- *Corresponding Author:
- K. Pavani Bharati
Department of Pharmacy Practice, JNTUK, Vizianagaram - 535 003, India
E-mail: pavani.craze@gmail.com
| Date of Submission | 13 July 2010 |
| Date of Revision | 10 January 2011 |
| Date of Acceptance | 15 January 2011 |
| Indian J Pharm Sci, 2011, 73 (1): 7-16 |
Abstract
Most commonly inherited bleeding disorder, first described in Aland Islands by Erik von Willebrand. It occurs as a result of decrease in plasma levels or defect in von Willebrand factor which is a large multimeric glycoprotein. Monomers of this glycoprotein undergo N-glycosylation to form dimers which get arranged to give multimers. Binding with plasma proteins (especially factor VIII) is the main function of von Willebrand factor. The disease is of two forms: Inherited and acquired forms. Inherited forms are of three major types. They are type 1, type 2, and type 3; in which type 2 is sub-divided into 2A, 2B, 2M, 2N. Type 1 is more prevalent than all other types. Mucocutaneous bleeding is mild in type 1 whereas it is mild to moderate in types 2A, 2B, and 2M. Type 2N has similar symptoms of haemophilia. The pathophysiology of each type depends on the qualitative or quantitative defects in von Willebrand factor. The diagnosis is based on von Willebrand factor antigen, von Willebrand factor activity assay, FVIII coagulant activity and some other additional tests. Results should be analyzed within the context of blood group. von Willebrand factor multimer analysis is essential for typing and sub typing the disease. The management of the disease involves replacement therapy, non-replacement therapy and other therapies that include antifibrinolytics and topical agents.
Keywords
ADAMTS, classification, diagnosis, pathophysiology, treatment, von Willebrand factor, von Willebrand disease
When a blood vessel is injured and starts bleeding, platelets together with some clotting factors form a plug at the region of injury. As a result, the blood vessel stops bleeding. The plasma protein which allows or helps the platelets to stick with each other and form a clump is the von Willebrand factor (VWF). It also carries factor VIII. When there is a decrease in plasma levels or defect in the von Willebrand factor, the ability of the blood to clot decreases leading to a heavy and continuous bleeding after an injury which is termed as von Willebrand disorder or disease (VWD). This may cause internal organ damage and rarely may lead to death [1].
VWD is the most commonly inherited bleeding disorder [2-4]. Although it is a form of haemophilia which is also a clotting disorder, haemophilia is mostly due to the deficiency of clotting factors. For instance, haemophilia A is due to factor VIII deficiency and haemophilia B is due factor IX deficiency. VWD is milder and common when compared to haemophilia [5].
History [6]
It is named after the Finnish doctor, Erik von Willebrand (1870-1949). He is the first to describe the hereditary bleeding disorder in the families in Aland Islands. He could not identify the actual cause for the disorder but was able to distinguish it from haemophilia and other bleeding disorders.
Von Willebrand Factor
Von willebrand factor (VWF) is a large multimeric glycoprotein present in plasma. It is synthesized in Weibel-Palade bodies in endothelium, α-granules of platelets (megakaryocytes) and sub-endothelial connective tissue [7].
Structure
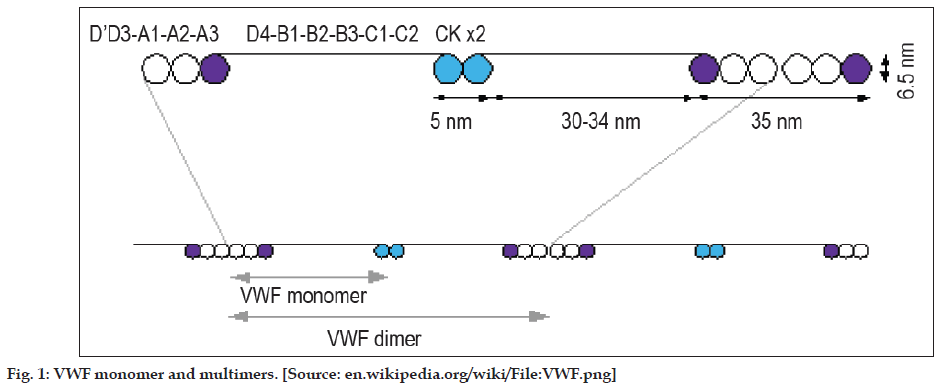
Each monomer of VWF contains 2050 amino acids with specific domains possessing specific functions [7]. These monomers undergo N-glycosylation in the endoplasmic reticulum leading to the formation of dimmers, Figure 1. These dimers in turn are arranged into multimers by the cross-linking of cysteine residues with the help of disulphide bonds. Formation of multimers takes place in Golgi apparatus. Each of these multimers consists of about 80 subunits 250kDa each. During the synthesis of VWF, large multimers and some cleavage products are also produced. Of these, only the larger multimers are functional whereas the cleavage products have no functional capacity [7]. Functions of each domain are tabulated in Table 1.
| Domains in a monomer | Function |
|---|---|
| D'/D3 | Binds to factor VIII |
| A1 | Binds to platelet GPIb receptor, heparin, possibly collagen. |
| A3 | Collagen |
| C1(RGD domain) | Binds to platelet integrin αIIbβ3 when activated. |
| Cysteine knot domain | With PDGF, TGFβ, βHCG |
Table 1: Functions of Each Domain
GPIb is platelet glycoprotein Ib complex, RGD is arginine-glycine-aspartate amino acid sequence in VWF, PDGF is platelet-derived growth factor, TGFβ is transforming growth factor-β and βHCG is β-human chorionic gonadotropin.
Functions
As already mentioned, the specific domains present in it are responsible for its functions. The main function is to bind with plasma proteins, especially factor VIII and coagulate blood [7]. Factor VIII in its inactive state binds to VWF in the circulation. If it is unbound, it rapidly degrades. When VWF is exposed in endothelium during an injury to blood vessel, it binds to collagen. When coagulation is stimulated, the platelet receptors get activated. VWF binds to these activated receptors. VWF binds to platelet glycoprotein Ib (GPIb) receptor when it forms a complex with glycoprotein IX (GPIX) and glycoprotein V (GPV). This occurs when there is a rapid flow in narrow blood vessels. Studies show that VWF uncoils and decelerate platelets under these conditions.
Catabolism
A disintegrin-like and metalloprotease domain with thrombospondin type 1 motifs (ADAMTS-13), a plasma metalloprotease breaks down VWF between tyrosine at position 842 and methionine at position 843 in A2 domain. As a consequence, the multimers are broken into smaller sub-units which can be degraded by other peptidases [8].
Classification
These are of two forms [4]. They are: Inherited forms and acquired form. Hereditary forms include three major types and a platelet type [4]. The three major forms are type 1, type 2, and type 3. The international society of thrombosis and homeostasis has classified VWD based on the definition of qualitative and quantitative defects [4]. According to this classification, type 2 VWD is again classified into four different types like type 2A, type 2B, type 2M and type 2N.
Epidemiology
The disease prevalence is about only 1%. More often, it can be detected in women based on the bleeding tendency during menstruation. The disease may be severe in people with ‘O’ blood group [9]. Type 1 includes [4] 60%-80% of the cases. Type 2 includes [4] 20-30%. Type 3 accounts [10] for less than 5% of all the cases. Acquired VWD occurs [10] most often in individuals over 40 years with no prior bleeding history.
Aetiology
It is an inherited disease where the parent carrying the gene may or may not be symptomatic. Type 1 and type 2 are inherited if the gene is passed on to the offspring from either of the parent. Type 3 is inherited only if the gene is passed from both the parents [1]. Acquired VWD is seen in patients with auto antibodies [4].
Clinical Manifestations
Children with VWD may have symptoms that are different from those of parent carrying the gene. It is the bleeding disorder that is commonly seen in women. Menorrhagia is seen in more than 70% of women with VWD and a half suffers from dysmenorrheal [11]. Different types of von Willebrand diseases have varying degrees of bleeding tendencies (nose bleeding, bleeding gums, easy bruising). Individual with type 3 VWD have a severe internal and joint bleeding, but this is very rare condition [1].
Typically type 1 VWD manifests mild mucocutaneous bleeding. Most common symptoms include bruising and epistaxis. Women experience a heavy menstrual bleeding in reproductive age and a heavy blood loss during delivery. If the VWF levels are lower than 15 IU/dl, the disease symptoms can be more severe [11,12]. Type 2A VWD individuals usually manifest mild to moderate mucocutaneous bleeding. Whereas type 2B VWD typically have mild to moderate mucocutaneous bleeding. Thrombocytopenia may be observed which becomes worsened during stress (severe infection/ surgery/pregnancy/if desmopressin is used). Like type 2B individuals, type 2M VWD also typically has mild to moderate mucocutaneous bleeding. When there is a low or absent VWF:RCo, the episodes of bleeding can be severe. Type 2N VWD symptoms are similar to those of mild hemophilia A which includes excessive bleeding at the time of surgery [10]. Acquired VWD individuals also present with mild to moderate bleeding [13,14].
Pathophysiology
VWF is active only in high blood flow condition and shear stress. Hence the organs with extensive small vessels such as skin, uterus, and gastrointestinal tract show deficiency of the factor. The pathophysiology of different forms of VWD can be given as followed.
Inherited forms
Type 1
It is a partial quantitative defect but the clotting impairment may not be seen clearly. Genetic changes in VWF are common in severe cases whereas in milder cases of type 1 VWD, complex spectrum of molecular pathology together with polymorphisms of VWF gene may be seen [15]. Individuals with type 1 VWD lead a normal life though they have low levels of VWF. These low levels are due to mutations that affect the gene expression. As a result of mutations, the intracellular transportation of VWF sub-units is impaired leading to severe, dominantly inherited type 1 VWD [16,17].
Type 1 VWD can also be caused by rapid clearance of VWF from the plasma of affected individuals. This decreases the cleavage time of circulating VWF multimer by ADAMTS-13. As a result, clearance shifts multimer distribution in plasma towards those that are initially secreted by the endothelial cells [18,19].
Bleeding tendency is mainly because of decreased levels of VWF. There is normal distribution of the high molecular weight multimers. Laboratory findings reveal that the ratio of activity of VWF and its antigen is proportionately decreased [20].
Type 2
It is a qualitative defect where there is no change in plasma VWF levels but characterized by a structural and functional defects based on which, it is further sub-divided into four types.
Type 2A
It is characterized by a decreased VWF mediated platelet adhesion. This is usually because of deficiency of high molecular weight multimers in the circulation. The deficiency of large multimers arises as a result of defective multimer assembly or increased cleavage of the multimers by ADAMTS-13 [21]. Defects in multimer assembly may occur due to homozygous or heterozygous mutations. These mutations result in the prevention of multimerisation in the Golgi apparatus [22-24]. The activity of ristocetin co-factor is low when compared to that of von Willebrand antigen [10].
Type 2B
It is characterized by a decreased level of large multimers in the plasma and a markedly increased proteolysis [25,26]. Like in type 2A, the proportion of ristocetin co-factor activity is lower [10] even in type 2B but the proteolytic activity does not affect the multimerisation in Golgi apparatus. The mutations that cause type 2B do not impair the multimer assembly but the multimers, after their secretion, get bound to the platelets after which they become cleaved by ADAMTS-13. These small multimers do not mediate effective platelet adhesion and inhibits directly the interaction of platelets with connective tissue [27].
Type 2M
It includes qualitative variants in which VWF dependant platelet adhesion is decreased without any deficiency of high molecular weight VWF multimers. The secretion and assembly of the multimers is almost normal. The mutations bring about a defect in the functions and result in the impairment of VWF binding to platelets. This ultimately leads to a decreased exposure of VWF to ADAMTS-13, thereby preserves the distribution of large multimers similar to that initially secreted from endothelial cells [28]. VWF:RCo is disproportionately low when compared to VWF:Ag in most of the patients with type M VWD [10].
Type 2N
In this, the variants have a marked decline in the binding affinity for factor VIII. The mutations that impair the binding affinity may be homozygous or compound heterozygous. In certain cases, both the alleles of VWF may have factor VIII binding mutations. But in most of the cases of type 2N, only one of the two alleles has the mutation while the other may express a little or no mutation [29]. In type 2N, the level of factor VIII is lower when compared to the VWF:Ag. This led to misdiagnosis as haemophilia A. The patients should be suspected only if they have clinical symptoms of hemophilia A with an autosomal rather than x-linked inheritance [4,30,31].
Type 3
It is caused by recessive mutation which leads to undetectable VWF level. Hence it is often termed as a severe form [29]. The mutations that usually cause type 3 VWD are missense and nonsense mutations [32]. It is characterized by severe mucosal bleeding with no detectable VWF antigen [4].
Acquired VWD
In this, the function of VWF is not inherited but its antibody complex is rapidly cleared from the circulation. It has a diverse pathology. VWF normally produced and removed from the circulation by tumor cell adhesion or VWF antibody-mediated large multimer disruption or protein digestion gradually [13,33]. Patients with aortic stenosis may develop VWD and may have gastrointestinal bleeding [4].
Diagnosis
Type 1 and type 2 people do not have major bleeding problems. Hence, the early diagnosis is difficult. Whereas, early diagnosis is easy in type 3 people as they have severe bleeding problems since infancy [1].
The diagnosis is established based on the personal and/or family history of abnormal bleeding and diagnostic test results. The screening tests like bleeding time and platelet function analyzer (PFA-100®, Dade Behring, Deerfield, I11) are less sensitive [21,34]. Diagnosis is mainly based on VWF activity assay (VWF:RCo), reduced VWF antigen (VWF:Ag), and FVIII coagulant activity (FVIII:C) [35].
The various tests [36] that are included in the diagnosis of VWD are: Bleeding history, total blood count, VWD profile testing (VWF:Ag, VWF:RCo, FVIII:C), ABO blood group. Optional tests if initial test results suggest VWD include: VWF multimer analysis, VWF:CBA, VWF:FVIIIB, RIPA, Genetic tests.
Bleeding history
It can be considered to be supportive for VWD when the patient has three different hemorrhagic symptoms or when the bleeding score is more than 3 (males) or 5 (females) [37,38]. Scoring systems and the importance of assessing the bleeding history and the chance of having VWD (especially type 1) have yet to be studied outside of defined population [35,37].
Total blood count
This includes assessing hemoglobin, hematocrit, platelet count (PC) and morphology, prothrombin time (PT), activated partial thromboplastin time (aPTT). Fibrinogen level or thrombin time (TT) can be measured optionally [39]. Usually, individuals with VWD have a normal thrombin time, prothrombin time, platelet count and fibrinogen level. Some individuals have a prolonged aPTT which is consistent with VWD whereas, it may be normal in mild or moderate cases [40,41]. Depending upon the type and severity of the disease, the hemoglobin and hematocrit levels vary from normal to decreased [42].
Platelet count and prothrombin time
In most of the individuals with VWD, the platelet count is normal whereas it is decreased in type 2 VWD patients [36]. With the use of PFA-100®, platelet counts yield a low closure time (CT). Closure time is the time taken by the platelets to occlude a hole impregnated with collagen or epinephrine. With this, one can assess the platelet adhesion or aggregation [43]. Majority of the individuals with VWD other than type 2N, have abnormal PFA-100® results. Its use for screening populations for VWD has not yet been established [34,44-46]. Patients with severe type 1 and type 3 VWD show abnormal PFA-100® results. On the other hand, mild to moderate type 1 VWD patients and some type 2 cases do not show abnormal results [47-49]. The individuals with VWD have a normal prothrombin time [10].
Activated partial thromboplastin time
The activated partial thromboplastin time is normal often. It may be prolonged incase of reduced levels of FVIII. The deficiency of FVIII is secondary to the deficiency of VWF. In type 1 VWD, the FVIII levels in plasma are slightly higher than VWF and may fall in the normal range. In type 2 individuals, other than type 2N, FVIII levels are 2-3 times higher than VWF. However, it is decreased in type 2N individuals. In type 3 individuals, the plasma levels of FVIII are below 10 IU/dl [50,51]. The normal FVIII levels in plasma are 50-150 IU/dl approximately [10].
VWD profile testing
This includes three tests which help in measuring the plasma levels of VWF (VWF:Ag), function of the VWF protein which is present as ristocetin cofactor activity (VWF:RCo) and the ability of the VWF to serve as carrier protein to maintain normal survival of FVIII. All these levels are markedly decreased or absent in type 3 VWD [36].
VWF:Ag (VWF antigen)
In this, the plasma concentration of VWF is measured by using methods like enzyme-linked immunosorbent assay (ELISA) or automated latex immunoassay (LIA). Results should be expressed in international units (IU), either as IU/dl or IU/ml [39]. Normal range of VWF:Ag is 50-200 IU/dl [10]. In type 1, 2A, 2B individuals, the levels are decreased whereas they may be normal or decreased in the case of type 2M. In type 2N VWD, VWF:Ag levels are normal [36].
VWF:RCo (ristocetin cofactor activity assay)
It is a function assay in which the ability of VWF to agglutinate with normal platelets is measured. This interaction between the VWF and the normal platelets is initiated by an antibiotic, ristocetin. Use of this antibiotic in clinical trials has been stopped as it causes thrombocytopenia. But in laboratory tests, it is still used since it is the most widely accepted functional test for VWF [39]. Normal range is 50-200 IU/dl [10]. The levels are decreased in type 1, 2A, 2B VWD and found to be normal in case of type 2N individuals. They may be normal or decreased in type 2M individuals [36].
FVIII:C (FVIII coagulant assay)
It is a functional FVIII assay [10] which is used to measure the ability of VWF to serve as a carrier protein for FVIII. Its normal range is 50-150 IU/ dl [10]. Its value may be decreased or normal in VWD. While it is always decreased in type 2N and type 3 VWD [36].
ABO blood group
Several studies have been conducted to know the influence of ABO blood group on plasma levels of VWF [52-54]. It was found that 66% of the variation in the plasma levels of VWF was determined genetically in which 30% of the genetic component was explained by ABO blood group [55]. Individuals with O group have lowest VWF levels while AB group individuals have highest levels. The influence of these blood groups on the plasma levels of VWF make it difficult to diagnose type 1 VWD since the normal range of VWF:Ag in O group individuals is below 50 IU/dl, which is generally considered as the lower normal limit [56]. The VWF:RCo level in individuals with O blood group is significantly lower than those in the non-O group [57].
VWF multimer analysis
Analyzing the multimer distribution is necessary for typing and sub-typing of VWD. The assay is laborious which involves protein electrophoresis followed by radioactive or immunofluorescent detection of the multimers on the gel [19,58]. This test is not done alone with the initial screening tests unless there is sufficient initial information which suggests VWD [21]. The multimer assays are designated as low resolution and high resolution. Low resolution systems differentiate larger multimers from intermediate and smaller multimers; whereas, high resolution systems differentiate each multimer band of smaller multimers into 3-8 satellite bands. Low resolution gel systems are primarily used to differentiate type 2 VWD variants from those of type 1 or 3. All sizes of multimers can be observed in type 1 VWD plasma. On the other hand, type 3 VWD plasma does not show any multimer distribution. Type 2A VWD plasma comprises only smaller multimers whereas type 2B comprises only larger multimers [39].
VWF:CBA (VWF collagen-binding assay)
This helps in measuring the binding of VWF to collagen. The A3 domain of VWF is the primary site of collagen binding. This assay depends upon the size of VWF multimers. Larger multimers bind more avidly than smaller. The performance of the assay, sensitivity to detect the disease and discrimination among subtypes depends on the source of collagen [59,60]. The VWF:CBA along with the assays of VWF:RCo and VWF:Ag improves the differentiation of type 1 from type 2A, 2B or 2M VWD [61,62].
VWF:FVIIIB (VWF:FVIII binding assay)
This assay detects the factor VIII binding defect on VWF [63]. In other words, it is used in measuring the ability of VWF to bind to the exogenously added factor VIII. This assay is used to diagnose type 2N VWD [30,64]. The amount of factor VIII bound is estimated by using chromogenic FVIII assay. This is then directly related to the individual’s VWF. In type 2N (homozygous or compound heterozygous), the VWF in the circulation does not bind with FVIII normally and hence the amount of FVIII is decreased [39].
RIPA (ristocetin-induced platelet aggregation)
It is mainly used to diagnose type 2B VWD [10,39]. It is generally done using low concentration ristocetin (usually <0.6 mg/ml). At this low concentration, ristocetin does not cause VWF binding and platelet aggregation. If it does cause, it shows that the respective individual has either type 2B or mutations in the platelet VWF receptor. The latter is termed as plate-type VWD or pseudo VWD and it can be differentiated from type 2B by VWF:PB (VWF:platelet binding) assay. In individuals with type 3 VWD, RIPA will be reduced at higher concentrations of ristocetin (1.1-1.3). This test is not sensitive enough to diagnose other types of VWD [39].
Genetic tests
Mutation analysis is used in identifying mutations in the VWF gene associated with types 2A, 2B, 2M, 2N and some other forms of type 1 and 3 VWD. It is useful in differentiating mild haemophilia A from type 2N. Mild haemophilia A has no VWF mutations and follows X-linked inheritance; whereas type 2N is due to VWF mutations and follows autosomal recessive inheritance. It is also used to differentiate type 2B from type 2M; type 2A from 2B [65,66]. Mutation analysis also helps in managing future pregnancies by determining the causative mutation in the families with type 3 VWD [36]. This analysis is less useful in diagnosing type 1 VWD which has a complex and variable genetic basis [66]. Most of the mutations in type 2B, 2M, and 2N VWD cluster in cDNA which directs the synthesis of specific regions of VWF [67]. Mutations cluster in A2 domain in common forms of type 2A VWD while they may be scattered throughout the gene in less common forms of type 2A [39].
Treatment
There are certain standard recommendations to guide therapy for VWD [68,69]. The mainstay of VWD treatment is the replacement of the deficient protein (VWF) [70]. The different therapies [71] used in treating VWD are: Non-replacement therapy, replacement therapy, and other therapies.
Non-replacement therapy, DDAVP (1-desamino-8- D-arginine vasopressin)
Desmopressin is a synthetic derivative of the antidiuretic, vasopressin. It is chemically known as 1-desamino-8-D-arginine vasopressin. Through its agonist effect on vasopressin V2 receptors, it stimulates the release of VWF from endothelial cells [72,73]. DDAVP increases the plasma concentration of VWF through cyclic AMP-mediated release of VWF from endothelial cell Weibel-Palade bodies [74]. FVIII levels are also increased but its storage and release mechanisms have not yet been elucidated fully [75,76]. Though DDAVP induces the release of tissue plasminogen activator (tPA), it is rapidly inactivated by plasminogen activator inhibitor (PAI-1) and so fibrinolysis or bleeding does not appear to be promoted after treatment by DDAVP [71].
It can be given intravenously or intranasally or can also be administered subcutaneously if available [71]. Indicated for most of the type 1 patients, some type 2A patients [1]. The standard dosing of DDAVP is 0.3 mg/kg intravenously in 30-50 ml of normal saline over 30 min [75,77]. Subcutaneous doses are identical to i.v. dose. Nasal instillation (Stimate®) contains 150 μg per metered nasal puff (0.1 ml of a 1.5 mg/ml solution). Dose is one puff for those whose body weight is <50 kg and two puffs (one to each nostril) for those who are >50 kg weight [71]. It is not indicated for type 2B VWD as there was fall in the platelet count after its use [78]. It is not clinical use in type 3 VWD because there is no clinical relevant rise in FVIII or VWF:RCo activities [79]. Minor side effects include transient hyper or hypotension, headache or gastrointestinal upset, facial flushing [70,73].
Replacement therapy
Humate-P® and Alphanate SD/HT® are the plasmaderived concentrates to replace VWF [71]. These products should not be interchanged with one another as they are not identical and differ in the ratios of FVIII to VWF [80,81].
Humate-P® is administered intravenously and is indicated for patients who cannot tolerate desmopressin or patients who need prolonged treatment. It can also be used in any variant of type 2 disease and severe type 3 cases [1]. When reconstituted at the recommended volume, each milliliter of the product contains 50-100 IU/ml VWF:RCo and 20-40 IU/ml FVIII activity [82]. Alphanate SD/HT®, upon reconstitution to the recommended volume, each milliliter of the product contains 40-180 IU/ml FVIII activity and not less than 16 IU/ml VWF:RCo activity [71]. Adverse reactions include urticaria, chest tightness, rash, pruritus and edema [83].
Other therapies, antifibrinolytics
Aminocaproic acid and tranexamic acid are the antifibrinolytics use in the VWD therapy. They act by inhibiting the conversion of plasminogen to plasmin and thereby inhibit fibrinolysis. Thus, they stabilize the clots that have formed [84]. These drugs can be given orally or intravenously to treat mild mucocutaneous bleeding in individuals with VWD. Adult dose of aminocaproic acid is 4-5 g as a loading dose given orally or intravenously 1 h before invasive procedures. It is then followed by 1 g/h given orally or intravenously or 4-6 g for every 4-6 h orally until bleeding is controlled or for 5-7 days post-operatively [70]. The total daily dose should not exceed 24 g/24 h to minimize potential side effects. Children require weight-based dosing which can also be used in adults (50-60 mg/kg) [70,85]. Tranexamic acid is given intravenously at a dose of 10 mg/kg every 8 hours [70]. Both drugs cause nausea, vomiting and rarely thrombotic complications [71].
Topical agents
Topical bovine thrombin (Thrombin-JMI) is used as a topical agent in the case of minor bleeding from the capillaries and small venules. Fibrin sealant (Tisseel VH®) is another topical agent which is used in certain surgical situations, but it is ineffective in treating heavy arterial bleeding. It gives good results when used as an adjunct to haemostasis in dental surgery in individuals with VWD [86,87]. Topical collagen sponges are also used in controlling bleeding wounds [88].
Conclusions
There is an evolution in the direction of genetic testing for the management of families with hereditary bleeding disorders including VWD. Testing centers and counselors play a vital role in supporting the patients. In spite of scientific development in clinical research in bleeding disorders, still there is a need for skilled clinicians and laboratory scientists with expertise in haemostasis. Training opportunities have to be developed for haemostasis specialists.
References
- National heart, lung, and blood institute diseases and conditions index.von Willebrand disease. Available from: http://www.nhlbi.nih.gov/health/dci/Diseases/vWD/vWD_All.html [Last accessed on 2010 May 14].
- Sadler JE, Mannucci PM, Berntorp E, Bochkov N, Boulyjenkov V,Ginsburg D, et al. Impact, diagnosis and treatment of von Willebranddisease. Thromb Haemost 2000;84:160-74.
- Sadler JE, Gralnick HR. Commentary: A new classification for von Willebrand disease. Blood 1994;84:676-9.
- Sadler JE. A revised classification of von Willebrand disease. Forthe subcommittee on von Willebrand Factor of the scientific andstandardization committee of the International society on thrombosisand haemostasis. Thromb Haemost 1994;71:520-5.
- Favaloro EJ, Soltani S, McDonald J. Potential Laboratory Misdiagnosisof Haemophilia and von Willebrand disorder owing to cold activationof blood samples for testing. Am J Clin Pathol 2004;122:686-92.
- von Willebrand EA. Hereditary pseudohaemophilia [English translation].Haemophilia 1999;5:223-31.
- Sadler JE. Biochemistry and genetics of von Willebrand factor. AnnuRev Biochem 1998;67:395-424.
- Levy GG, Motto DG, Ginsburg D. ADAMTS 13 turns 3. Blood2005;106:11-7.
- Anonymous molecular basis of von Willebrand disease and its clinicalimplications. Haematologica 2004;89:1036.
- Goodeve AC, James P. Von Willebrand Disease. Available from: http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=geneandpart=vonwillebrand [Last accessed on 2010 June 14].
- Kadir RA, Chi C. Women and von Willebrand disease: Controversiesin diagnosis and management. Semin Thromb Hemost 2006;32:605-15.
- James P, Lillicrap D. Genetic testing for von Willebrand disease: TheCanadian experience. Semin Thromb Hemost 2006;32:546-52.
- Federici AB. Acquired von Willebrand syndrome: An underdiagnosedand misdiagnosed bleeding complication in patients withlymphoproliferative and myeloproliferative disorders. Semin Hematol 2006;43:s48-58.
- Nichols WL, Hultin MB, James AH, Manco-Johnson MJ, MontgomeryRR, Ortel TL, et al. von Willebrand disease (VWD): Evidence-baseddiagnosis and management guidelines, the National Heart, Lung, andBlood Institute (NHBLI) Expert panel report (USA). Haemophilia2008;14:171-232.
- James PD, Notley C, Hegadorn C, Leggo J, Tuttle A, Tinlin S, et al.The mutual spectrum of type 1 von Willebrand disease: Results fromCanadian cohort study. Blood 2007;109:145-54.
- Eikenboom JC, Matsushita T, Reitsma PH, Tuley EA, Castaman G,Briet E, et al. Dominant type 1 von Willebrand disease caused bymutated cysteine residues in the D3 domain of von Willebrand factor.Blood 1996;88:2433-41.
- Bodó I, Katsumi A, Tuley EA, Eikenboom JC, Dong Z, SadlerJE. Type 1 von Willebrand disease mutation Cys1149Arg causesintracellular retention and degradation of heterodimers: A possiblegeneral mechanism for dominant mutations of oligomeric proteins.Blood 2001;98:2973-9.
- Mannucci PM, Lombardi R, Castaman G, Dent JA, Lattuada A,Rodeghiero F, et al. von Willebrand disease “Vicenza” with largerthan-normal (supranormal) von Willebrand factor multimers. Blood1998;71:65-70.
- Studt JD, Budde U, Schneppenheim R, von Depka Prondzinski M,Ganser A, Barthels M. Quantification and facilitated comparision ofvon Willebrand factor multimer patterns by densitometry. Am J ClinPathol 2001;116:567-74.
- Triplett DA. Laboratory diagnosis of von Willebrand’s disease. MayoClin Proc 1991;66:832-40.
- Favaloro EJ. Appropriate laboratory assessment as a critical facet inthe proper diagnosis and classification of von Willebrand disorder. BestPract Res Clin Haematol 2001;14:299-319.
- Gaucher C, Die’val J, Mazurier C. Characterization of von Willebrandfactor gene defects in two unrelated patients with type IIC vonWillebrand disease. Blood 1994;84:1024-30.
- Schneppenheim R, Thomas KB, Krey S, Budde U, Jessat U, Sutor AH,et al. Identification of a candidate missense mutation in a family withvon Willebrand disease type IIC. Hum Genet 1995;95:681 6.
- Ruggeri ZM, Nilsson IM, Lombardi R, Holmberg L, Zimmerman TS.Aberrant multimeric structure of von Willebrand factor in a new variantof von Willebrand’s disease (type IIC). J Clin Invest 1982;70:1124-7.
- Zimmerman TS, Dent JA, Ruggeri ZM, Nannini LH. Subunitcomposition of plasma von Willebrand factor. Cleavage is present innormal individuals, increased in IIA and IIB von Willebrand disease,but minimal in variants with aberrant structure of individual oligomers(types IIC, IID, and IIE). J Clin Invest 1986;77:947-51.
- Ruggeri ZM, Pareti FI, Mannucci PM, Ciavarella N, Zimmerman TS. Heightened interaction between platelets and factor VIII/von Willebrandfactor in a new subtype of von Willebrand’s disease. N Eng J Med1980;302:1047-51.
- Lankhof H, Damas C, Schiphorst ME, IJssdijk MJ, Bracke M, SixmaJJ, et al. Functional studies on platelet adhesion with recombinant vonWillebrand factor type 2B mutants R543Q and R543W under conditionsof flow. Blood 1997;89:2766-72.
- Ciavarella G, Ciavarella N, Antoncecchi S, De Mattia D, RanieriP, Dent J, et al. High-resolution analysis of von Willebrand factormultimeric composition defines a new variant of type I von Willebranddisease with aberrant structure but presence of all size multimers (typeIC). Blood 1985;66:1423-9.
- Sadler JE, Budde U, Eikenboom JC, Favaloro EJ, Hill FG, HolmbergL, et al. Update on the pathophysiology and classification of vonWillebrand disease: A report of the subcommittee on von Willebrandfactor. J Thromb Haemost 2006;4:2103-14.
- Schneppenheim R, Budde U, Krey S, Drewke E, Bergmann F, LechlerE, et al. Results of a screening for von Willebrand disease type 2Nin patients with suspected haemophilia A or von Willebrand diseasetype 1. Thromb Haemost 1996;76:598-602.
- Geil JD. Von Willebrand Disease. Available from: http://emedicine.medscape.com/article/959825-overview [Last accessed on 2010May 14].
- Eikenboom JC, Ploos van Amstel HK, Reitsma PH, Briet E. Mutationsin severe, type III von Willebrand’s disease in the Dutch population:Candidate missense and non sense mutations associated with reducedlevels of von Willebrand factor messenger RNA. Thromb Haemost1992;68:448-54.
- Franchini M, Lippi G. Acquired von Willebrand syndrome: An update.Am J Hematol 2007;82:368-75.
- Quiroga T, Goycoolea M, Munoz B, Morales M, Aranda E, Panes O,et al. Template bleeding time and PFA-100 have low sensitivityto screen patients with hereditary mucocutaneous hemorrhages:Comparative study in 148 patients. J Thromb Haemost 2004;2:892-8.
- Sadler JE, Rodeghiero F. ISTH SSC Subcommittee on von WillebrandFactor. Provisional criteria for the diagnosis of VWD type 1. J ThrombHaemost 2005;3:775-7.
- Pruthi RK. A practical approach to genetic testing for von WillebrandDisease. Mayo Clin Proc 2006;81:679-91.
- Rodeghiero F, Castaman G, Tosetto A. The discriminant power ofbleeding history for the diagnosis of type 1 VWD: An international,multicenter study. J Thromb Haemost 2005;3:2619-26.
- Tosetto A, Rodeghiero F, Castaman G. Impact of plasma VWF levelsin the diagnosis of type 1 VWD: Results from a multicenter Europeanstudy(MCMDM-1VWD). J Thromb Haemost 2007;5:715-21.
- National Heart, Lung, and Blood Institute, U.S. Department of Healthand Human services. The diagnosis, evaluation, and management of vonWillebrand disease. Available from: http://www.nhlbi.nih.gov/guidelines/vwd/3_diagnosisandevaluation.htm [Last accessed on 2010 July 1].
- Federici AB. Diagnosis of inherited von Willebrand disease: A clinicalperspective. Semin Thromb Haemost 2006;32:555-65.
- Favaloro EJ, Lillicrap D, Lazzari MA, Cattaneo M, Mazurier C, WoodsA, et al. von Willebrand disease: Laboratory aspects of diagnosis andtreatment. Haemophilia 2004;10(Suppl 4):164-8.
- Quest diagnostics. Von Willebrand disease. Laboratory support ofdiagnosis. Clinical focus. Available from: http://www.questdiagnostics.com/hcp/intguide/jsp/showintguidepage.jsp?fn=CF_vWD.htm#Additional Information for Tests Useful in von Willebrand Disease [Last accessedon 2010 July 1].
- Ledford-Kraemer MR, Editor. Clotting Times 2006;5:2-12. Availablefrom http://www.centralizeddiagnostics.cl/Literatura/docs/literatura/von%20Willebrands%20Disease%20CTSep2006%20.pdf [Last accessedon 2010 July 1].
- Cattaneo M, Federici AB, Lecchi A, Agati B, Lombardi R, Stabile F,et al. Evaluation of the PFA-100® system in the diagnosis andtherapeutic monitoring of patients with von Willebrand disease. ThrombHaemost 1999;82:35-9.
- Cattaneo M, Lecchi A, Agati B, Lombardi R, Zighetti ML. Evaluationof platelet function with the PFA-100 system in patients with congenitaldefects of platelet secretion. Thromb Res 1999;96:213-7.
- Fressinaud E, Veyradier A, Truchaud F, Martin I, Boyer-NeumannC, Trossaert M, et al. Screening for von Willebrand disease with anew analyzer using high shear stress: A study of 60 cases. Blood1998;91:1325-31.
- Dean JA, Blanchette VS, Carcao MD, Stain AM, Sparling CR,Siekmann J, et al. von Willebrand disease in a pediatric-basedpopulation. Comparision of type 1 diagnostic criteria and use of thePFA-100® and a von Willebrand factor/collagen-binding assay. ThrombHaemost 2000;84:401-9.
- Posan E, McBane RD, Grill DE, Motsko CL, Nicholas WL.Comparision of PFA-100® testing and bleeding time for detectingplatelet hypofunction and von Willebrand disease in clinical practice.Thromb Haemost 2003;90:483-90.
- Schlammadinger A, Kerenyi A, Muszbek L, Boda Z. Comparisionof the O’Brien filter test and the PFA-100® platelet analyzer in thelaboratory diagnosis of von Willebrand’s disease. Thromb Haemost2000;84:88-92.
- Kunicki TJ, Baronciani L, Canciani MT, Gianniello F, Head SR,Mondala TS, et al. An association of candidate gene haplotypes andbleeding severity in von Willebrand disease type 2A, 2B, and 2Mpedigrees. J Thromb Haemost 2006;4:137-47.
- Michiels JJ, van de Velde A, van Vliet HH, van der Planken M,Schroyens W, Berneman Z. Response of von Willebrand factorparameters to desmopressin in patients with type 1 and type 2congenital von Willebrand disease: Diagnostic and therapeuticimplications. Semin Thromb Haemost 2002;28:111-32.
- Gastineau DA, Moore SB. How important are ABO-related variationsin coagulation factor levels? Transfusion 2001;41:4-5.
- McCallum CJ, Peake IR, Newcombe RG, Bloom AL. Factor VIII levels and blood group antigens. Thromb Haemost 1983;50:757.
- Mohanty D, Ghosh K, Marwaha N, Kaur S, Chauhan AP, Das KC.Major blood group antigens - a determinant of factor VIII levels inblood? Thromb Haemost 1984;51:414.
- Orstavik KH, Magnus P, Resiner H, Berg K, Graham JB, Nance W.Factor VIII and factor IX in a twin population: Evidence for a majoreffect of ABO locus on factor VIII level. Am J Hum Genet 1985;37:89-101.
- Gill JC, Endres-Brooks J, Bauer PJ, Marks WJ Jr, Montgomery RR.The effect of ABO blood group on the diagnosis of von Willebranddisease. Blood 1987;69:1691-5.
- Moeller A, Weippert-Kretschmer M, Prinz H. Influence of ABO bloodgroup on primary hemostasis. Tranfusion 2001;41:56-60.
- Budde U, Pieconka A, Will K, Schneppenheim R. Laboratory testingfor von Willebrand disease: Contribution of multimer analysis todiagnosis and classification. Semin Thromb Haemost 2006;32:514-21.
- Favaloro EJ. Detection of von Willebrand disorder and identificationof qualitative von Willebrand factor defects: Direct comparision ofcommercial ELISA-based von Willebrand factor activity options. Am JClin Pathol 2000;114:608-18.
- Neugebauer BM, Goy C, Budek I, Seitz R. Comparision of twovon Willebrand factor collagen-binding assays with different bindingaffinities for low, medium, and high multimers of von Willebrandfactor. Semin Thromb Haemost 2002;28:139-48.
- Favaloro EJ, Bonar R, Kershaw G, Sioufi J, Hertzberg M, Street A,et al. Laboratory diagnosis of von Willebrand’s disorder: Quality anddiagnostic improvements driven by peer review in a multilaboratory testprocess. Haemophilia 2004;10:232-42.
- Favaloro EJ. Collagen binding assay for von Willebrand factor(VWF: CBA): Detection of von Willebrand disease (VWD), and thediscrimination of VWD subtypes, depends on collagen source. ThrombHaemost 2000;83:127-35.
- Nishino M, Girma JP, Rothschild C, Fressinaud E, Meyer W. Newvariant of von Willebrand disease with defective binding to factor VIII.Blood 1989;74:1591-9.
- Rodgers SE, Lerda NV, Favaloro EJ, Duncan EM, Casey GJ,Quinn DM, et al. Identification of von Willebrand disease type 2N(Normandy) in Australia: A cross-laboratory investigation using different methods. Am J Clin Pathol 2002;118:269-76.
- James P, Lillicrap D. The role of molecular genetics in diagnosing vonWillebrand disease. Semin Thromb Haemost 2008;34:502-8.
- Keeney S, Bowen D, Cumming A. The molecular analysis of vonWillebrand disease: A guideline from UK Haemophilia Centre Doctors’Organisation Haemophilia Genetics Laboratory Network. Haemophilia 2008;14:1099-111.
- Montgomery RR. Structure and function of von Willebrand factor. In:Colman RW, Hirsh J, Marder VJ, Clowes AW, George JN, editors.Hemostasis and thrombosis: Basic principles and clinical practice. 4thed. Philadelphia: Lippincott Williams and Wilkins; 2001. p. 249-74.
- Federici AB, Castaman G, Mannucci PM, for the Italian Associationof Haemophilia Centres (AICE). Guidelines for the diagnosisand management of von Willebrand disease in Italy. Haemophilia2002;8:607-21.
- Pasi KJ, Collins PW, Keeling DM, Brown SA, Cumming AM, DolanGC, et al. Management of von Willebrand disease: A guideline fromthe UK Haemophilia Centre Doctors’ Organisation. Haemophilia 2004;10:218-31.
- Mannucci PM. Treatment of von Willebrand disease. N Eng J Med2004;351:683-94.
- National Heart, Lung, and Blood Institute, U.S. Department of Healthand Human services. The diagnosis, evaluation, and management ofvon Willebrand disease. Available from: http://www.nhlbi.nih.gov/guidelines/vwd/4_managementofvwd.htm [Last accessed on 2010July 5].
- Kaufmann JE, Vischer UM. Cellular mechanisms of the haemostaticeffects of desmopressin (DDAVP). J Thromb Haemost 2003;1:682-9.
- Mannucci PM. Desmopressin (DDAVP) in the treatment of bleedingdisorders: The first 20 years. Blood 1997;90:2515-21.
- Kaufmann JE, Oksche A, Wollheim CB, Gunther G, Rosenthal W,Vischer UM. Vasopressin-induced von Willebrand factor secretionfrom endothelial cells involves V2 receptors and cAMP. J Clin Invest 2000;106:107-16.
- Mannucci PM, Ruggeri ZM, Pareti FI, Capitanio A. 1-Deamino-8-d-arginine vasopressin: A new pharmacological approach to themanagement of haemophilia and von Willebrand’s disease. Lancet 1977;1:869-72
- Montgomery RR, Gill JC. Interactions between von Willebrand factorand factor VIII: Where did they first meet? J Pediatr Haematol Oncol2000;22:269-75.
- de la Fuente B, Kasper CK, Rickles FR, Hoyer LW. Response ofpatients with mild and moderate haemophilia A and von Willebrand’sdisease treatment with desmopressin. Ann Intern Med 1985;103:6-14.
- Holmberg L, Nilsson IM, Borge L, Gunnarsson M, Sjorin E. Platelet aggregation induced by 1 deamino-8-D-arginine vasopressin (DDAVP)in type IIB von Willebrand’s disease. N Eng J Med 1983;309:816-21.
- Federici AB, Mazurier C, Berntorp E, Lee CA, Scharrer I, GoudemandJ, et al. Biological response to desmopressin in patients with severetype 1 and type 2 von Willebrand disease: Results of multicenter European study. Blood 2004;103:2032-8.
- Agrawal YP, Dzik W. The VWF content of factor VIII concentrates.Transfusion 2001;41:153-4.
- Chang AC, Rick ME, Ross PL, Weinstein MJ. Summary of a workshopon potency and dosage of von Willebrand factor concentrates.Haemophilia 1998;4(Suppl 3):1-6.
- Dobrkovska A, Krzensk U, Chediak JR. Pharmacokinetics, efficacyand safety of Humate-P in von Willebrand disease. Haemophilia1998;4(Suppl 3):33-9.
- Lillicrap D, Poon MC, Wlaker I, Xie F, Schwartz BA. Efficacy andsafety of the factor VIII/von Willebrand factor concentrate, Haemate-P/Humate-P: Ristocetin cofactor unit dosing in patients with vonWillebrand disease. Thromb Haemost 2002;87:224-30.
- Miller RA May MW, Hendry WF, Whitfield HN, Wickham JE. The prevention of secondary haemorrhage after prostatectomy: The valueof antifibrinolytic therapy. Br J Urol 1980;52:26-8.
- Mannucci PM. Haemostatic drugs. N Eng J Med 1998;339:245-53.
- Federici AB, Sacco R, Stabile F, Carpenedo M, Zingaro E, MannucciPM. Optimising local therapy during oral surgery in patients with vonWillebrand disease: Effective results from a retrospective analysis of 63cases. Haemophilia 2000;6:71-7.
- Rakocz M, Mazar A, Varon D, Spierer S, Blinder D, Martinowitz U.Dental extractions in patients with bleeding disorders. The use of fibringlue. Oral Surg Oral Med Oral Pathol 1993;75:280-2.
- Zwischenberger JB, Brunston RL Jr, Swann JR, Conti VR. Comparisionof two topical collagen-based haemostatic sponges during cardiothoracicprocedures. J Invest Surg 1999;12:101-6.