- *Corresponding Author:
- A. Jayshree
Centre of Advanced Study in Botany, University of Madras, Guindy Campus, Chennai-600 025, India
E-mail: jayshreeannamalai@yahoo.com
| Date of Submission | 29 May 2016 |
| Date of Revision | 31 August 2016 |
| Date of Acceptance | 08 September 2016 |
| Indian J Pharm Sci 2016;78(5):575-581 |
This is an open access article distributed under terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Algae are rich sources of structurally novel and biologically active metabolites. Primary and secondary metabolites produced by these organisms have drawn interest of the pharmaceutical industries. In this regard we have put forth an investigation to evaluate phenolic and flavonoid content in methanol extract of fresh algal biomass followed by antioxidant, antimicrobial and anticancer activities. Determination of total phenol and flavonoid content was followed by the 2,2-diphenyl-1-picryhydrazyl radicals scavenging, phosphomolybdate and reducing power assay for evaluating antioxidant potency. Agar well diffusion method for antibacterial activity and trypan blue test along with MTT assay was done for anticancer activity. Among two microalgae, Chlorella vulgaris had efficiently high amount of total phenol and flavonoid content. At 1000 µg/ml concentration of extract, C. vulgaris and Chlamydomonas reinhardtii exhibited 92.57 and 83.38% diphenyl-1-picryhydrazyl free radical scavenging activity and 97.90 and 82.74% total antioxidant potency. C. reinhardtii showed more efficient zone of inhibition against Gram negative bacteria: Pseudomonas aeruginosa, Escherichia coli and Staphylococcus aureus than C. vulgaris. In trypan blue test, C. vulgaris showed marked cytotoxicity against breast cancer, MCF-7 cell lines with IC50 value of 23.45 µg/ml than C. reinhardtii; the IC50 values were confirmed further by MTT assay. In the study, flavonoids were found to perform crucial role as anticancer agent with R2 values 0.755 and 0.941 for C. vulgaris and C. reinhardtii; significantly higher than phenol coefficient values. Thus, both of the microalgae in dietary supplements will be effective mediators in scavenging free radicals, inhibiting human pathogens and antiapoptotic protein of tumor cells.
Keywords
Phenols, flavonoids, anticancer property, free radical scavenging, anti-apoptotic protein
Chlorella vulgaris is a single-celled eukaryotic green micro algae, known to be first form of a plant with a well-defined nucleus emerged over 2 billion years ago [1]. Chlorella contains the highest amount of chlorophyll of any known plant. Chlamydomonas reinhardtii, another unicellular algae though not much explored as nutrient supplement; it is well known model organism with its three genomes (nuclear, plastidial and mitochondrial) completely sequenced [2]. It finds its application mostly in molecular biology especially in studies involving flagellar motility, chloroplast dynamics, biogenesis and genetics [3]. C. vulgaris is well determined nutrient-dense superfood containing 60% protein, 18 amino acids, 20 vitamins and minerals like iron, calcium, potassium, magnesium and phosphorous [4]. One of its unique properties is a phytonutrient called Chlorella Growth Factor [1]. These two microalgae are known for high volume production of β-carotene.
Besides being food supplements microalgae, Chlorella sp., Chlamydomonas sp. have been proved to have antibacterial activity in vitro against both Gram positive and Gram negative bacteria [5]. The production of extracellular antibiotic metabolites by marine algae has been well studied in recent years. They are also been reported that a wide range of antifungal activities were obtained from extracts of green microalgae [6]. Eventually, microalgae are almost an untapped resource of natural antioxidants due to their enormous biodiversity, much more diverse than higher plants. They contain various biologically active compounds that are been used as source of food, feed and medicine [7]. Microalgae are proposed as an alternative molecular pharming system. This relatively new platform offers several advantages, including short time from transformation to scaling up; rapid growth and ease of cultivation; safety, because microalgae do not harbor human pathogens, many are Generally Regarded As Safe (GRAS) organisms, and grow in axenic conditions facilitating production of biopharmaceuticals; homogeneity of protein, antibiotics and phytochemicals production with the use of controlled bioreactors [8].
In recent times, studies suggest that there is an inverse relationship between dietary intake of antioxidant rich foods and the incidence of human disease. The search to replace these synthetic antioxidants with natural antioxidants has become an essential deed in immune pharmacy discovery since these components are suspected carcinogens [9]. Antioxidants are presumed to have several positive health effects that include prevention of cardiovascular disorders, ageing related diseases such as Alzheimer and certain types of cancer [10]. Microalgae in this regard are exceptionally rich source of pharmacologically active metabolites with antineoplastic, antitumor, antibacterial, antifungal and antiviral properties [7]. In the present study, antioxidant activity of methanol extracts of C. vulgaris and C. reinhardtii was investigated along with the aspect to determine its potency to inhibit the growth of human pathogens and proliferation of breast cancerous cell line, MCF-7. Apart from investigation, it has been initiated to compare a well-known model microalgae sp., C. reinhardtii with well-known single cell protein, C. vulgaris for its antioxidant, antibacterial and anticancer properties.
Materials and Methods
Algal strains, C. vulgaris and C. reinhardtii were obtained from Algal Culture Collection, Center for Advanced Studies in Botany, University of Madras, Chennai. Test microorganisms, Escherichia coli MTCC-1687, Proteus vulgaris MTCC-742, Staphylococcus aureus MTCC-96, Pseudomonas aeruginosa MTCC-1688 and Bacillus subtilis MTCC-441 were obtained from Microbial Type Culture Collection (MTCC), Chandigarh. Human breast cancer MCF-7 (GDC055) cell lines were obtained from National Centre for Cell Sciences, Pune. Muller Hinton agar was purchased from Hi-Media, India. Methanol was of ACS grade, purchased from Merck, Mumbai. Fetal bovine serum was purchased from Gibco Laboratories. RPMI-1640 was purchased from Gibco/BRL Invitrogen (Caithershurg, MD). L-Ascorbic acid, gallic acid, quercetin, aluminium chloride, potassium acetate Folin-Ciocalteu’s phenol reagent, trypsin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and dimethylsulfoxide (DMSO) were purchased from Sisco Research Laboratories Pvt. Ltd., Mumbai. Double distilled water was used for the all the experiments. Sample absorbance was read using Beckman DU 64 UV/Vis spectrophotometer.
Preparation of algal extracts
Algal cultures, C. vulgaris and C. reinhardtii were maintained at 24±1° in a thermostatically controlled room and illuminated with cool inflorescence lamps (Phillips 40 W, cool daylight 6500 K) at an intensity of 2000 lux in a 12:12 h light dark regime. At log phase, algal biomass was harvested. About 10 g of the collected fresh algal biomass of each culture equal to 1 g of dried one were completely homogenized and extracted with 100 ml of methanol solvent. Clarification of algal mixture was carried out by filtration method using Whatman No.1 filter paper. The clarified extracts were evaporated under a vacuum at 50° using rotary evaporator. The crude extracts were stored in the dark in a vial and kept at 4° until further analysis.
Total phenols and flavonoids
In total phenol content determination, standard procedure was followed using Folin-Ciocalteu’s reagent and sample absorbance was measured at 725 nm [11]. Gallic acid was used as standard for a calibration curve; the total phenol was expressed in gallic acid equivalents. The total flavonoid was determined by measuring the sample absorbance at 415 nm [12]. Quercetin was used as standard for a calibration curve; the total flavonoid content was expressed in quercetin equivalents. Each of the above assays using algal extracts was performed in triplicate.
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay
The free radical scavenging activity of different fractions were measured in vitro by using 0.1 mM solution of DPPH in 95% methanol [13]. To 1 ml of this solution, 1 ml of each fractions dissolved in methanol at different concentrations were added and allowed to stand at room temperature for 30 min in dark. Then absorbance was measured at 517 nm against methanol blank and percentage of scavenging inhibitions were determined by comparing with ascorbic acid as standard. Percentage inhibition (I%)=(Ac–As)/Ac×100, where, Ac is the absorbance of the control and As is the absorbance of the sample.
Total antioxidant capacity
The antioxidant activities of different fractions of methanol extracts were evaluated based on the reduction of Mo (VI)-Mo (V) and subsequent formation of a green phosphate/Mo (V) complex at acidic pH [14]. Initially different concentrations of 0.3 ml extract were combined with 3 ml of reagent solutions containing 0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate. Then the reaction mixtures were incubated at 95° for 90 min and the absorbance were measured at 695 nm against methanol blank after cooling to room temperature. The total antioxidant activity was expressed as the number of equivalents of ascorbic acid.
Reducing power assay
The reducing power of different fractions of methanol extracts (1.0 ml) was assayed by the addition of 2.5 ml of phosphate buffer (50 mM, pH 7.0) and 2.5 ml of 1% potassium ferricyanide [15]. After incubation of reaction mixtures at 50° for 20 min, 2.5 ml of trichloroacetic acid (10%) was added to the mixtures and centrifuged at 3000 rpm for 10 min. From the supernatant, 1.25 ml was mixed with 1.25 ml of distilled water and 0.25 ml FeCl3 solution (0.1% w/v) [8]. Finally the absorbance was measured at 700 nm against methanol blank; increased absorbance values indicate higher reducing power.
Preparation of active bacterial suspension
Inhibition of microbial growth by methanol extracts was assessed by agar well diffusion assay. Standard aseptic microbiological methods were followed throughout this antibacterial study. Bacterial inoculums were prepared as per 0.5 McFarland standard [16] with test organisms: E. coli, P. vulgaris, S. aureus, P. aeruginosa and B. subtilis in Muller Hinton broth. Dried crude methanol extract was dissolved in 100% DMSO at mg/ml concentration.
Agar well diffusion bioassay
The antimicrobial activity of the methanol extracts dissolved in DMSO were determined by agar well diffusion technique using Mueller Hinton agar medium [17]. As per McFarland standard, sterile cotton tipped swab was dipped in the freshly prepared culture with the dilution of 108 cells/ml and swabbed on sterile molten Muller Hinton agar. Five wells were made per plate on swabbed plates using cork-borer (0.85 cm). From this 50, 75 and 100 μl extracts were loaded in three wells and in other two wells, 100 µl of streptomycin at mg/ml concentration (positive control) and 100 µl DMSO (negative control) were added. Then the plates were incubated at 37° for 24 h in aerobic condition and diameter of the inhibition zones were measured with caliper. Tests were performed under sterile conditions in duplicate and repeated three times.
Cell proliferative studies
Trypan blue exclusion test was performed by following method. Human breast cancer MCF-7 (GDC055) cell lines were maintained in RPMI-1640 supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin (100 μg/ml) in a humidified atmosphere of 50 μg/ml CO2 at 37°. This semiquantitative method was used to determine the viable cell numbers before and after treatment of algal extracts. The cancer cells were seeded at a density of 2×104 cells/well and were treated with different concentrations of the algal extract for 72 h at 37° in the presence of 5% CO2. After 72 h, 20 μl of medium and equal volume of trypan blue were mixed. The viable and dead cells were counted by Neubauer haemacytometer [18].
In determining the cytotoxicity of algal extracts against Human breast cancer MCF-7 cell lines, MTT assay was used as a quantitative method [19]. Cancer cells at the density of 1×105/well were plated in 100 μl of medium/well in 96-well plates and incubated for 48 h at 37°. When cells reached the confluence, various concentrations of algal extracts dissolved in 0.1% DMSO was added and incubated. The algal extracts were removed after incubation and washed with phosphate-buffered saline (pH 7.4). Twenty microliter per well (5 mg/ml) of 0.5% MTT phosphate buffered saline solution was added. After 4 h incubation, 0.04 M HCl/isopropanol were added. The absorbance at 570 nm was measured with a microplate reader (Bio-Rad, Richmond, CA), to determine viable cells; wells untreated with algal extracts were read as blank. The experiment was performed in triplicate. The effect of the samples on the proliferation of human breast cancer cells was expressed as the % cell viability, using the following formula: % cell viability=A570 of treated cells/A570 of control cells×100.
Results and Discussion
Total phenol and flavonoid content in the methanol extract of C. vulgaris was determined to be 220 mg GAE/g extract DW and 131.15 mg QE/g extract DW whereas for C. reinhardtii it was 150 mg GAE/g of extract DW and 80.76 mg QE/g of extract DW.
Free radical scavenging ability of methanol extract of C. vulgaris was maximum of 92.57% at 1000 µg/ml with IC50 value of 397.01 µg/ml; C. reinhardtii was maximum of 83.38% at 1000 µg/ml with IC50 value of 423.44 µg/ml and standard (ascorbic acid) of 94.08 % at 1000 µg/ml with IC50 value of 127.52 µg/ml (Table 1). The phosphomolybdenum assay is based on the reduction of Mo (VI) to Mo (V) in the presence of antioxidants resulting in the formation of a green phosphate/Mo (V) complex exhibiting maximum absorption at 695 nm. Based on reduction ability, radical scavenging activity of methanol extract of C. vulgaris was maximum of 92.90% at 1000 µg/ml with IC50 value of 54.78 µg/ml; C. vulgaris was maximum of 82.74% at 1000 µg/ml with IC50 value of 73.06 µg/ml and standard same as above, respectively (Table 1).
| Concentration (µg/ml) | DPPH assay | Phosphomolybdate assay | Standard (Ascorbic acid) | ||
|---|---|---|---|---|---|
| C. vulgaris | C. reinhardtii | C. vulgaris | C. reinhardtii | ||
| 62 125 250 500 1000 |
8.17±0.19 11.53±0.27 27.01±0.63 62.97±1.47 92.57±2.16 |
6.25±0.08 12.49±0.32 24.67±0.89 59.04±0.52 83.38±1.23 |
56.58±1.32 78.13±1.82 89.33±2.08 92.25±2.15 97.90±2.28 |
42.43±0.69 61.48±1.90 75.82±1.00 79.97±2.34 82.74±1.32 |
23.82±0.56 49.013±1.14 64.54±1.50 78.60±1.83 94.08±2.20 |
| IC50 (µg/ml) | 397.01 | 423.44 | 54.78 | 73.06 | 127.52 |
Results are expressed as the average of triplicate determination±standard deviation
Table 1: Dpph Assay and Total Antioxidant Assay
Reducing power of C. vulgaris extract increased to 0.245 at 1000 μg/ml, in C. reinhardtii extract increased to 0.197 at 1000 μg/ml where as standard had absorbance of 0.630 at 1000 μg/ml (Table 2); the reducing power of the extracts increased in dose dependent manner.
| Concentration (µg/ml) | Absorbance value | ||
|---|---|---|---|
| Reducing power assay | Standard (Ascorbic acid) | ||
| C. vulgaris | C. reinhardtii | ||
| 62 125 250 500 1000 |
0.114±0.002 0.133±0.003 0.136±0.003 0.182±0.004 0.245±0.005 |
0.098±0.003 0.118±0.002 0.124±0.001 0.156±0.003 0.197±0.004 |
0.298±0.006 0.345±0.008 0.423±0.009 0.489±0.011 0.630±0.147 |
Results are expressed as the average of triplicate determination±standard deviation
Table 2: Antioxidant Activity by Reducing Power Assay.
The correlation coefficient (R2) between the antioxidant activity and phenolic content of C. vulgaris was assessed and found to be very small or negligible with phenol (R2=-1.889) whereas with that of flavonoid was R2=0.964. Whereas, in case of C. reinhardtii also, R2 between the antioxidant activity and phenolic content (R2=-1.990) was found to be negligible with phenol and with flavonoid was R2=0.917.
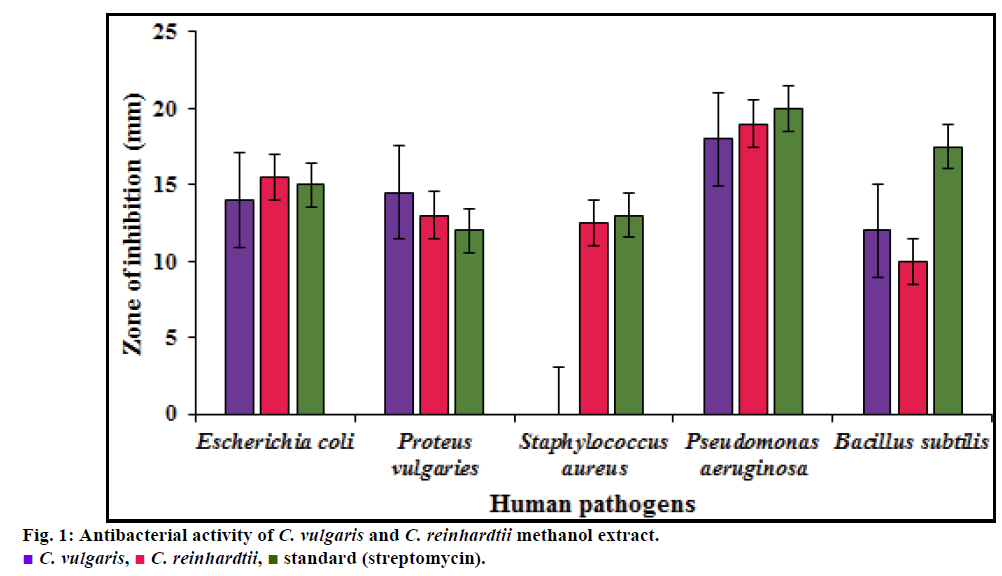
Methanol extract of C. vulgaris showed narrow antibacterial activity against Gram positive bacteria, exhibiting no inhibition against S. aureus and minimum inhibition of 12 mm against B. subtilis at the concentration of 100 µg/ml while C. reinhardtii exhibited 10.5 mm. Among Gram negative bacteria, maximum antibacterial potency was observed against P. vulgaris with inhibition zone diameter of 14.5 mm when compared with that of inhibition by standard, streptomycin (12 mm). E. coli and P. aeruginosa also showed varying susceptibility to extract. Methanol extract of C. reinhardtii showed better antibacterial activity against Gram negative bacteria, E. coli (17 mm) and P. aeruginosa (19 mm) than C. vulgaris exhibiting inhibition zone of 14 mm and 18 mm respectively. Among Gram positive bacteria, methanol extract of C. reinhadtii exhibited minimum zone of inhibition of 13.5 mm against S. aureus, while this was competing inhibition to standard (14 mm) and potential than C. vulgaris which exhibited no zone of inhibition as stated above (fig. 1).
Methanol extracts of C. vulgaris showed marked cytotoxicity against MCF-7 cell lines than C. reinhardtii with IC50 values of 23.45 µg/ml and 46.87 µg/ml. MTT assay confirmed the trypan blue test values exhibiting similar IC50 values (fig. 2). In the present study, both C. vulgaris and C. reinhardtii revealed flavonoids to exhibit potential anticancer activity with high correlation coefficient values of R2=0.755 and 0.941, respectively. Flavonoids tend to be significant cause for exhibiting anticancer activity than phenol.
Phenolic compounds act as antioxidants by chelating metal ions, preventing radical formation and improving the antioxidant endogenous system [20]. The total phenolic content in C. reinhardtii CC 124 treated to glass beads and steel ball were 42 and 51 GAE mg/ml [5]. These total phenolic contents were more or less similar to the phenolic content obtained C. reinhardtii in the present study suggesting variation is based on extraction methods. Polyphenols represent a diverse class of compounds including flavonoids (i.e. flavones, flavonols, flavonones, flavononols, chalcones and flavan-3-ols), lignins, tocopherols, tannins and phenolic acids [21]. Flavonoids, the largest group of phenolic compounds is known to contain a broad spectrum of chemical and biological activities including antioxidant and free radical scavenging properties [22].
In human cells, reactive oxygen species (ROS) are formed by endogenous factors resulting in extensive oxidative damage, age related degenerative conditions, cancer and a wide range of other human diseases. Natural antioxidants from plant origin can react rapidly with ROS and retard the extent of oxidative deterioration. Antioxidant activity of Steel ball and glass bead treated extracts of C. reinhardtii CC 124 were 107.34 and 90.69% compared to that of standard (butylated hydroxyl anisole) 359.01%, respectively [5]. Furthermore, antioxidants from natural sources can also increase the shelf life of foods. Therefore, the consumption of antioxidant and addition of antioxidant to food materials could protect the body as well as the foods [23]. Microalgae would serve as a continuous and reliable source of natural products, comprising antioxidants because they can be cultivated in bioreactors on a large scale. Furthermore, the qualities of microalgal cells can be controlled by using clean nutrient media for growing the microalgae avoiding use of herbicides, pesticides and any other toxic substances. The value of microalgae as a source of natural antioxidants is further enhanced by the relative ease of purification of target compounds [24].
Phenolic compounds such as flavonoids, phenolic acids and tannins are considered to be the major contributors exhibiting antioxidant capacity and are assessed to have diverse biological activities such as antiinflammatory, antiatherosclerotic and anticarcinogenic activities [11]. Therefore in C. vulgaris among two phenolic compounds, flavonoid may be a major contributor of antioxidant capacities. In a study by Syed et al. [25] the chloroform extract of C. vulgaris showed minimum zone of inhibition with zone diameter of 15, 13, 12 mm for E. coli, Bacillus sp. and Pseudomonas sp. respectively. Methanol extract of C. reinhardtii was found to be more efficient in inhibiting gram negative bacteria than positive strains. It showed maximum inhibition against P. aeruginosa, E. coli and S. aureus with the inhibition zone diameter of 19, 15.5 and 12.5 mm respectively. The inhibition against P. vulgaris and B. subtilis was comparatively lower than C. vulgaris extract. Steel ball extract of C. reinhardtii CC 124 also showed similar results as that present study exhibiting inhibition efficiently against gram negative bacteria than gram positive bacteria [5].
Development of anticancer agents from plant sources started in early 1950s with the discovery of Vinca alkaloids, vinblastine and vincristine and the isolation of the cytotoxic podophyllotoxins [26]. The extracts induced high antiproliferative activity in a dose dependent manner. The study is supported by rodent model studies of Yuan and Walsh [27] which demonstrated the protective effects of dietary kelps and other red and green algae against mammary, intestinal and skin carcinogenesis. Recent studies suggest C. vulgaris as an effective natural chemotoxic agent against tumour cells by inducing apoptosis [28]. ChlorellaPR1 efficiently reduced murine melanoma B16F10 cells viability by 50% with 5.5 µg/ml concentration of DMSO extract [29]. Thus, potential algal derived anticancer drugs will have major footprint in the treatment of cancer.
From the study, it is concluded that both C. vulgaris and C. reinhardtii have a significant level of flavonoids and phenols, which turn out to be potential scavenger of free radicals at the concentration of 1000 µg/ml. The crude extracts tend to inhibit the growth of breast cancer cell lines and human pathogens. C. vulgaris are potential in inhibiting B. subtilis and P. vulgaris while C. reinhardtii are more efficiently inhibiting E. coli, P. aeruginosa and S. aureus. This suggests the use of these two microalgae against both gram positive and negative bacteria with minimum inhibition at the concentration of 100 µl. Anticancer activity in the range of 23-46 µg/ml extracts of C. vulgaris and C. reinhardtii efficiently reduced cell viability by 50%. Overall comparison between two proposed microalgae reveal that C. vulgaris are rich in flavonoid content and are able to exhibit better free radical scavenging and anticancer activity than C. reinhardtii, in agreement to the literature studies. However, C. reinhardtii is found to exhibit better inhibition of both gram negative and positive bacteria than C. vulgaris. Thus, further research on chemical characteristics of antioxidant and other bioactive components of microalgae, C. vulgaris and C. reinhardtii would lead to the formulation of effective drugs in treatment of cancer and dreadful bacterial infections
Financial support and sponsorship
Nil
Conflicts of interest
The author declares no competing interests.
References
- Nick GL. Addressing human exposure to environmental toxins with Chlorella pyrenoidosa - medicinal properties in whole foods. Townsend Letter for Doctors and Patients 2003;237:28-32.
- Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 2007;318:245-51.
- Behrens J, von Kries JP, Kuhl ML, Bruhn, Wedlich D. Functional interaction of β-catenin with the transcription factor LEF-1. Nature 1996;382:3638-42.
- Bengwayan PT, Laygo JC, Pacio AE, Poyaoan JLZ, Rebugio JF, Yuson ALL. A comparative study on the antioxidant property of chlorella (Chlorella sp.) tablet and glutathione tablet. E Int Sci Res J 2010;2:12-25.
- Renukadevi KP, Saravana PS, Angayarkanni J. Antimicrobial and antioxidant activity of Chlamydomonas reinhardtii sp. Int J Pharm Sci Res 2011;2:1467-72
- Kuda T, Kunii T, Goto H, Suzuki T, Yano T. Changes of radical-scavenging capacity and ferrous reducing power in chub mackerel Scomber japonicus and Pacific saury Cololabis saira during 4°C storage and retorting. Food Chem 2007;103:900-5.
- Fedorov SN, Ermakova SP, Zvyagintseva TN, Stonik VA. Anticancer and cancer preventive properties of marine polysaccharides: some results and prospects. Mar Drugs 2013;11:4876-901.
- Specht E, Miyake-Stoner S, Mayfield S. Micro-algae come of age as a platform for recombinant protein production. Biotechnol Lett 2010;32:1373-83.
- Goiris K, De Vreese P, De Cooman L, Muylaert K. Rapid screening and guided extraction of antioxidants from microalgae using voltammetric methods. J Agric Food Chem 2012;60:7359-66.
- Shibata T, Hama Y, Miyasaki T, Ito M, Nakamura TJ. Extracellular secretion of phenolic substances from living brown algae. Appl Phycol 2006;18:787-94.
- Alhakmani F, Kumar S, Khan SA. Estimation of total phenolic content, in vitro antioxidant and antiinflammatory activity of flowers of Moringa oleifera. Asian Pac J Trop Biomed 2013;3:623-27.
- Kalita P, Tapan BK, Pal TK, Kalita R. Estimation of total flavonoids content (TFC) and antioxidant activities of methanol whole plant extract of Biophytum sensitivum Linn. J Drug Deliv Ther 2013;3:33-37.
- Amudha M, Rani S. Evaluation of in vitro antioxidant potential of Cordia retusa. Indian J Pharm Sci 2016;78:80-86.
- Rao AS, Reddy SG, Babu PP, Reddy AR. The antioxidant and antiproliferative activities of methanol extracts from Navara rice bran. BMC Complement Altern Med 2010;10:4.
- Jayanthi P, Lalitha P. Reducing power of the solvent extracts of Eichhornia crassipes (Mart.) Solms. Int J Pharm Sci 2011;3:126-28.
- Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by standard single disc diffusion method. Am J Clin Pathol 2003;45:493-96.
- Mattana, CM, Satorres, SE, Sosa A, Fusco M, Alcaráz LE. Antibacterial activity of extracts of Acacia aroma against methicillin-resistant and methicillin-sensitive Staphylococcus. Braz J Microbiol 2010;41:581-87.
- Mosaddegh M, Gharanjik BM, Naghibi F, Esmaeili S, Pirani A, Tehrani BE, et al. A survey of cytotoxic effects of some marine algae in the Chabahar coast of Oman Sea. Res J Pharmacognosy 2014;1:27-31.
- Chandini SK, Ganesan P, Bhaskar N. In vitro activities of three selected brown seaweeds of India. Food Chem 2008;107:707-13.
- Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 2010;15:7313-52.
- Al-Azzawie HF, Mohamed-Saiel SA. Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Science 2006;78:1371-77.
- Shalaby EA. Algae as promising organisms for environment and health. Plant Signal Behav 2011;6:1338-50.
- Kumar S, Pandey AK. Chemistry and biological activities of Flavonoids: An overview. Sci World J 2013;2013:1-16.
- Amarowicz R, Pegg RB, Moghaddam PR, Barl B, Weil JA. Free radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem 2004;84:551-62.
- Syed S, Arasu A, Ponnuswamy I. The uses of Chlorella vulgarisas antimicrobial agent and as a diet: the Presence of bio-active compounds which caters the vitamins, minerals in general. Int J Biosci Biotechnol 2015;7:185-90.
- Cragg GM, Newman DJ. Plants as a source of antiancer agents. J Ethnopharmacol 2005;100:72-79.
- Yuan YV, Walsh NA. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem Toxicol 2006;44:1144-50.
- Panahi Y, Mostafazadeh B, Abrishami A, Saadat A, Beiraghdar F, Tavana S, et al. Investigation of the effects of Chlorella vulgaris supplementation on the modulation of oxidative stress in apparently healthy smokers. Clin Lab 2013;59:579-87.
- Gupta P, Sinha D, Bandopadhyay R. Isolation and screening of marine microalgae Chlorella sp. pr1 for anticancer activity. Int J Pharm Pharm Sci 2014;6:517-19.
 C. vulgaris,
C. vulgaris,  C. reinhardtii,
C. reinhardtii,  standard (streptomycin).
standard (streptomycin).