- *Corresponding Author:
- N. Lin
Center for Theory of Traditional Chinese Medicine, Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing, China
E-mail: linna888@163.com
| Date of Submission | 06 October 2012 |
| Date of Revision | 02 March 2013 |
| Date of Acceptance | 10 March 2013 |
| Indian J Pharm Sci 2013;75(3):270-276 |
Abstract
Coptidis Rhizoma (Coptis chinensis) has been reported to have antioxidative effect on hemolysis of erythrocytes induced by acetylphenylhydrazine in mice and rats. However, the ability of Coptidis Rhizoma to protect structure and function of erythrocytes membrane and morphology of erythrocytes against oxidative damage remains unknown. In this study, we undertook a characterization of antioxidative activity in erythrocytes membrane of Coptidis Rhizoma using an in vivo model of acetylphenylhydrazine-induced mice together with in vitro studies with 2,2-azo-bis (2-amidinopropane) dihydrochloride-induced erythrocytes for further morphology characterization. Acetylphenylhydrazine-induced mice were treated intragastrically with Coptidis Rhizoma at doses of 0.3, 0.6, and 1.2 g/kg per day for 3 days and at the dose of 0.6 and 1.2 g/kg it showed that there was an increasing trend in membranes cytoskeletal proteins of band I-IV, especially a significant upregulation in band II. Significant increase in phosphatidylserine and phosphatidylcholine content at the dose of 1.2 g/kg Coptidis Rhizoma was obsereved. At all doses of Coptidis Rhizoma, the declined membrane fluidity of acetylphenylhydrazine-induced mice was significantly increased. In addition, at the dose of 1.2 g/kg Coptidis Rhizoma treatment showed a significant increase in Ca 2+ /Mg 2+ -ATPase activity and there was an increasing trend in the activity of Na + /K + -ATPase. In vitro, Coptidis Rhizoma protected erythrocytes from 2,2-azo-bis (2-amidinopropane) dihydrochloride-induced hemolysis in a dose-dependent manner at concentrations of 0.25-1.5 mg/ml, and also significantly inhibited the 2,2-azo-bis (2-amidinopropane) dihydrochloride-induced morphological alterations in mice erythrocytes. These results demonstrate that Coptidis Rhizoma is capable of protecting erythrocytes against oxidative damage probably by acting as an antioxidant and maintaining membrane integrity.
Keywords
Coptidis rhizoma, erythrocytes membranes, hemolysis, oxidative damage
Growing evidence suggests that oxidative damage to cell components may play an important role in the alteration of normal physiological processes and development/progression of many human diseases [1]. Plasma membrane is a dynamic organelle system tightly controlling cellular structure and function. Its structure and functions are susceptible to alterations as a consequence of interactions with xenobiotics. A well documented example is malondialdehyde (MDA), a highly reactive and bifunctional molecule as the characterized product of the lipid peroxidation of erythrocytes, which is shown to crosslink erythrocytes phospholipids and proteins, impair the membrane-related functions. This ultimately leads to diminishing erythrocytes survival [2-4].
The rhizome of Coptis chinensis Franch, which is officially recognized in the Chinese Pharmacopoeia as Coptidis Rhizoma (CR), has been used for thousands years in China as a herb for the treatment of reducing heat, purging fire, and removing toxins due to its various activities such as of antihypertensive, antidiabetic, antiinflammatory, hypolipidemic, and antioxidant effects [5], among which the antioxidative effects of CR were evaluated by the DPPH assay, FRAP assay, and ABTS assay in vitro [6]. It has been reported previously that CR showed antioxidative hemolysis of erythrocytes induced by acetylphenyhydrazine (APH) in mice and rats, which was indicated by a significant reduction in serum bilirubin levels, the amounts of plasma-free hemoglobin, the number of reticulocytes in whole blood, a significant decrease in MDA content, an increase in superoxide dismutase (SOD), glucose-6-phosphate dehydrogenase (G6PD) activity and glutathione (GSH) content [7,8]. However, the prevention of oxidative damage structurally and functionally of erythrocytes membrane is still little known, in particular its link to morphology of erythrocytes. Therefore, this study was conducted to determine the effect of CR on oxidative damage of erythrocytes membrane by using 2,2-azo-bis (2-amidinopropane) dihydrochloride (APPH)-induced mice model in vivo and observing AAPH-induced erythrocytes oxidative hemolysis and morphological alterations in vitro. Based on this study, we further elucidate the preventive effect of CR on oxidative damage of erythrocytes, as part of an ongoing effort to identify novel and potent agents for the prevention and treatment hemolytic disease caused by oxidative stress.
Materials and Methods
AAPH was obtained from JK chemical company, Greensboro, USA; APH (Lot 971015) was purchased from Beijing Chemical Reagent Company, Beijing, China; primaquine was obtained from Sigma-Aldrich, St. Louis, MO, USA; enzymes of Na+/K+-ATPase and Ca2+/Mg2+-ATPase kits were purchased from Jiancheng, Nanjing, China.
Herbal preparation
Coptidis Rhizoma was purchased commercially (Medicine Co. Ltd., Beijing, China). The extract was prepared by boiling the herb in water for 1 h, followed by an additional re-extraction of the plant material by the above method. Water extracts were combined and concentrated to a concentration including 1.2 g crude herb per milliliter, mixed well before administration. The extract was concentrated in vacuum and dissolved in phosphate buffered saline (PBS, pH 7.4, 1 g/ml) for erythrocytes assays in vitro. All samples were stored at −4°.
In vivo experiments
Four week old male KM mice (Animal Science Laboratory of Peking University Health Science department, PR China) were intraperitoneally injected with APH 0.03 g/kg. The mice were randomly assigned into six groups: APH-injected mice without any treatment (APH, n=10), normal mice without APH injection as control, APH-injected mice treated with 0.058 g/kg primaquine (PQ, n=10), and APHinjected treated with CR intragastrically at a daily dose of 1.2 g/kg, 0.6 g/kg, or 0.3 g/kg (n=10, in per group) for 3 days. Dose calculations followed guidelines correlating dose equivalents on the basis of 1/5, 1/10, 1/20 of the LD50 dose (6.0 g/kg) [9].
Untreated control mice received distilled water only. All animals were maintained on a 12 h light/ dark cycle under constant temperature (24±2°) and humidity (55±5%) and were allowed free access to food and water. All procedures for consideration of animal welfare were reviewed and approved by the ethical committee of China Academy of Traditional Chinese Medicine.
Preparation of erythrocytes and membranes
Animals were sacrificed after 3 days of treatment, thereafter blood was collected into heparinized tubes. Plasma and the buffy coat were carefully removed after 10 min centrifugation at 500×g and 4°. Erythrocytes were harvested after repeated washing in PBS and suspended in a known volume of isotonic buffer. The packed cells were hemolysed by diluting 1:10 with hypotonic sodium phosphate buffer (5 mM, pH 8), then the membranes were obtained by centrifugation at 10 000×g after 30 min. Pellet membranes were washed three times by repeated centrifugation at 10 000×g for 30 min. Packed membranes were suspended in a known volume of isotonic buffer [10].
HPLC analysis of phospholipid
A precise amount of erythrocyte membranes 0.5 ml was added to 3 ml of chloroform:methanol (2:1) mixture and then 1 ml methanol was added, shaken well for 2 min, followed by adding 1 ml of water and shaked well for 2 min, then centrifugated at 1500×g for 5 min, the lower portion was collected and dried with nitrogen. One hundred microliter of chloroform was added and diluted with 2 ml of hexane-isopropanol (3:1). Phospholipid content of membranes was determined by HPLC, chromatographic separation was performed using a Hypersil SiO2 column (250×4.6 mm). The mobile phase, acetonitrile:methanol:85% phosphoric acid (500:50:9, v/v/v) was delivered isocratically at 1.5 ml/min, and was measured at λex=203 nm, followed by quantification of PS, phosphatidylethanolamine (PE), PC in the membranes.
Electrophoresis of membrane proteins
Polyacrylamide gel electrophoresis with dodecyl sulphate (SDS-PAGE) was carried out employing the discontinuous buffer system of Laemmli [11]. The monomer concentration in the mini slab gels was 4% stacking and 10% resolving gel. Samples were dissociated by mixing them in SDS sample buffer (62.5 mM Tris-HCl, pH 6.8, containing 2% SDS, 5% 2-mercaptoethanol, 10% glycerol and bromophenol blue as tracking dye) and then boiled for 1 min. Protein concentration was determined according to Lowry [12] and 15 μg of protein was loaded to gel. A standard protein (molecular weight: 14,400~97,400 Da) was included in the slab gel. At the end of electrophoresis, the gels were stained with 0.2% Coomassie Brilliant Blue in 30% methanol/10% acetic acid. The relative quantity of each band was measured by Alpha Ease FC (Fluorchem FC2) software.
Detecting Na+/K+-ATPase and Ca2+/Mg2+-ATPase enzymes
After 3 days of treatment, blood was collected from the retroorbital plexus and the erythrocytes membranes were collected according to above procedure. Assays for ATP enzyme were performed using the test combination kits from Nanjing JianCheng, Nanjing City, PR China [13].
Measurement of fluidity and deformation of erythrocytes
Erythrocytes were suspended in a 15% polyvinylpyrrolidone (PVP) buffer (w/v, molecular weight 30 kDa, 61 mM NaCl, 0.8 mM Na2HPO4, and 0.2 mM KH2PO4, pH 7.4, and viscosity 15 mPa·s) and adjusted to 2×107 cells/ml to measure deformation index (DI) using an ektacytometer (LBY-BX2, Precil, Beijing, China) at shear rates ranging from 50 to 150 s−1 [14].
Erythrocyte ghosts measuring 0.5 ml was incubated with 2 μM of 1,6-diphenyl-1,3,5-hexatriene (DPH), at 37º for 30 min, and then centrifuged at 10,000 g for 10 min. After the removal of the supernatant, the ghosts were washed in PBS three times and suspended in 1 ml PBS. The fluorescence intensity was measured in a spectrofluorometer (Hitachi, Japan) at 25º, with the Excitation and Emission wavelengths were 360 nm and 440 nm, respectively, and the slot breadth of 5 nm. The fluorescent polarization was calculated by the following equation: FP=IVV−GIVH/IVV+GIVH, where IVV and IVH are the fluorescence intensities observed in parallel and perpendicular directions, respectively, with respect to the polarization direction of the exciting wave. G is an apparatus constant depending on the emission wavelength. Membrane lipid fluidity is inversely proportional to fluorescence polarization [15].
in vitro assay for antihemolytic activity of CR
Eight male KM mice (weight: 22~24 g) were fed commercial food for 1 week. Blood was collected into heparinized tubes. Erythrocytes were harvested as per the above mentioned procedure. Crude erythrocytes were then washed three times with 10 parts PBS. Packed erythrocytes were thereafter suspended in 40 parts PBS solution. Erythrocyte suspensions (1.25 ml) were mixed with 30 μl of PBS solution containing CR at the final concentrations of 0.025, 0.05, 0.1, 0.25, 0.5, 1, 1.5, 2, 2.5 mg/ml. Erythrocyte suspension (1.25 ml) was mixed with 30 μl of PBS solution alone and was used as a control sample. Two hundred millimolars AAPH in PBS solution (250 μl) was then added to the mixture, followed by incubation in a water bath at 37° for 3 h with gentle shaking. After incubation, the sample was centrifuged at 1000 g for 10 min. The absorbance (A) of the supernatant at 540 nm was recorded with a UV/Vis recording spectrophotometer (Bio-Teck, America) [16,17]. Antihemolysis percentage was calculated by the following equation: Inhibition=(AAAPH−ACR)/ AAAPH×100.
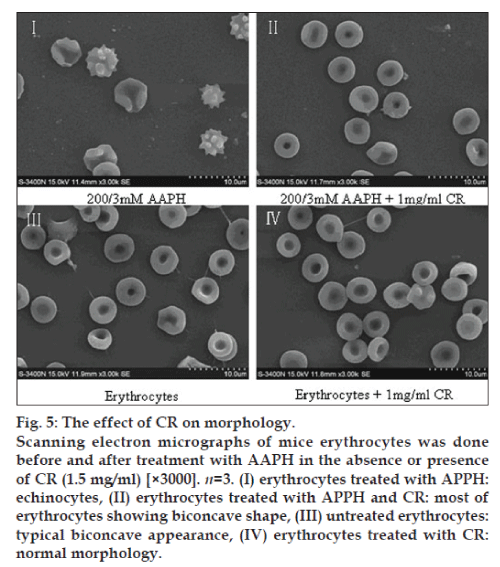
Scanning electron microscopy
Packed erythrocytes (1.5 ml) were incubated with AAPH (200/3 mM) with or without CR (1.5 mg/ml) for 40 min at 37°. After incubation, erythrocytes were fixed with 2.5% glutaraldehyde. Following incubation overnight at 4°, the fixed samples were washed twice with saline solution and then smeared on grease-free glass slides and air-dried. The slides were dehydrated in an ascending series of acetone (30-100%) and then transferred to aluminium stubs with double sided adhesive tape. The cells were gold coated for 3 min at 10-1 Torr in a Polaron E 5000 sputter device. The observations and photographic records were performed with an electron microscope (Model: LEO 435 VP; LEO Electron Microscopy Ltd., Cambridge, UK) [18].
Data analysis
All data were expressed as mean standard deviation and were calculated using one-way ANOVA, within SPSS 11.0 software package. Values were considered significantly different if P<0.05.
Results
Effect of CR on phospholipid
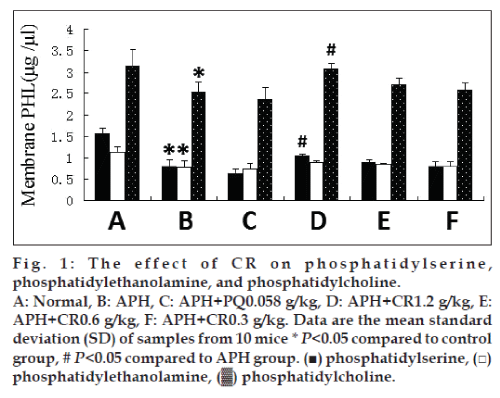
To characterize preventive oxidative damage activities of CR in vivo, we treated APH-induced heamolysis in mice with CR. Phospholipid content was measured with HPLC. The content of PS, PE, and PC were significantly reduced in membranes of mice injected with 0.03 g/kg APH. CR treatment had modest effects on content of PS, PE, and PC. There was a trend of increasing content with an increasing dose of CR, but only the highest dose, 1.2 g/kg, resulted in a significant difference (P<0.05) in PS and PC compared with untreated APH-induced mice. This had an increasing trend on the PE content, but the difference was not significant (fig. 1).
Effect of CR on membrane protein
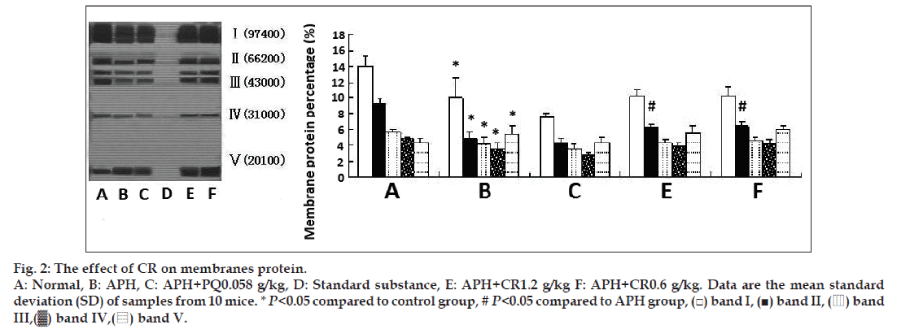
Results in fig. 2 present the extent of protection offered by CR to membranes cytoskeletal proteins against APH-induced oxidative damage. Treatment with single APH led to significantly decreased intensities of band Ι-ΙV and an increase in band V. Treatment with CR showed there was an increasing trend in membrane cytoskeletal proteins for band Ι-ΙV and only a significant increase (P<0.05) in band II was found. However, treatment with CR did not affect the band V intensities compared with that of single APH-induced mice.
Effect of CR on Na+/K+-ATPase and Ca2+/ Mg2+-ATPase
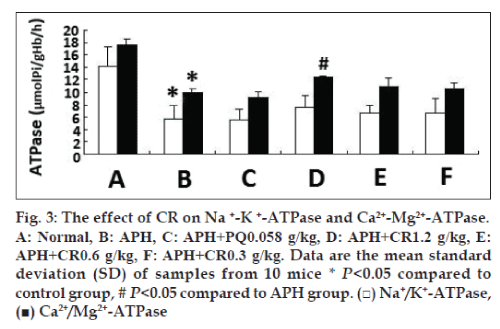
Due to APH-induced structural changes in erythrocytes membrane, we examined the correlative function. The activity of Na+/K+-ATPase and Ca2+/ Mg2+-ATPase was significantly reduced in mice injected with 0.03 g/kg APH, and increased in mice treated with CR at any dose tried, whereas only the highest dose 1.2 g/kg CR treatment had the significant increase on Ca2+/Mg2+-ATPase activity (P<0.05). However, no significant increase in Na+/K+-ATPase was found, identifying an upregulating effect developed as the dose of CR increased (fig. 3).
Effect of CR on fluidity and deformation
The effect of CR treatment on fluidity of membranes and deformation of erythrocytes in APH-induced mice was assessed and result is shown in Table 1. The FP value in APH-induced mice was significantly higher than that of the control group, which indicative of fluidity decreased. The fluidity increased significantly in APH-induced mice treated with CR at all of three dose tried (P<0.05). The DI in APH-induced group was significantly lower than that of the control mice group, which showed deformability was reduced. However, there was no significant effect on deformability at any dose of CR compared with untreated APH-induced animals. In contrast, primaquine significantly decreased erythrocytes deformability of APH-induced mice.
| Group | FP | DI |
|---|---|---|
| Control | 0.077±0.017 | 0.353±0.007 |
| APH | 0.184±0.051a | 0.332±0.006c |
| APH+PQ | 0.100±0.068 | 0.313±0.007cd |
| APH+CR 1.2 g/kg | 0.057±0.030b | 0.336±0.010c |
| APH+CR 0.6 g/kg | 0.064±0.030b | 0.329±0.012c |
| APH+CR 0.3 g/kg | 0.067±0.043b | 0.330±0.013c |
For quantization, 10 mice were analyzed from each group; Data are the mean±standard deviation (SD) from 10 samples; a,cP<0.05 compared to control group, bdP<0.05 compared to single APH-induced animals, APH: Acetylphenyhydrazine, PQ: Primaquine, CR: Coptidis Rhizoma, DI: Deformation index.
Table 1: The effect of cr on fluidity and deformation of erythrocytes
Effect of CR on antihemolytic activity
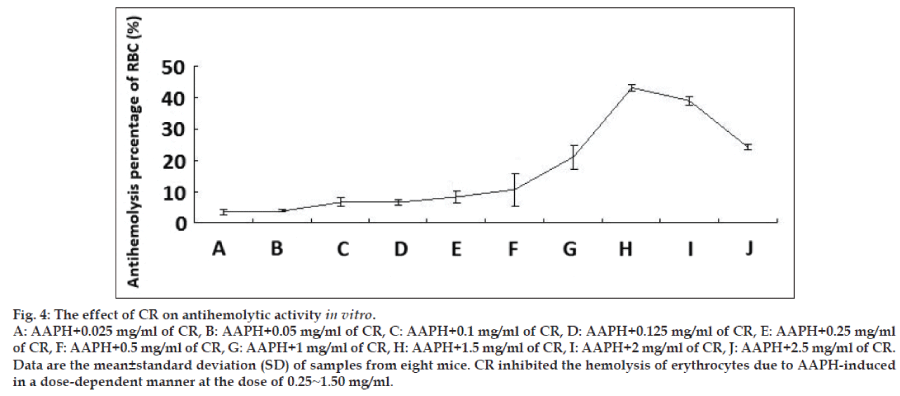
To investigate whether CR has preventative oxidative damage activities on erythrocytes in vitro, the previously reported method was used to determine the hemolysis of erythrocytes mediated by AAPH [16,17]. The addition of AAPH (a peroxyl radical initiator) to the erythrocytes suspension caused the oxidation of lipids and proteins in cell membranes and thereby induced hemolysis. The antihemolytic effect of CR is shown in fig. 4, CR inhibited the hemolysis of erythrocytes due to AAPH-induced peroxyl radicals in a dose-dependent manner at the dose of 0.25~1.50 mg/ml. The 1.5 mg/ml dose resulted in the highest difference with a 40% inhibition hemolysis compared with untreated erythrocytes. However, the inhibited effects decreased as the concentration increased.
Effect of CR on morphology
The scanned electron micrographs (SEM) of erythrocytes treated in vitro with AAPH and CR are shown in fig. 5. Normal erythrocytes appear as typical discocytes, while exposure to AAPH resulted in a significant change in the cell shape and distinct echinocyte formation. CR per se did not cause any morphological alterations in the cells, under the experimental conditions. CR significantly inhibited the AAPH-induced morphological alterations.
Discussion
The biomembranes may be most susceptible to free radical attack due to its content of polyunsaturated fatty acids. An altered cell membrane composition can cause a greater sensitivity to peroxidative stress, with increase in membrane fragility [19]. Structure determines function, membrane fluidity is not only the basic feature of biomembranes but also a sensitive indicator for reflected cell function. Membrane fluidity at a normal level is conducive to maintaining cell membranes material transport, permeability, deformation, and activity [20]. In the present study, we chose erythrocytes and its membranes as the models in vivo and in vitro to study the efficacy of CR on APH and AAPH-induced lipid/protein peroxidation, 0.03 g/kg APH induced the damage to membranes phospholipids and proteins of normal structure. Treatment with CR showed a significant increase in band, PS and PC content at the dose of 1.2 g/kg CR (figs. 1 and 2). At the three doses of CR significantly increased the decline membranes fluidity in APH-induced mice (Table 1). 1.2 g/ kg CR treatment had a significant increase on Ca2+/Mg2+-ATPase activity in APH-induced mice (fig. 3). Together with our former results [7,8] suggest CR prevents oxidative damage in erythrocytes membrane phospholipids and proteins through removing toxic free radicals, inhibiting lipid peroxidation and improving antioxidant capacity, resulting in a reduction of erythrocytes hemolysis. To our best knowledge, the present study was the first to demonstrate that CR in vivo strengthen the antioxidative effect on structure and function of erythrocytes membrane.
It is known that AAPH-induced hemolysis in erythrocytes is a function of incubation time and proportional to the concentration of free radicals [21,22]. Oxidants induce alterations in the erythrocytes membranes as manifested by a decreased cytoskeletal protein content which can lead to abnormalities in erythrocytes shape [23]. The present study revealed that CR antihemolysis activity in erythrocytes from oxidation incubated with AAPH in a dose-response relationship. A peaked at a concentration of 1.5 mg/ml, the weaker response elicited at higher doses suggests that CR exerts cytotoxicity at relatively higher concentrations. Our SEM observations showed that AAPH induced morphological alterations in erythrocytes from a discoid to an echinocytic form and with a decrease in erythrocytes quantity. Significant protection was offered by CR in terms of retaining the morphological integrity of the erythrocytes as well as reducing the extent of lipid peroxidation when subjected to oxidative stress.
Few researchers suggested deeming CR as oxidizing agents like primaquine, because they think administration of CR may cause hemolytic jaundice to infants with inborn G6PD deficiency [24,25]. On this issue, scholars conducted relevant clinical and experimental researches, but have not yet reached to any consensus [26-28]. In our study, we found primaquine further increased oxidant damage to APH-induced animals, However, CR significantly enhances the functions of antioxidant system in hemolytic rats [7,8], together with the findings of antioxidant hemolysis of erythrocytes, these results do not support the view that CR acted as an oxidant like primaquine, whereas it showed significantly antioxidant activity. These results are also consistent with the CR containing formulas with antioxidant activity in clinic, such as Huanglianjiedu decoction [29,30].
In conclusion, the results indicated that CR could protect erythrocytes structure, function, and morphological alterations against oxidative damage. These findings provide evidence that CR plays a role in antioxidant protection in erythrocytes by maintaining membrane integrity.
References
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. ProcNatlAcadSci USA 1993;90:7915-22.
- Hebbel RP, Leung A, Mohandas N. Oxidation-induced changes in Microrheologic properties of the red blood cell membrane. Blood 1990;76:1015-20.
- Chiu D, Kuypers F, Lubin B. Lipid peroxidation in human red cells. SeminHematol 1989;26:257-76.
- Jain SK, Hochstein P. Polymerization of membrane components in aging red blood cells. BiochemBiophys Res Commun 1980;92:247-54.
- Jung HA, Min BS, Yokozawa T, Lee JH, Kim YS, Choi JS. Anti-Alzheimer and antioxidant activities of CoptidisRhizoma alkaloids. Biol Pharm Bull 2009;32:1433-8.
- Yuan WJ, Li CF, Ding QJ, Kang WY. Studies on the antioxidant activity of Coptischinensis. Guihaia 2009;29:694-7.
- Wang Y, Qiao L, Liu C, Lin N. Effect and its mechanism of Coptischinensison oxidative hemolysis of erythrocytes in miceinduced by acetylphenylhydrazine. ZhongguoZhong Yao ZaZhi 2010;35:2449-52.
- Liu CF, Qiao L, Wang YW, Lin N. Coptischinensis and berberine on the oxidative hemolysis of red blood cells in rats and effects of antioxidant system. Chin J ExpTradit Med Formul 2010;16:251-4.
- Yang SY, Wang XH. A research on the erupted fetal diseases caused by traditional Chinese drugs: Discussion from the issue that Chinese goldthread rhizome is prohibited in Singapore. J Tradit Chin Med 2008;28:235-40.
- Dodge JT, Mitchell C, Hanahan DJ. The preparation and chemical characterization of haemoglobin-free ghosts of human erythrocytes. Arch BiochemBiophys 1963;100:119-30.
- Laemmli UH. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680-5.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J BiolChem 1951;193:265-75.
- Wang Y, XuPx. The effects of intermittent hypoxia training on Na+-K+-ATPase and Ca2+-Mg2+-ATPase activities of erythrocyte membrane. ActaSci Nat 2011;44:44-8.
- Zhao T, Guo J, Li H, Huang W, Xian X, Ross CJ, et al. Hemorheological abnormalities in lipoprotein lipase deficient mice with severe hypertriglyceridemia. BiochemBiophys Res Commun 2006;341:1066-71.
- Niranjan TG, Krishnakantha TP. Membrane changes in rat erythrocyte ghosts on ghee feeding. Mol Cell Biochem 2000;204:57-63.
- Sekiya N, Goto H, Shimada Y, Endo Y, Sakakibara I, Terasawa K. Inhibitory effects of triterpenes isolated from Hoelen on free radical-induced lysis of red blood cells. Phytother Res 2003;17:160-2.
- Sekiya N, Hikiami H, Nakai Y, Sakakibara I, Nozaki K, Kouta K, et al. Inhibitory effects of triterpenes isolated from Chuling (Polyporusumbellatus FRIES) on free radical-induced lysis of red blood cells. Biol Pharm Bull 2005;28:817-21.
- Singh N, Rajini PS. Antioxidant-mediated protective effect of potato peel extract in erythrocytes against oxidative damage. ChemBiol Interact 2008;173:97-104.
- Angela MR, Paola AC, Gigliola M, Simona M, Stefania Z, Sara T, et al. Effects of long-term space flight on erythrocytes and oxidativestress of rodents. PLos One 2012;7:1-9.
- Kanti BP, Syed IR. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxid Med Cell Longev 2010;3:2-12.
- Sugiyama H, Fung KP, Wu TW. Purpurogallin as an antioxidant protector of human erythrocytes against lysis by peroxyl radicals. Life Sci 1993;53:39-43.
- Miki M, Tamai H, Mino M, Yamamoto Y, Niki E. Free-radical chain oxidation of rat red blood cells by molecular oxygen and its inhibition by α-tocopherol. Arch BiochemBiophys 1987;258:373-80.
- Battistelli M, Sanctis RD, Bellis RD, Cucchiarini L, Dacha M, Gobbi P. Rhodiolarosea as antioxidant in red blood cells: Ultrastructural and hemolyticalbehaviour. Eur J Histochem 2005;49:243-54.
- Wong HB. Singapore kernicterus. Singapore Med J 1980;21:556-67.
- Health Sciences Authority. Health supplements guidelines in Singapore. Singapore, Health Supplement Unit, Centre for Drug Administration. Health products Regulation Group, 2005.
- Yeung CY. Changing pattern of neonatal jaundice and kernicterus in Chinese neonates. Chin Med J 1997;110:448-54.
- Fok TF. Neonatal jaundice-traditional Chinese medicine approach. J Perinatol 2001;21(Suppl 1):S98-100.
- Gao XS, Chen FX, Yang SY, Pi XX, Li JR, Lin N, et al. Comprehensive report of berberine-induced hemolytic jaundice toxicity and its prevention and treatment. Chin J Chin Mater Med 2002;27:71-4.
- Wang LJ, Xu Q. Anti-oxidative activity of Huanglianjiedu decoction, a traditional of Chinese formula. J China Pharm Univ 2001;32:51-3.
- Du QQ, Zhang X, Song FR, Liu ZQ, Liu SY. HPLC-ESI-MS analysis of ginsenosides and antioxidant activity during combination of Ginseng with aconitum carmichaeli and CoptisChinensis. Chem J Chin Univ 2010;31:1332-6.