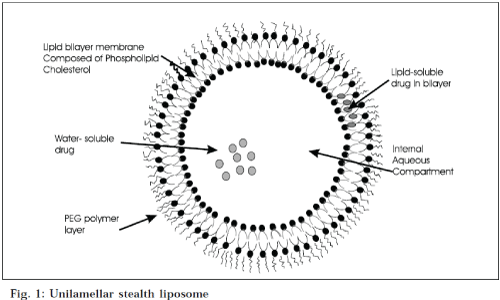
Liposomes are simple microscopic vesicles in which lipid bilayer structures are present with an aqueous volume entirely enclosed by a membrane, composed of lipid molecule. There are a number of components present in liposomes, with phospholipid and cholesterol being the main ingredients. The type of phospholipids includes phosphoglycerides and sphingolipids, and together with their hydrolysis products [11]. Classification of liposomes on the basis of lamellae and composition is shown in Table 1, and on the basis of size and number of lamellae in Table 2 (Fig. 1).
| Type | Composition | Characteristics | References |
|---|---|---|---|
| Conventionalliposomes | Neutral and or negatively chargedphospholipids + cholesterol | Subject to coated-pit endocytosis, contents ultimatelydelivered to lysosomes if they do not fuse from theendosomes, useful for RES targeting; rapid andsaturable uptake by RES; short circulation half life;dose dependent pharmacokinetics. | 12,13 |
| PH sensitiveliposomes | Phospholipids such as phosphatidylethanolamine, dioleoylphosphatidylethanolamine with either CHEMS1or OA2 . | Subject to coated-pit endocytosis at low pH, fuse withcell or endosome membrane and release their contentsin cytoplasm; suitable for intracellular delivery of weakbase and macromolecules; biodistribution and pharmacokinetics similar to conventional liposomes. | 14 |
| Cationic liposomes | Cationic lipids | Possibly fuse with cell or endosome membranes; suitablefor delivery of negatively charged macromolecules(DNA, RNA); ease of formation; structurally unstable;transfection activity decreases with time; toxic at highdose, mainly restricted to local administration. | 15 |
| Long circulatingliposomes (or) Stealth liposomes | Neutral high transition temperature,lipid, cholesterol+ 5-10% of PEG-DSPE3 ,GMI4, HPI5 | Hydrophilic surface coating, low opsonisation and thus low rate of uptake by RES; long circulating half life (40 hrs); dose independent pharmacokinetics upto 10micromoles/mouse lipid dose. |
16 |
| Immuno-liposomes | Conventional or long circulating liposomes with attached Ab or recognition sequence. | Subject to receptor-mediated endocytosis; cell specific binding (targeting); can release contents extracellularly near the target tissue and drugs diffuse through plasma membrane to produce their effects. | 17 |
| Magnetic liposomes | Phosphatidyl choline, cholesterol small amounts of a linear chain aldehyde and colloidal particles of magnetic iron oxide | Liposomes that indigenously contain binding sites for attaching other molecules like antibodies on their exterior surface. Can be made use of by an external vibrating magnetic field in their deliberate, on site, rupture and immediate release of their components. | 18 |
| Temperature (or) heat sensitive liposomes | dipalmitoyl phophatidylcholine | Temperature (or) dipalmitoyl Vesicles showed maximum release at 41°Y, the phase transition temperature of dipalmitoyl phophatidylcholine Liposomes released the entrapped content at the target cell surface upon a brief heating to the phase transition temperature of the liposome membrane | 19 |
Table 1: liposome classification based on composition and mode of drug delivery.
| Type | Size range | Characteristics | References |
|---|---|---|---|
| Multilamellar vesicles (MLV) | 0.1-0.3 µm | More than one bilayer; moderate aqueous volume to lipid ratio 1:4:1mole lipid); (greater encapsulation of lipophilic drug; mechanically stable upon long term storage; rapidly cleared by RES; useful for targeting the cellsof RES; simplest to prepare by thin film hydration of lipids in presence of an organic solvent. | 20, 21 |
| 1. Oligolamellar vesicles or paucilamellar vesicles | Intermediate between LUV & MLV | 14 | |
| 2. Multivesicular liposomes | Separate compartment are present in a single MLV | 22 | |
| 3. Stable plurilamellar vesicles | Have unique physical &biological properties due to osmotic compression. | 23 | |
| Large unilamellar vesicles (LUV) | 0.1-10 µm | Single bilayer, high aqueous volume to lipid ratio (7:1mole lipid) useful for hydrophilic drugs; high capture of macromolecules; rapidly cleared by RES; prepared by detergent dialysis, ether injection, reverse phase evaporation, or active loading methods. | 24 |
| Small unilamellar vesicles (SUV) | ≤0.1 µm | Single bilayer, homogeneous in size; thermodynamically unstable; susceptible to aggregation and fusion at low or no charge; limited capture of macromolecules; low aqueous volume to lipid to ratio (0.2:1.5:1mole lipid); | 25 |
Table 2: Liposome Classification By Size and Number of Lamellae
All methods of preparation of liposomes involve dissolution of cholesterol, lecithin, and charge in organic solvent, followed by drying it to a thin film, and then dispersion of film in an aqueous medium to obtain liposome suspension at a critical hydrating temperature. The hydrating temperature used to prepare liposomes should be above the phase transition temperature of phospholipid used i.e. temperature at which there is transition from gel to liquid phase. It can be altered by using phospholipid mixtures, or by adding sterols e.g. cholesterol. Gel state vesicular delivery system can be improved by adding cholesterol to the lipid in case of liposomes, or to the surfactant in case of niosomes, discussed later on in the paper. This temperature can give good clues to vesicular delivery, system stability, and permeability. The methods of preparation have been classified to the three basic modes of dispersions
• Physical dispersion involving hand shaking and nonhand shaking methods [26,27]
• Solvent dispersion involving ethanol injection, ether injection, double emulsion vesicle method, reverse phase evaporation vesicle method, and stable plurilamellar vesicle method [28-34]
• Detergent solubilization [35,36]
The liposomes are characterized for their physical attributes i.e. size, shape, and size distribution [37-40], surface charge [41], percent capture [42,43], entrapped volume [44], lamellarity through freeze fracture microscopy and P-NMR [45], phase behavior [46], drug release [47,48], quantitative determination of phospholipids [49] and cholesterol analysis [50].
Cationic liposomes (CLs) are used as gene vectors (carriers) in worldwide human clinical trials of non-viral gene therapy. These lipid-gene complexes were found to have the potential of transferring large pieces of DNA of up to 1 million base pairs into cells [51,52]. The outcome of a study carried with doxorubicin expressed in the kinetic model, revealed a 5-6 fold larger rate constant of cell killing potency for the encapsulated drug, versus the free drug. [53] Liposomes are available in sizes ranging from 20 nm to greater than 1μm, and therefore provide an opportunity to be administered by the intranasal route, for controlled drug delivery to the respiratory tract [54]. The fact was also recognized by other scientists when they administered cytosine arabinoside-entrapped liposomes by the same route, and found that the drug remained within the lungs for a considerable time [55,56]. Inhalation devices like nebulizers produce an aerosol of droplets containing liposomes. Some other drugs encapsulated in liposomes are pentamidine [57], sodium cromoglycate [58], and salbutamol [59], and administered by same route. A study carried out by Medina et al., demonstrated the potential of the pleural route, as a technique for mediastinal mode targeting using the avidin/biotin-liposome system [60]. The results of a study by Voinea et al., suggest that superoxide dismutase entrapped in liposomes, is effective in scavenging superoxide anions, increases nitric oxide bioactivity, and improves the vasorelaxation of resistance arteries in diabetic hamsters [61]. The findings by Joshi and Misra [62] demonstrate that liposome of budesonide can be prepared with a high entrapment value, stabilized by lyophilization, and delivered as an aerosolized dry powder inhalation..
Liposomes as carriers for topical and transdermal delivery, have been reviewed in detail by Touitou et al. [63] One of the earliest applications on topically administered liposome-entrapped triamcinolone, was reported by Mezei et al. [64] After this, topical application of liposome has been explored on various categories of drugs like steroids65,66, non-steroidal antiinflammatory drugs [67,68], local anesthetics [69] and antimicrobial agents [70].
Manosroi et al., [71] demonstrated that amphoterecin B entrapped in charged liposomes, showed sustained skin absorption, and concluded that the positively charged liposome might be the best formulation for AmB, due to its higher stability than other formulations. In addition to use of liposomes in the treatment of psoriasis [72] encapsulation of associated antigens epitopes into sterically stabilized liposomes, result in effective immunogenic formulations suitable for clinical use in active specific tumour immunotherapy [73].
Application of liposomes in topical ocular drug delivery has also given considerable attention [74]. Liposomes offer advantages over most ophthalmic preparations in being completely biodegradable and non-toxic. Smolin et al. [75] reported that idoxiuridine entrapped in liposomes, was more effective in treatment of acute and chronic herpetic keratitis in albino rats, than the unentrapped drug. Schaeffer et al. [76] reported that the transcorneal flux of liposome-entrapped penicillin G, indoxol, and carbachol, were approximately double than that of the unentrapped drug. Immunoliposomes bearing antibody against the cell surface viral glycoprotein, was suggested as targeting carriers in the treatment of ocular herpetic keratitis [77] Liposomes have the ability to intimately make contact with the corneal and conjunctival surfaces, and thereby increase the ocular drug absorption. Guo et al. [78] confirmed the importance of +ve charge in corneal retention of liposomes. Substantial reduction in retinal toxicity of cytarabin in liposomal form, was reported by Liu et al, [79] who suggested that this combination offers great promise in the treatment of ocular proliferative disorders (as an alternative to fluorouracil). The in vitro corneal penetration and in-vivo corneal absorption of acyclovir-containing liposome systems were investigated. Results of in vitro studies demonstrated that, positively charged liposomes resulted in a high penetration of acyclovir than those of negatively charged liposomes, and free drug solution. An in vivo study indicated that extent of acyclovir concentration in the cornea was higher than those negatively charged liposomes, and free drug solution [80].
Liposomes as a potential delivery system for the oral administration of insulin, have been extensively studied [81,82]. It was observed by many scientists, that the liposomes had protective effects against proteolytic digestive enzymes like pepsin and pancreatin [83,84], and they can increase the intestinal uptake of macromolecules and hence are capable of enhancing insulin uptake [85].
Liposomes with a specifically modified design, i.e. longcirculating and especially actively targeting liposomes, stand a better chance in becoming truly tumoritropic carriers of photosensitizers, and can hence be used successfully in photodynamic therapy [86].
Liposomal drug delivery system is advantageous in the fulfillment of the aspects related to protection of the drug, controlled release of the active moiety along with the targeted delivery, and cellular uptake via endocytosis [87-90]. Besides the merits, liposomes also pose certain problems associated with degradation by hydrolysis [91], oxidation [92], sedimentation, leaching of drug; and aggregation or fusion [93] during storage. Approaches that can be used to increase liposome stability involve efficient formulation and lyophilization. Formulation involves the selection of the appropriate lipid composition and concentration of the bilayer, in addition to the aqueous phase ingredients, such as buffer, antioxidants, metal, chelators, and cryoprotectants. Charge-inducing lipids, such as phosphatidylglyceride be incorporated into the liposome bilayer to decrease fusion, while cholesterol and sphingomyelin can be incorporated in formulations, in order to decrease the permeability and leakage of encapsulated drugs. Buffers at neutral pH can decrease hydrolysis. Addition of antioxidants such as sodium ascorbate, can decrease the oxidation. Freeze-dried liposome formulations should incorporate a lipoprotectantlike non-reducing disaccharide, such as trehalose, and sucrose. Some problems associated with clinical applications of liposomes, are difficulties experienced in sterilization and large-scale production. Moreover, it is difficult to obtain large quantities of sterile products with defined and reproducible properties, which display adequate chemical and physical stability. The cost and purity of phospholipid is another limiting factor. They are suitable for parenteral administration but oral administration is not possible, because of inability of liposomes to survive to the action of bile salts and phospholipids [94].
Niosomes or non-ionic surfactant vesicles
Rigorous conditions required for handling liposomes under cryogenic atmosphere have prompted the use of non- ionic surfactant in vesicular drug delivery system, in lieu of phospholipids. Thus, the new vesicular delivery system consisting of unilamellar or multilamellar vesicles called niosomes, was introduced. In this case, an aqueous solution is enclosed in a highly ordered bilayer made up of non- ionic surfactant, with or without cholesterol and dicetyl phosphate, and exhibit a behaviour similar to liposomes in vivo. The bilayered vesicular structure is an assembly of hydrophobic tails of surfactant monomer, shielded away from the aqueous space located in the center and hydrophilic head group, in contact with the same. Addition of cholesterol results in an ordered liquid phase formation which gives the rigidity to the bilayer, and results in less leaky niosomes. Dicetyl phosphate is known to increase the size of vesicles, provide charge to the vesicles, and thus shows increase entrapment efficiency. Other charge-inducers are stearylamine and diacylglycerol, that also help in electrostatic stabilization of the vesicles. Niosomes have unique advantages over liposomes. Noisomes are quite stable structures, even in the emulsified form [95,96]. They require no special conditions such as low temperature or inert atmosphere for protection or storage, and are chemically stable. Relatively low cost of materials makes it suitable for industrial manufacture. A number of non- ionic surfactants have been used to prepare vesicles viz. polyglycerol alkyl ether, glucosyl dialkyl ethers, crown ethers, ester linked surfactants, polyoxyethylene alkyl ether [97-102], Brij [103,104], and a series of spans and tweens [105-108].
Niosomes entrap solute in a manner analogous to liposomes. They are osmotically active, and are stable on their own, as well as increase the stability of the entrapped drugs [109,110]. Handling and storage of surfactants require no special conditions. Niosomes possess an infrastructure consisting of hydrophilic and hydrophobic moieties together, and as a result, can accommodate drug molecules with a wide range of solubilities [111]. They exhibit flexibility in structural characteristics (composition, fluidity, size,), and can be designed according to the desired situation [112]. Niosomes improve the oral bioavailability of poorly absorbed drugs [113-115], and enhance skin penetration of drugs [116-119]. They can be made to reach the site of action by oral [120,121] [oral absorption of niosomes is better as compared to liposomes as replacement of phospholipids by nonionic surfactants has made niosomes less susceptible to the action of bile salts], parenteral [122,123], as well as topical routes [124-127]. They allow their surface for attachment of hydrophilic moieties in the bilayer, to bring about changes in-vivo, by incorporation of hydrophilic groups such as poly (ethylene glycol), concanavalin A, and polysaccharide to the non-ionic surfactant, thus acting as stealth or long circulating niosomes [128,129]. Niosomal dispersion in the aqueous phase can be emulsified in non-aqueous phase to regulate delivery rate of drug, and administer to normal vesicles in extended non-aqueous phase [130].
Niosomal surfactants are biodegradable, biocompatible, and non- immunogenic. Niosomes improve the therapeutic performance of drug molecules by delayed clearance from the circulation, protecting the drug from biological environment and restricting effects to target cells.
Niosomes can be formulated by lipid layer hydration method, or by reverse phase evaporation method, or by transmembrane pH gradient uptake process (remote loading), to form multilamellar vesicles. Other methods include hand shaking, ether injection, and sonication [131,132]. These methods are based on whether the drug is actively or passively entrapped in vesicles. In passive trapping, the technique drug and lipids are codispersed with a fraction of drug being entrapped, according to hydrophobicity and electrostatic charge. If the drug is hydrophilic, it will be entrapped in the internal aqueous phase, and the hydrophobic drug will primarily be entrapped in the lipid region. Active trapping can be achieved in response to ion gradients placed across niosomal membranes. This allows drug entrapment after the niosomal carrier has been formulated.
Similar to liposomes, there are 3 major types of niosomes –multilamellar vesicles (MLV, size >0.05 μm), small unilamellar vesicles (SUV, size -0.025-0.05 μm), large unilamellar vesicles (LUV, size >0.10 μm). MLVs vesicles exhibit increased-trapped volume and equilibrium solute distribution, and require hand-shaking method. They show variations in lipid compositions. SUVs are commonly produced by sonication, and French Press procedures. Ultrasonic electrocapillary emulsification or solvent dilution techniques can be used to prepare SUVs. The injections of lipids solubilised in an organic solvent into an aqueous buffer, can result in spontaneous formation of LUV. But the better method of preparation of LUV is reverse phase evaporation, or by detergent solubilisation method [133-136].
Niosomes are characterized for different attributes such as vesicle diameter using light microscope, photon correlation microscopy, freeze capture microscopy, entrapment efficiency, and in vitro release rate. Other aspects studied are drug stability, drug leakage in saline and plasma on storage, pharmacokinetic aspect, toxicity, etc.
Varshosaz et al. [137] prepared niosomes of sorbitan monoesters (span 20, 40, 60, and 80), using the film hydration method without sonication, and demonstrated that vesicles containing span 60 showed the highest protection of insulin against proteolytic enzymes. Micromanipulation of the external bilayers of niosomes allows the formation of tethers, which are fluid state lipid/ surfactant lamellar nanotubes, and can be used as mode of transport of a vesicle within a flexible microtube [136] Niosomes can be used as stable carriers for tretinoin, when incorporated in P90 or span vesicles. The results presented a half-life shorter, or very close to that of the free drug [138]. Niosomal gel formulation containing nimesulide, have also demonstrated enhanced antiinflammatory activity, compared to plain drug gel and marketed formulation [139], due to increased permeation of the drug by niosomal gel formulation. An antileishmanial property of bacopasaponin C was maximal without any side effects, in the form of niosomal vesicular systems [140] A study of daunorubicinhydrochloride encapsulated in niosomes by Balasubramanium et al., suggests that the multilamellar vesicles obtained by the reverse evaporation process, resulted in vesicles that resisted the immediate lysis in the Kupffer cells, whereby a prolonged drug concentration was achieved which enhanced the cell lysis [141]. Hao et al., prepared niosomes, which have high encapsulation capacity for soluble drugs, starting from span 60 and cholesterol, using evaporation-sonication method, and demonstrated that niosomes prepared in this way not only have high encapsulation capacity, but it is also expected that side effects of drugs may be reduced [142]. Niosomes have also been used as carriers for iobitridol, a diagnostic agent used for X-ray imaging [143] Non-ionic surfactant vesicles (niosomes), were prepared and appended with a polysaccharide cap using hydrophobic anchors. Hydrophobized polysaccharides, O-palmitoyl pullulan (OPPu) and cholesteroyl pullulan (CHPu), were anchored onto propranolol HCl containing preformed niosomes for oral drug delivery [144]. The niosomal formulation displayed higher and sustained plasma drug level profile, compared to free drug solution, and hence act as promising carriers for 5-fluorouracil [145].
Like liposomes, aqueous suspension of niosomes may exhibit aggregation, fusion, leaching or hydrolysis of entrapped drugs, thus limiting the shelf- life of niosomes dispersion. Niosome preparation is time-consuming, requires specialized equipment, and is inefficient, particularly if smaller quantities are required for particular application or dose..
Transfersomes
Liposomal as well as niosomal systems, are not suitable for transdermal delivery, because of their poor skin permeability, breaking of vesicles, leakage of drug, aggregation, and fusion of vesicles [146,147]. To overcome these problems, a new type of carrier system called “transfersome”, has recently been introduced, which is capable of transdermal delivery of low as well as high molecular weight drugs148. Transfersomes are specially optimized, ultradeformable (ultraflexible) lipid supramolecular aggregates, which are able to penetrate the mammalian skin intact. Each transfersome consists of at least one inner aqueous compartment, which is surrounded by a lipid bilayer with specially tailored properties, due to the incorporation of “edge activators” into the vesicular membrane [149,150]. Surfactants such as sodium cholate, sodium deoxycholate, span 80, and Tween 80, have been used as edge activators [151-153]. It was suggested that transfersomes could respond to external stress by rapid shape transformations requiring low energy. These novel carriers are applied in the form of semi-dilute suspension, without occlusion. Due to their deformability, transfersomes are good candidates for the non-invasive delivery of small, medium, and large sized drugs. Multiliter quantities of sterile, well-defined transfersomes containing drug can be, and have been prepared relatively easily.
Materials commonly used for the preparation of transferosomes are phospholipids (soya phosphatidyl choline, egg phosphatidyl choline), surfactant (tween 80, sodium cholate) for providing flexibility, alcohol (ethanol, methanol) as a solvent, dye (Rhodamine-123, Nile-red) for confocal scanning laser microscopy (CSLM), and buffering agent (saline phosphate buffer pH 7.4), as a hydrating medium.
Transfersomes are prepared in two steps. First, a thin film, comprising phospholipid and surfactant is prepared, hydrated with buffer (pH 6.5) by rotation, and then brought to the desired size by sonication. The concentration of surfactant is very crucial in the formulation of transfersomes, because at sublytic concentration, these agents provide flexibility to vesicles membrane, and at higher concentration, cause a destruction of vesicles. In the second step, sonicated vesicles are homogenized by extrusion through a polycarbonate membrane [154].
Transfersomes are characterized for different physical properties such as vesicle diameter using photon correlation spectroscopy or dynamic light scattering method [155], entrapment efficiency [156], vesicle diameter [157,158], degree of deformability or permeability, in vitro drug release, confocal scanning laser microscopy (CSLM) study for investigating the mechanism of penetration of transfersomes across the skin, for determining histological organization of the skin, shapes and architecture of the skin penetration pathways, and for comparison and differentiation of the mechanism of penetration of transfersomes with liposomes, niosomes, and micelles. Other parameters studied are in vivo fate [159], pharmacokinetic aspects [160-162], toxicity studies, etc.
Transfersomes have been proposed for a variety of applications in humans. They are used as a carrier for protein and peptides like insulin, bovine serum albumin, vaccines, etc. The delivery of these large biogenic molecules into the body is difficult. When given orally, they are completely degraded in the GI tract, and when used in a degradation preventing formulation, their uptake in the gut becomes problematic and extremely insufficient. These are the reasons why nearly alltherapeutic peptides still have to be introduced into the body through an injection needle, in spite of the inconvenience of this method. To overcome the above problems, numerous attempts have therefore been made for delivery of peptides and proteins across the skin [163]. All recent approaches, either chemical (penetration enhancers, lipid vesicles), or physicals (iontophoresis, sonophoresis), have some limitations.
Proteins and other molecules, normally do not cross the intact mammalian skin. Despite this, it elicits antibodies against the subcutaneously applied proteins, such as fluorescein-isothiocyanate-labelled bovine serum albumin (FITC-BSA), if these macromolecules are associated with the specially optimized and ultradeformable agent carriers [164]. A judicious combination of the integral membrane proteins and the ultradeformable membrane, also provides a solution to the problem of the noninvasive delivery of such molecules. Incorporation of gap junction protein (GJP) into transfersomes for example, results in a maximum immune response to this type of macromolecules [165]. Delivery of peptides by transfersomes provides a very successful means for the noninvasive therapeutic use of such large molecular weight drugs on the skin [166]. Insulin-loaded transfersomes were prepared and evaluated, and it was found that transfersomesassociated insulin (transfersulinTM) is carried across the skin with an efficacy of >50%, and often >80%, if properly optimized. After each transfersulin application on the intact skin, the first signs of systemic hypoglycemia are observed after 90 to 180 minutes, depending on the specific carrier composition [167]. It was reported that the formulation of interleukin-2 and interferon-α containingtransfersomes, are able to deliver sufficient concentrations for immunotherapy [168]. The same concept was used for transdermal immunization, using transfersomes loaded with soluble protein like integral membrane protein, gap junction protein, bovine serum albumin, etc. Corticosteroids are used topically for a large variety of dermatological conditions, but the dermally administered corticosteroids typically fail to deliver a sufficiently large drug amount into the body. Use of highly concentrated, or even supersaturated drug solution on skin, leads to the problem of drug precipitation, and higher chances of the adverse effects [169].
Transfersomes improve the site specificity, overall drug safety, and lower the doses several times than the currently available formulations for the treatment of skin diseases. Because of their good penetration power and flexibility, transfersomes formulations are used for effective delivery of non-steroidal anti-inflammatory agents like ibuprofen [170] and diclofenac [171]. Transfersomes not only increase the penetration of diclofenac through intact skin, but also carry these agents directly into the depth of the soft tissues under the application site. Cevc[171], developed formulation of tamoxifen, the most common agent for the treatment of all stages of breast cancer, is based on ultradeformable vesicles, and applied on the shaved murine back. Most of the epidermallyapplied transfersomes penetrated the skin, leaving less than 5% of the drug-derived radioactivity on the body surface. Such delivery of tamoxifen, lowers the incident of side effects like depression and thrombosis. Recently, the impact of the combined use of ultradeformable liposomes and iontophoresis on the penetration of tritiated estradiol, was compared with saturated aqueous solution [172]. The tritium exchange study showed that extent of exchange correlated well with current density and time of application, with some shielding of estradiol by liposomal structure. Transfersomes enhanced passive estradiol penetration after occlusion. Estradiol flux was increased linearly with current density, although being delivered against electro-osmotic flow. Elastic vesicles with rigid vesicles, in terms of their interaction, was compared with human skin, and reported that unlike rigid vesicles, there is no ultra structural changes takes place in the human skin on application of elastic vesicles [173]. It was reported, that in vitro transport of pergolide from L595 #x2013; PEG-8-L, elastic vesicle showed highest skin permeation of pergolide, having a steady-state flux of 137.9 ng/h/cm2, [174]. Transfersomes have been reported to improve transdermal delivery of drugs, when applied nonocclusively [175,176]. Transfersomes have also been reported to improve the therapeutic efficacy of cyclosporine, and the site specificity and safety of corticosteroids [177,178].
But like liposomes, transfersomes have certain limitations
1. Transfersomes are chemically unstable because of their predisposition to oxidative degradation,
2. Lack of purity of the natural phospholipids comes in the way of adoption of transfersomes as drug delivery vehicles and
3. Transfersomes formulations are expensive to prepare.
Pharmacosomes
The limitations of transfersomes can be overcome by the “pharmacosome” approach. The prodrug conjoins hydrophilic and lipophilic properties, and therefore acquires amphiphilic characters, and similar to other vesicle forming components, was found to reduce interfacial tension, and at higher concentrations exhibits mesomorphic behavior. These are defined as colloidal dispersions of drugs covalently bound to lipids, and may exist as ultrafine vesicular, micellar, or hexagonal aggregates, depending on the chemical structure of druglipid complex [179]. Many constraints of various classical vesicular drug delivery systems, such as problems of drug incorporation, leakage from the carrier, or insufficient shelf life, can be avoided by the pharmacosome approach. The idea for the development of the vesicular pharmacosome, is based on surface and bulk interactions of lipids with drug. Any drug possessing an active hydrogen atom (-COOH, -OH, -NH2, etc.) can be esterified to the lipid, with or without spacer chain. Synthesis of such a compound may be guided in such a way that strongly result in an amphiphilic compound, which will facilitate membrane, tissue, or cell wall transfer, in the organism. The salient features of pharmacosomes are
• Entrapment efficiency is not only high but predetermined, because drug itself in conjugation with lipids forms vesicles.
• Unlike liposomes, there is no need of following the tedious, time-consuming step for removing the free, unentrapped drug from the formulation.
• Since the drug is covalently linked, loss due to leakage of drug, does not take place. However, loss may occur by hydrolysis.
• No problem of drug incorporation
• Encaptured volume and drug-bilayer interactions do not influence entrapment efficiency, in case of pharmacosome. These factors on the other hand have great influence on entrapment efficiency in case of liposomes
• The lipid composition in liposomes decides its membrane fluidity, which in turn influences the rate of drug release, and physical stability of the system.
However, in pharmacosomes, membrane fluidity depends upon the phase transition temperature of the drug lipid complex, but it does not affect release rate since the drug is covalently bound
• The drug is released from pharmacosome by hydrolysis (including enzymatic).
• Phospholipid transfer/exchange is reduced, and solubilization by HDL is low.
• The physicochemical stability of the pharmacosome depends upon the physicochemical properties of the drug-lipid complex.
• Due to their amphiphilic behavior, such systems allow, after medication, a multiple transfer through the lipophilic membrane system or tissue, through cellular walls piggyback endocytosis and exocytosis.
• Following absorption, their degradation velocity into active drug molecule depends to a great extent on the size and functional groups of drug molecule, the chain length of the lipids, and the spacer. These can be varied relatively precisely for optimized in vivo pharmacokinetics.
• They can be given orally, topically, extra-or intravascularly.
Mantelli et al.,[180] compared the effect of diglyceride prodrug on interfacial tension, with the effect produced by a standard detergent dodecylamine hydrochloride, and observed similar effect on lowering of surface tension . Above the critical micelle concentration (CMC), the prodrug exhibits mesomorphic lyotropic behaviour, and assembles in supramolecular structures. The prepared prodrugs are generally characterized for their structural conformation (by IR, NMR spectrophotometry, thin layer chromatography (TLC), melting point determination), partition coefficient [181], surface tension [182], and prodrug hydrolysis. Hand-shaking method and ether injection method, have been utilized for preparing vesicles. In hand-shaking method, the dried film of the drug-lipid complex (with or without egg lecithin) deposited in a round bottom flask upon hydration with aqueous medium, readily gives a vesicular suspension. In ether injection method, organic solution of the drug-lipid complex, was injected slowly into the hot aqueous medium, wherein the vesicles are readily formed. Like other vesicular systems, pharmacosomes are characterized for different attributes such as size and size distribution, nuclear magnetic resonance (NMR) spectroscopy, entrapment efficiency, in vitro release rate, stability studies, etc. The approach has successfully improved the therapeutic performance of various drugs i.e. pindolol maleate, bupranolol hydrochloride, taxol, acyclovir, etc [183,184].
Pharmacosomes bearing unique advantages over liposome and niosome vesicles, have come up as potential alternative to conventional vesicles. The system, yet requires greater efforts towards investigating the nonbilayer phases, and exploring the mechanism of action. Furthermore, the effect of covalent linkages and addition of spacer group on rate of in vivo hydrolysis and subsequent pharmacokinetics is to be exhaustively studied, in order to exploit more advantages of this system. Like other vesicular drug delivery systems, pharmacosomes, on storage, undergo fusion and aggregation, as well chemical hydrolysis. Some other emerging vesicular systems in addition to the above, are listed in Table 3.
| Vesicular systems | Description | Application | Reference |
|---|---|---|---|
| Enzymosomes | Liposomal constructs engineered to provide a mini bioenvironment in which enzymes are covalently immobilized or coupled to the surface of liposomes. | Targeted delivery to tumor cells | 186 |
| Virosomes | Liposomes spiked with virus glycoprotein, incorporated into the liposomal bilayers based on retro viruses derived lipids. | Immunological adjuvants Ligand mediated drug | 187 |
| Ufasomes | Vesicles enclosed by fatty acids obtained from long chain fatty acids (oleic acid, linoleic acid) by mechanical agitation of evaporated films in the presence of buffer solutions. | targeting | 188 |
| Cryptosomes | Lipid vesicles with a surface coat composed of PC and of suitable polyoxyethylene derivative of phosphatidyl ethanolamine. | Ligand mediated drug targeting | 189 |
| Emulsomes | Nanosize lipid particles (bioadhesivenanoemulsion) consisted of microscopic lipid assembly with apolar core. | Parenteral delivery of poorly water soluble drugs | 190 |
| Discomes | Niosomes solubilized with non-ionic surfactant solution (polyoxyethylenecetyl ether class). | Ligand mediated drug targeting | 191 |
| Aquasomes | Three layered self-assembly compositions with ceramic carbon nanocrystalline particulate core coated with glassy cellobiose. | Specific targeting, molecular shielding | 192 |
| Ethosomes | Ethosomes are lipid “soft, malleable vesicles” embodying a permeation enhancer and composed of phospholipid, ethanol and water. | Targeted delivery to deep skin layers | 193 |
| Genosomes | Artificial macromolecular complexes for functional gene transfer. Cationic lipids are most suitable because they possess high biodegradability and stability in the blood stream. | Cell specific gene transfer | 194 |
| Photosomes | Photolyase encapsulated in liposomes, which release the contents, by photo-triggered charges in membrane permeability characteristics. | Photodynamic therapy | 195 |
| Erythrosomes | Liposomal systems in which chemically crosslinked human erythrocytes cytoskeletons are used as a support to which lipid bilayer is coated. | Effective targeting of macromolecular drugs | 196 |
| Hemosomes | Haemoglobin containing liposomes engineered by immobilizing haemoglobin with a polymerisable phospholipids. | High capacity oxygen carrying system | 197 |
| Proteosomes | High molecular weight multi-subunit enzyme complexes with catalytic activity, which is specifically due to the assembly pattern of enzymes. | Better catalytic activity turnover than non-assciated enzymes. | 198 |
| Vesosome | Nested bilayer compartments in vitro via the “interdigitated” bilayer phase formed by adding ethanol to a variety of saturated phospholipids. | Multiple compartments of the vesosome give better | 199 |
| Archaeosomes | Vesicles composed of glycerolipids of archaea with potent adjuvant activity. | protection to the interior contents in serum Potent adjuvant activity | |
| 200 |
Table 3: Some Emerging Vesicular Systems.
Conclusion
Vesicular systems have been realized as extremely useful carrier systems in various scientific domains. Over the years, vesicular systems have been investigated as a major drug delivery system, due to their flexibility to be tailored for varied desirable purposes. In spite of certain drawbacks, the vesicular delivery systems still play an important role in the selective targeting, and the controlled delivery of various drugs. Researchers all over the world continue to put in their efforts in improving the vesicular system by making them steady in nature, in order to prevent leaching of contents, oxidation, and their uptake by natural defense mechanisms. Current research trends are generally based on using different approaches (like pegylation, biotinylation etc.) for cellular targeting. Certainly, the last word has not yet been said about vesicular drug delivery systems.
References
- Li, V.H.K., Robinson, J.R. and Lee, V.H.L., In; Controlled Drug Delivery: Fundamentals and Applications, 2nd Edn., Vol 29, Marcel Dekker, Inc., NY, 1987, 7.
- Goldberg, E. P. Eds., In; Targeted Drugs, 2nd Edn., Wiley, New York, 1983, 312.
- Gregoriadis, G., Nature, 1977, 265, 407.
- Poste, G., Kirsch, R. and Koestler, T., In; Gregoriadis, G. Eds; Liposomes Technology Vol 3, CRC Press Inc., Baco Raton. Fl, 1983, 29.
- Poznansky, M. J. and Juliano, R. L., Pharmacol. Rev., 1983, 36, 277.
- Bangham, A. D., Standish, M..M. and Watkins, J. G., J. Mol. Biol., 1965, 13, 238.
- Ogihara-Umeda I., Sasaki T., Toyama H., Oda K., Senda M., Nishigori H. Cancer Detect Prev., 1997; 21(6):490
- Park, J.W., Hong, K., Kirpotin, D.B. and Benz C.C., Adv.Pharmacol ., 1997, 40, 399.
- Kao, .GY., Change, L.J., Allen, T.M., Cancer Gene Ther . 1996, 3(4), 250-6.
- Todd, J. A., Modest, E. J., Rossow, P. W. and Tokes, Z. A., Biochem.Pharmacol., 1982, 34, 541.
- Eible, H., Chem. Phys. Lipids, 1980, 16, 455.
- Gregoriadis, G. and Ryman, B.E., Eur. J. Biochem., 1972a, 24, 485.
- .Gregoriadis, G. and Ryman, B.E., Biochemistry, 1972b, 129, 123.
- Mathiowitz.,Eds; Encyclopedia of Controlled Drug Delivery, Vol I, John Wiley & Sons, Inc., New York, 1999, 461.
- Felgnu, P.L. and Ringold, G. M., Nature, 1994, 337, 387.
- Gregoriadis, G., In; McCormack, B., Eds, Targeting of Drugs: Strategies for Stealth Therapeutic Systems, Plenum, New York, 1998.
- Plautz, G.E., Proc. Natl. Acad. Sci. USA., 1993, 90, 4645.
- Elmi, M.M. and Sarbolouki, M.N., Int. J. Pharm., 2001, 215, 45.
- Sullivan, S.M. and Huang L., Proc. Natl. Acad. Sci., 1986, 83, 6117.
- Bonte, F., In; Puisieux, F., Couvreur, P., Delattre, J., Devissaguet, J.P., Eds; Liposomes, NewSystems and New Trends in their Applications, Editions de Sante, Paris, 1995, 75.
- Alving, C.R., Proc. Natl. Acad. Sci. USA., 1978, 75, 2959.
- Kim, S., Biochim. Biophys. Acta, 1983, 728, 338.
- Gruner, S. M., Lenk, R. P., Janoff, A. S. and Ostro, M. J., Biochemistry, 1985, 24, 2833.
- Reeves, J. P. and Dowben, R. M., J. Cell Physiol., 1969, 73, 49.
- Sharma, A. and Sharma, U. S., Int. J. Pharm., 1997, 154, 123.
- Mayer, L.D., Hope, M. J., Cullis, P.R. and Janoff, A.S., Biochim.Biophys. Acta, 1985, 817, 193.
- Kremer, J.M., Eskai, M.W., Pathmamanoharan, G. and Wiersema, P.H., Biochemistry, 1977, 16, 3932.
- Batzre, S. and Korn, E. D., Biochem. Biophys. Acta, 1973, 298, 1015.
- Deamer, D.W., Ann. N.Y.Acad. Sci, 1978, 308, 250.
- Deamer, D.W. and Bangham, A. D., Biochim. Biophys. Acta, 1976, 443, 629.
- Schieran, H., Rudolph, S., Fiukelstein, M., Coleman, P. and Weisman, G., Biochim. Biophys. Acta, 1978, 646, 4.
- Kim, S. and Martin, G. M., Biochim. Biophys. Acta , 1981, 646, 4.
- Batalle Memorial Inst., British Patent Appl. No. 2001929A, 1979.
- Arnardottir, H.B., Sveinsson, S.L. and Kristmundsdottir, T., Int. J.Pharm., 1995, 117, 237
- Korenbrot, J. I., Ann. Rev. Physiol., 1977, 39, 17.
- Razin, S., Biochim. Biophys. Acta, 1972, 265, 241.
- Hargreaves, W. R. and Deamer, D. W., Biochemistry, 1978, 17, 3759.
- Huang, C. H., Biochemistry, 1969, 8, 344.
- Sharma, P., Tyrell, D. A. and Ryman, B.T., Biochem. Soc. Tran., 1977, 5, 1146.
- Fraley, R., Wyatt, J. P. and Papahadjopoulus, D., In; Knight, C. G. Eds, Liposomes: From Physical Structues to Therapeutics Applications, Elsevier, North Holland, Biomedical Press, Amsterdam, New York, Oxford, 1981, 69.
- Bangham, A.D., Hill, M.V. and Miller, N.G., Methods Membr.Biol., 1974, 1, 1.
- Rosier, R.N., Gunter, T.E., Tucker, D.A. and Gunter, K.K., Anal.Biochem., 1979, 120, 113.
- Gunter, K. K., Gunter, T. E., Jarkowski, A. and Rosier, R. N., Anal.Biochem., 1982, 120, 113.
- Pidgeon, C., Hunt, A. H. and Dittrich, K., Pharm. Res., 1986, 3, 23.
- Hope, M. J., Bally, M. B., Webb, G. and Cullis, P. R., Biochim.Biophys. Acta., 1985, 812, 55.
- Cullins, P.R. and Hope, M. J., In; Vance, D. E., Vance, J. E. Eds., Biochemistry of lipid and membrane, Benzain/Cumning Inc., 1985, 56.
- Ganesan, M.G., Weiner, N.D., Flynn, G. I. and Ho, N. F. H., Int. J.Pharm., 1984, 20, 139.
- Egbaria, K. and Weiner, N. D., Adv. Drug Delivery Rev., 1990, 5, 287.
- Bartlett, G. R. J., J. Biol. Chem. , 1959, 234, 466.
- Brooks, C. J. W., Machachlan, J., Cole, W. J. and Lawric, T. D. V., Proc. Symp. Analysis of Steroids, Hungary, 1984, 349.
- Ewert, K., Slack, N.L., Ahmad, A., Evans, H.M., Lin, A.J., Samuel, C.E. and Safinya, C.R., Curr. Med. Chem ., 2004, 11, 133.
- May, S. and Ben-Shaul, A., Curr. Med. Chem. 2004, 11,151.
- Eliaz, R.E., Nir, S., Marty, C. and Szoka F.C., J. Cancer Res., 2004, 64,711.
- Kellaway, I.W. and Farr, S.J., Adv. Drug. Deliv. Rev., 1990, 5,149.
- McCulllough, H.N. and Juliano, R.L., Natl.Cancer Inst ., 1979, 163, 727.
- Juliano, R.L. and McCulllough, H.N., J. Pharmacol. Exp. Ther. , 1980, 214, 381.
- Debs, R.J., Straubinger, R.M., Brunette, E.N., Lin, E.J., Montgomery, A.B., Friend, D. S. and Papahadjopoulos, D.P., Am. Rev. respir.Dis., 1987, 135, 731.
- Taylor, K.M.G., Taylor, G., Kellaway, I.W. and Stevens, J., Int J.Pharm, 1990, 58, 49.
- Radhakrish, R., Mihalko, P.J. and Abra, R.M., US patent 4895719, 1990.
- Medina, L.A., Klipper, R., Phillips, W.T. and Goins, B., Nucl. Med.Biol., 2004, 31, 41.
- Voinea, M., Georgescu, A., Manea, A., Dragomir, E., Manduteanu, I., Popov, D. and Simionescu, M., Eur. J. Pharmacol., 2004,484,111.
- Joshi, M.R. and Misra, A., AAPS Pharm.Sci.Tech., 2001, 2,25.
- Touitou, E., Junginger, H.E., Weiner, N.D., Nagai, T. and Mezei, M., J. Pharm. Sci., 1994, 83(9), 1189.
- Meizei, M. and Guleshekharan, V., 39th Int. Cong. Pharm. Sci., F.I.P., Brithton, U.K., 1979.
- Knepp, V.M., Szoka, P.C. Jr. and Guy, R.H., J. Control. Rel ., 1990, 12, 25.
- Jacobes, M., Martin, G. P. and Marriott, C., J. Pharm.Pharmacol., 1988, 40, 829.
- Kriwet, K., Muller-Goymann, C.C., Int. J. Pharm., 1995, 125, 231.
- Moghimi, S.M. and patel, H.M., J. Microencap, 1993, 10, 155.
- Sharma, b.B., Jain, S.K. and Vyas, S.P., J. Microencap, 1994, 11, 279.
- Nishihata, T., Kotera, K.K., Nakano, Y. and Yamazaki, M., Chem.Pharm. Bull., 1987, 35, 3807.
- Manosroi, A., Kongkaneramit, L. and Manosroi, J., Int. J. Pharm., 2004, 270, 279.
- Trotta, M., Peira, E., Carlotti, M.E. and Gallarate, M., Int. J.Pharm., 2004,270,119.
- Adamina, M., Bolli, M., Albo, F., Cavazza, A., Zajac, P., Padovan, E., Schumacher, R., Reschner, A., Feder, C., Marti, W.R., Oertli, D., Heberer, M. and Spagnoli, G.C., Brit. J. Cancer., 2004, 90, 263.
- Gregoriadis, G. and Florence, A. T., Drugs, 1993, 45, 15.
- Smolin, J.E., Okumoto, M., Feiler, S. and Condon, D., Am. J.Ophthalmol , 1981, 91, 220.
- Schaeffer, H.E., Brietfelter, J.M. and Krohn, D.L., Invest.Ophthalmol. Vis. Sci., 1983, 23, 530.
- Schaeffer, H.E. and Krohn, D.L., Invest. Ophthalmol. Vis. Sci., 1982, 22, 220.
- Guo, L.S.S., Radhakrishnan, R. and Rehmann, C. T., J. Liposome Res., 1989/90, 3, 319.
- Liu, K.R., Peyman, G.A. and Khoobehi, B., Invest. Ophthalmol.Vis. Sci., 1989, 30, 1527.
- Law, S.L., Huang, K.J. and Chiang, C.H., J. Control. Release, 2000, 63, 135.
- Dapergolas, G. and Gregoriadis, G., Lancet, 1976, 2, 824.
- Patel, H.M., Stevenson, R.W., Parons, J.A. and Ryman, B.E., Biochim.Biophys.Acta , 1982, 716, 188.
- Patel, H.M. and Ryman, B. E., Biochem. Soc. Trans ., 1977, 5, 1739.
- Hashimoto, A. and Kawada, J., Endocrinol Japan , 1979, 26, 337.
- Zhenghong, W.V., Qi-neng,P., Yi, W., Jia-ming, L., ActaPharmacol. Sin., 2004, 25(7), 966.
- Derycke, A.S. and de Witte, P.A., Adv. Drug. Deliv. Rev., 2004, 56,17.
- Allen, T. M., Hansen, C. B. and Stuart, D. D., In; Lassic, D. D., Papahadjopoulos, D., Eds., Medical Applications of Liposomes, Elsevier, Amsterdam, 1978, 297.
- Allen, T. M., Ahmed, I., Lopes de Menezes, D. E. and Moase, E. H., Biochem.Soc. Trans., 1995, 23, 1073.
- Kirpotin, D. B., In; lasic, D. D., Papahadjopoulos, D. Eds; Medical Applications Of Liposomes, Elsevier, Amsterdam, 1998, 325.
- Mayer, L. D., Cullis, P. R. and Bally, M. B., In; Lasic, D. D., Papahadjopoulos, D., Eds.; Applications of Liposomes, Elsevier, Amsterdam, 1998, 231.
- Frfkjaer, S., Hjorth, E. and Wfatis, O., In; Gregoriadis, G., Eds; Liposome Technology, Vol 1, CRC Press, Boca Raton, FL, 1984, 235.
- Hunt, C. and Tsang, S., Int. J. Pharm., 1981, 8, 101.
- Wong, M. and Thompson, T., Biochemistry , 1982, 21, 4133.
- Nakhla, T., Marek, M. and Kovalcik, T., Drug DeliveryTechnology , 2002, 2(4), 135.
- Baille, A. J., Florence, A. T., Hume, L. R., Muihead, G. and Rogerson, J., J. Pharm. Pharmacol., 1985, 37, 863.
- Yoshioka, T., Sternberg, B. and Florence, A. T., Int. J. Pharm., 1994, 105, 1.
- Handjani-vila, R. M., Ribier, A., Rondot, B. and Vanlerberghe, G., Int. Cosmet. Sci., 1979, 1, 303.
- Baille, A. J., Coombs, G. H., Dolan, T. F. and Laurie, T., J. Pharm.Pharmacol., 1986, 38, 502.
- Kiwada, H., Niimura, H. and Kato, Y., Chem. Pharm. Bull., 1985, 33, 753.
- Echegoyen, L. E., Hernandaz, J. C., Kaifer, A. E., Gokel, G. W. and Echegoyen, L., J. Chem. Soc. Chem. Commun., 1988, 12, 836.
- Hunter, J. A., Dolan, T. F., Coombs, G. H. and Baille, A. J., J.Pharm. Pharmcol., 1988, 40, 161.
- Hofland, H.E.J., Bouwstra, H. and Junginger, H.E., Pro. Int.Contrl. Rel. Bioact. Mater. 1988, 406.
- Hofland, H. E. J., Bouwstra, J. A., Verhoef, J. C., Buckton, G., Chowdry, B. Z., Ponec, M., Junginger, H. E., J. Pharm.Pharmacol., 1992, 44, 287.
- Raja Naresh, R. A., Singh, U. V., Udupa, N. and Pillai, G. K., IndianDrugs , 1993, 30, 275.
- Parthasarthi, G., Udupa, N. and Pillai, G. K., Indian J. Pharm.Sci., 1994, 56, 90.
- Chandraprakash, K. S., Udupa, N., Umadevi, P. and Pillai, G. K., Int. Pharm., 1990, 61, R1.
- Chandraprakash, K. S., Udupa, N., Umadevi, P. and Pillai, G.K., Indian J. Pharm. Sci., 1992, 54, 197.
- Namdeo, A. and Jain, N. K., Indian J. Pharm. Sci., 1996, 58, 41.
- Rogerson, A., Cummings, J., Willmott, N. and Florence, A. T., J.Pharm. Pharmacol., 1988, 40, 337.
- Baille, A.J., Florence, A.T., Hume, L. R., Muirhead, G. T. and Rogerson, A., J. Pharm. Pharmacol. (suppl.), 1984, 36, 48.
- Udupa, N. and Pillai, G. K., Pharmag., 1991, 3, 45.
- Chitnis, M. P., Menon, R. S. and Gede, R. P., Tumori., 1984, 70, 313.
- Azmin, M. N., Florence, A. T., Handjani-vila, R. M., Stuart, J. F. B., Vanlerberghe, G. and Whittaker, J. S., J. Pharm. Pharmacol., 1985, 37, 237.
- Chandraprakash, K. S., Udupa, N., Pillai, G. K. and Umadevi, P., J.Drug Targeting , 1993, 1, 133.
- Cook, J. M. and Florence, A. T., Eur. J. Cancer Clin. Oncol. , 1988, 24, 48.
- Cappel, M. J. and Kreuter, J., Int. J. Pharm., 1991, 69, 143.
- Fang, J. Y.,Yu ,S. Y.,Wu ,P. C. and Huang,Y. B., Int. J. Pharm., 2001, 215, 91.
- Connor, J., Norley, N. and Huang, L., Biochim. Biophys. Acta, 1986, 884, 474.
- Carafa, M., Santucci, E. and Lucania, G., Int. J. Pharm, 2002, 231, 21.
- Rentel, C-O, Bouwstra, J. A., Naisbett, b. and Junginger, H. E., Int.J. Pharm., 1999, 186, 161.
- Sihorkar, V. and Vyas, S. P., Pharmazie, 2000, 55, 107.
- Namdeo, A. and Jain, N. K., J. Microencapsulation, 1999, 16, 731.
- Ruckmani, K., Jayakar, B. and Ghosal, S. K., Drug Develop. Ind.Pharm., 2000, 26, 217.
- Saettone, M. F., Perini, G., Carafa, M., Santucci, E. and Alhaique, F., STP Pharma. Sci., 1996, 6,94.
- Gayathri Devi, S., Venkatesh. andUdupa, N., Indian J. Pharm.Sci., 2000, 479.
- Jagtap, A. and Inamdar, D., Indian J. Pharm. Sci., 2001, 63, 49.
- Desai, T. R. and Finlay, W. H., Int. J. Pharm., 2002, 241, 311.
- Hofland, H. E. J., Bouwstra, J. A., Ponec, M., Bodera, H. E., Spies, E., Verhoef, J. C. and Junginger, H. E., J. Control. Release, 1991, 16, 155.
- Dufes, C., Schatzlein, A. G., Tetley, L., Gray, A. I., Watson, D. G., Olivier, J. C., Couet, W. and Uchegbu, I. F., Pharm. Res., 2000, 17, 1250.
- Albert, E. C., Mathur, R. and Wallach, D. E. H., W09217179USPatent 491128, 1992.
- Kiwada, H., Niimura, H. and Kato, Y., Chem. Pharm. Bull., 1985b, 33, 2475.
- Khandare, J. N., Madhavi, G. and Tamhankar, B. M., EasternPharmacist, 1994, 37, 61.
- Uchegbu, I. F., Bouwstra, J. and Florence, A. T., J. Pharm.Pharmacol. (Suppl.), 1992, 1052.
- Rocks, M. F. M., Vissie, H. G. J., Zwikker, J. W., Verkley, A. J. and Nolte, R. J. M., J. Amer. Chem. Soc., 1983, 106, 4509.
- Kippenberger, D., Rosenquist, K., Odberg, L., Tundo, P. and Findler, J. H., J. Am. Chem. Soc., 1983, 105, 1129.
- Yoshioka, T. and Florence, A. T., Int. J. Pharm., 1994, 168, 117.
- Varshosaz, J., Pardakhty, A., Hajhashemi, V.I., Najafabadi, A.R., Drug Deliv., 2003, 10, 251.
- Nasseri, B. and Florence, A.T., J. Control. Release, 2003, 92, 233.
- Manconi, M., Valenti, D., Sinico, C., Lai, F., Loy, G. and Fadda, A.M., Int. J. Pharm., 2003, 260, 261.
- Shahiwala, A. and Misra, A., J. Pharm. Sci., 2002, 5, 220.
- Sinha, J., Raay, B., Das, N., Medda, S., Garai, S., Mahato, S.B. and Basu, M.K., Drug Delivery , 2002, 9, 55.
- Balasubramaniam, A., Kumar, V.A. and Pillai, K.S., Drug Develop.Ind. Pharm., 2002, 28,1181.
- Hao, Y., Zhao, F., Li, N., Yang, Y., and Li, K. Int. J. Pharm., 2002, 244, 73.
- Muller, D., Foulon, M., Bonnemain, B. and Vandamme, T.F., J.Microencapsul., 2000,17, 227.
- Sihorkar, V. and Vyas, S. P., Pharmazie., 2000, 55, 107.
- Namdeo, A. and Jain, N. K., J. Microencapsul., 1999, 16, 731.
- Cevc, G., Blume, G. and Schatzlein, A., J. Control. Release, 1997, 45, 211.
- Lasch, J., Laub, P. and Wohlrab, W., J. Control. Release, 1991, 18, 55.
- Schatzlein, A. and Cevc, G., In; Cevc, G. Paltauf, E. Eds., Phospholipids characterization, metabolism, and novel biological applications, AOCS press, Champaign 1995, 191.
- Planas, M. E., Gonzalez, P., Rodriguez, S., Sanchez, G. and Cevc, G., Anesth. Analg., 1992, 95, 615.
- Cevc, G., Biochemistry , 1991a, 30/29, 7186.
- Cevc, G. and Blume, G., Biochem. Biophys. Acta, 1992, 1104, 226.
- El-Maghraby, G.M.M., Williams, A.C. and Barry, B.W., J. Pharm.Pharmacol., 1999, 51, 1123.
- El-Maghraby, G.M.M., Williams, A.C. and Barry, B.W., Int. J.Pharm., 2000, 196, 63.
- Cevc, G., Grbauer, D., Schatzlein, A. and Blume, G., Biochem.Biophys. Acta, 1998, 1368, 201.
- Fry, D.W., White, J.C. and Goldman, I. D., J. Anal. Biochem., 1978, 90, 809.
- New, R.R.C., “Liposomes: A practical approach”, Oxford University Press, Oxford, 1990, 1.
- Gamal, M., El Maghraby, M., Williams, A.C. and Barry, B.W., J.Pharm. Pharmacol., 1999, 51, 1123.
- Schatzlein, A. and Cevc, G., Brit. J. Dermatol., 1998, 138, 583.
- Cevc, G. and Marsh, D., Phospholipid bilayers physical principles and models. Wiley Intersciences, New York, 1995a, 235.
- W. J., Water, K. A., “Prediction of percutaneous penetration”, Vol. 3b, STS Publishing, Cardiff, 1993, 226.
- Cevc, G., Schatzlein, A. and Blume, G., J. Control. Release, 1995b, 36, 3.
- Cevc, G., Grbauer, D., Schatzlein, A., Blume, G. and Paul, A., Adv.Drug Del. Rev., 1996, 18, 349.
- Hadgraft, J. and Guy, R.H. (Eds), “Transdermal drug delivery: Development issues and research initiative”, Marcel Dekker, New York, 1989, 1.
- Paul, A. and Cevc, G., Vaccine Research, 1995, 4, 145
- Paul, A., Cevc, G. and Bachhawat, B. K., Eur. J. Immunol, 1995, 25, 3521.
- Cevc, G., Biochem. Biophys. Acta., 1990, 1031, 311.
- Cevc, G. In; Gregoriadis, G. Ed., “Liposome Technology”, CRC Press, Boca Raton, FL, 1992b, 1.
- Hafer, C., Goble, R., Deering, P., Lechmer, A. and Breut, J., Anticancer Research, 1999, 19, 1505.
- Davis, A. F. and Hadgraft, J., Int. J. Pharm., 1991, 76, 1.
- Cevc, G., Crit. Rev. Ther. Drug Carrier Syst., 1996, 13, 257.
- Jain, S., Jaio, N., Bhadra, D., Tiwari, A. K. and Jain, N. K., CurrentDrug Delivery , 2(3), 2005, 223.
- Essa, E. A., Bonner, M. C. and Barry, B. W., Int. J. Pharm., 2002, 240, 55.
- Honeywell-Nguyen, P.L., De Graff, A.M., Groenink, H.W.W. and Bouwstra, A.J., Biochim. Biophys. Acta., 2002, 1573, 130.
- Honeywell-Nguyen, P.L. and Bouwstra, A. .J., J. Control.Release, 2003, 86, 145.
- Touitou, E., Dayan, N., Bergelson, L., Godin, B. and Eliaz, M., J.Control. Release, 2000, 65, 403.
- Jain, S., Umamahashwari, R. B., Bhandra, D., Tripathi, P., Jain, P. and Jain, N.K., Indian J. Pharm. Sci., 2003, 65, 223.
- Jain, S., Jain, S. and Jain, N.K., In; Proceedings of 28th Conference of CRS, USA, 2001, 5207.
- Guo, J., Ping, Q., Sun, G. and Jiao, C., Int. J. Pharm., 2000, 194, 201.
- Vaizoglu, O. and Speiser, P. P., Acta Pharm Suec., 1986, 23, 163.
- Mantelli, S., Speiser, P. and Hauser, H., Chem. Phys. Lipids, 1985, 37, 329.
- Leo, A. and Hansche, F. D., Chem. Rev., 1971, 71, 525.
- Yadav, J. B., Advanced practical physical chemistry, Goel Publishing house, Meeruth, India, 1990, 74.
- Steve, A., U. S. patent US S, 534, 499 (C1 S14-25, A61K31/70), 1996, 9july, p 11.
- Taskintuna, I., Banker, A. S., Flores-Aguilar, M., Lynn, B. G., Alden, K. A., Hostetler, K. Y. and Freeman, W. R., Retina, 1997, 17, 57.
- Vingerhoeds, M. H., Haisma, H. J., Belliot, S.O., Smit, H.P., Crommelin, D.J.A. and Storm, G., Pharm. Res., 1996, 13, 604.
- Huckriede, A., Bungener, L., Daemen, T. and Wilschut, J., MethodsEnzymol., 2003, 373, 74.
- Babizhayev, M.A., Arch. Biochem. Biophys., 1988, 266, 446.
- Blume, G. and Cevc, G., Biochim. Biophys. Acta, 1993 , 1146, 157.
- Kretschmar, M., Amselem, S., Zawoznik, E., Mosbach, K., Dietz, A., Hof, H. and Nichterlein, T., Mycoses, 2001, 44, 281.
- Vyas, S.P., Jaitely, V. and Kanuja, P., Indian J. Exp. Biology, 1997, 35, 212.
- Khopade, A.J., Jain, S. and Jain, N.K., Int. J Pharm., 2002, 241, 145.
- Godin, B. and Touitou, E., Crit. Rev. Ther. Drug CarrierSyst., 2003, 20, 63.
- Zhdanov, R.I., Podobed, O.V. and Vlassov, V.V., Bioelectrochemistry, 2002, 58, 53.
- Petit-Frere, C., Clingen, P.H., Grewe, M., Krutmann, J., Roza, L., Arlett, C.F. and Green, M.H., J. Invest. Dermatol., 1998 , 111, 354.
- Cuppoletti, J., Mayhew, E., Zobel, C.R. and Jung C.Y., Proc. Natil.Acad. Sci. USA, 1981, 78, 2786.
- Gornicki, A., Gen. Physiol. Biophys., 2003, 22,121.
- Logan, I.R, Sapountzi, V., Gaughan, L, Neal., D.E., Robson, C.N., J.Biol. Chem ., 2004, 279, 11696.
- Kisak, E.T., Coldren, B., Evans, C.A., Boyer, C. and Zasadzinski, J.A., Curr.Med. Chem., 2004, 11, 199.
- Krishnan, L., Sal, S., Patil, G.B. and Sprott, G.D., J. Immunol., 2001, 166, 1885.