- *Corresponding Author:
- O. Gholami
Department of Physiology and Pharmacology, Faculty of Medicine, Sabzevar University of Medical Sciences, Sabzevar, Iran
E-mail: omidghphd@gmail.com
| Date of Submission | 21 June 2016 |
| Date of Revision | 24 October 2016 |
| Date of Acceptance | 12 December 2016 |
| Indian J Pharm Sci 2016;78(6):827-833 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Induction of apoptosis in tumor cells is one of the mechanisms of chemotherapy. Myeloid cell leukemia type 1 is one of the Bcl-2 protein families that make an important role in chemo resistance to drugs. Thus down-regulation of myeloid cell leukemia type 1 gene is one of the aims in chemotherapy. Umbelliprenin and auraptene are naturally coumarins. Cytotoxicity and apoptosis induction is one of their effects. In this study we examined the cytotoxicity of these two coumarins against some cancerous cell lines by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay method and compare them together. In next step, we tested the effect of auraptene. On myeloid cell leukemia type 1 gene expression in Jurkat cells by real time polymerase chain reaction method. We found that umbelliprenin and auraptene. Have cytotoxic effect against cancerous cell lines. Auraptene is more cytotoxic than umbelliprenin. We also found that auraptene down-regulates myeloid cell leukemia type 1 gene expression in a pattern that different from umbelliprenin.
Keywords
Umbelliprenin, auraptene, cytotoxicity, apoptosis, myeloid cell leukaemia type 1
Cancer is the uncontrolled growth of abnormal cells in the body. Chemotherapy and radiotherapy are highly effective methods of cancer treatment, but these methods exert severe side effects [1]. Gradual resistance of cancer cells against these treatment is one of the main emerging problems in cancer treatment [2]. In this regard, achieving a new approach to improve cancer treatment results is one of the aims of immunopharmacological studies [3]. Apoptosis or programmed cell death is a strictly controlled pathway that is responsible for removal of unwanted cells, old and injured cells [4]. The induction of apoptosis in tumour cells can lead to their own destruction, so apoptosis has been suggested as an efficient mechanism by which malignant tumour cells can be removed when treated with antineoplastic drugs [5].
The B-cell lymphoma 2 (Bcl-2) protein family acts as a key regulator in the intrinsic or mitochondrial apoptosis pathway [6]. Myeloid cell leukaemia type 1 (Mcl-1) is one of the most important members of the Bcl-2 protein family. Mcl-1 is highly expressed in a variety of human cancer cell lines including breast, CNS, colon, lung, ovarian, prostate, renal and melanoma [7].
Overexpression of Mcl-1 gene is exploited by cancer cells to survive, and as a mechanism for developing resistance to diverse chemotherapeutic agents [8]. There are multiple drugs that display a mechanism of action that involves down-regulation of Mcl-1. Studies with these drugs show the importance of this protein in maintenance and progression of the cancer phenotype [8].
Many anticancer drugs are basically derived from herbal plants. Genus Ferula includes herbal plants that traditionally used as food and/or medicine in Iran and other Asian countries. Many Ferula species, including F. asafetida, synthesize terpenyloxy coumarins. Coumarins are a wide class of natural and synthetic compounds that showed variety of pharmacological activities including antiinflammatory [8], antioxidant [9], antinociceptive [ 10], hepatoprotective [11], antithrombotic [12], antiviral [13], antimicrobial [14,15], antituberculosis [16], anticarcinogenic [17], antidepressant [18], antihyperlipidemic [19] and anticholinesterase [20] activities.
Umbelliprenin and auraptene are terpenyloxy coumarins synthesized by F. asafoetida and citrus species. These 2 coumarins have similar structure, with the only difference being the higher length of the 7-prenyloxy chain, which contains 15 instead of 10 carbons [21]. In the present study, we examined the cytotoxicity of umbelliprenin and auraptene on various cancerous cell lines by 3-(4,5-dimethylthiazol-2- yl)-2,5 diphenyltetrazolium bromide (MTT) method and compared those together. We also tested the effect of auraptene on Mcl-1 gene expression in Jurkat cells by real time polymerase chain reaction (PCR) method and compared it with the effect of umbelliprenin on gene expression.
Materials and Methods
Cell lines were prepared from national cell bank of Iran (Pasteur institute, Tehran, Iran). HeLa (cervical cancer cell line), Jurkat (T cell leukaemia cell line), MCF-7 (breast cancer cell line) and KYSE-30 (oesophageal carcinoma cell line) were cultured in RPMI 1640 (Gibco™, Cat. Number: 11875-093) and incubated at 95% humidity, with 5% CO2 at 37° in the culture medium containing 10% foetal bovine serum (FBS, Gibco™, Cat.Number:10082-147) and 50 U/ml penicillin/streptomycin (Sigma Aldrich, Cat. Number: 4458). After that cells were frozen in FBS containing 10% dimethyl sulfoxide (DMSO) and stored in liquid nitrogen (5×106 cells/vial). The viability of cryopreserved cells was determined by trypan blue staining, immediately upon thawing. Only cells whose viability exceeded 93% (range of 93.4-99%) were used in this study. Umbelliprenin and auraptene preparations were obtained from Biotechnology Research Center and School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran.
MTT assay
The effects of auraptene and umbelliprenin on the growth of cell lines were examined using the MTT assay. Cells were sub-cultured in 96-well plates at a density of 104 cells (for MCF-7, HeLa, KYSE-30) or 5×104 cells (Jurkat) per well with or without auraptene and umbelliprenin (10, 20, 40 μg/ml) for 24 and 48 h in a final volume of 100 μl. Auraptene and umbelliprenin were dissolved in DMSO. Immediately before use, it was diluted in the culture medium to obtain a final DMSO concentration of 0.5% (v/v). Then, the medium was changed with fresh medium supplemented with 20 μl of MTT (5 mg/ml in phosphate-buffered saline (PBS)). Plates were incubated at 37° for 2 h. After addition of 100 μl DMSO to each well, plates were agitated for 1 min. Spectrophotometric absorbance at 570 nm was measured. Cell viability was calculated using the formula. %viable cells=(OD of drug-treated sample/OD of untreated sample)×100.
RNA isolation and real-time quantitative PCR analysis
Jurkat cells were incubated by auraptene (10, 20, 40 μg/ml) in 37° and 5% CO2 for 1, 2 and 3 h. Total RNA was isolated using RNX-Plus kit in accordance with the manufacturer's instructions (Sinagene®). The quantity and quality of the total RNA was verified with the Pico Drop spectrophotometer (Alpha Biotech, Cambridge, UK) according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized from 2 μg total RNA treated with DNase I using TaKaRa kit (PrimeScript First Strand cDNA Synthesis Kit).
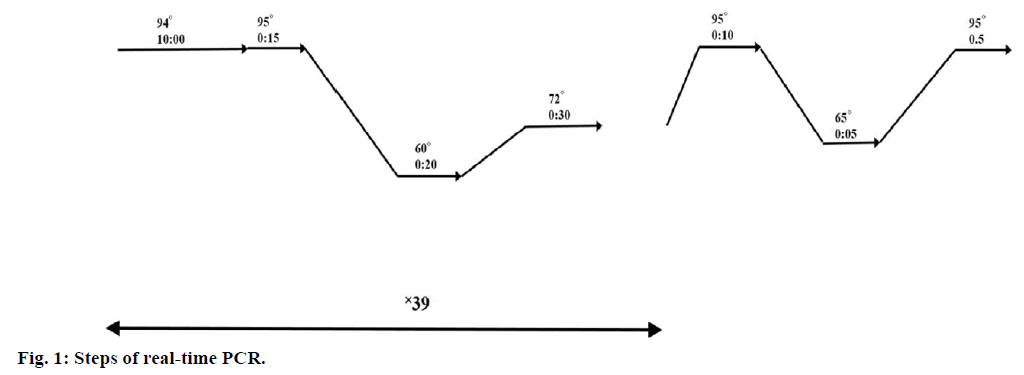
Real-time PCR was performed in 3 replicates in 20 μl reaction volumes using 0.2 μl cDNA, 10 μ SYBR Green master mix (YEKTA TAJHIZ AZMA, SYBR Green qPCR Master Mix 2x) and 0.4 μl of each primer on a Bio-Rad real time CR System. PCR was performed using Mcl-1 primers (5′-CCA AGA AAG CTG CAT CGA ACC AT-3′ and 5′-CAG CAC ATT CCT GAT GCC ACC T-3′) and GAPDH primers (5′-GGA AGG TGA AGG TCG GAG T-3′ and 5′-GTC ATT GAT GGC AAC AAT ACC ACT-3′) as internal control. Conditions of real-time PCR steps are presented in Figure 1.
Statistical analysis
One way analysis of variance (ANOVA) test was used for statistical analysis by SPSS16 software. The P-value was considered significant when it was <0.05. Error bars indicate standard deviations. Number of readings (N) for MTT assay and real-time PCR were 3 replicate. Calculations were done with GraphPad Prism 5 (GraphPad Software, San Diego).
Results and Discussion
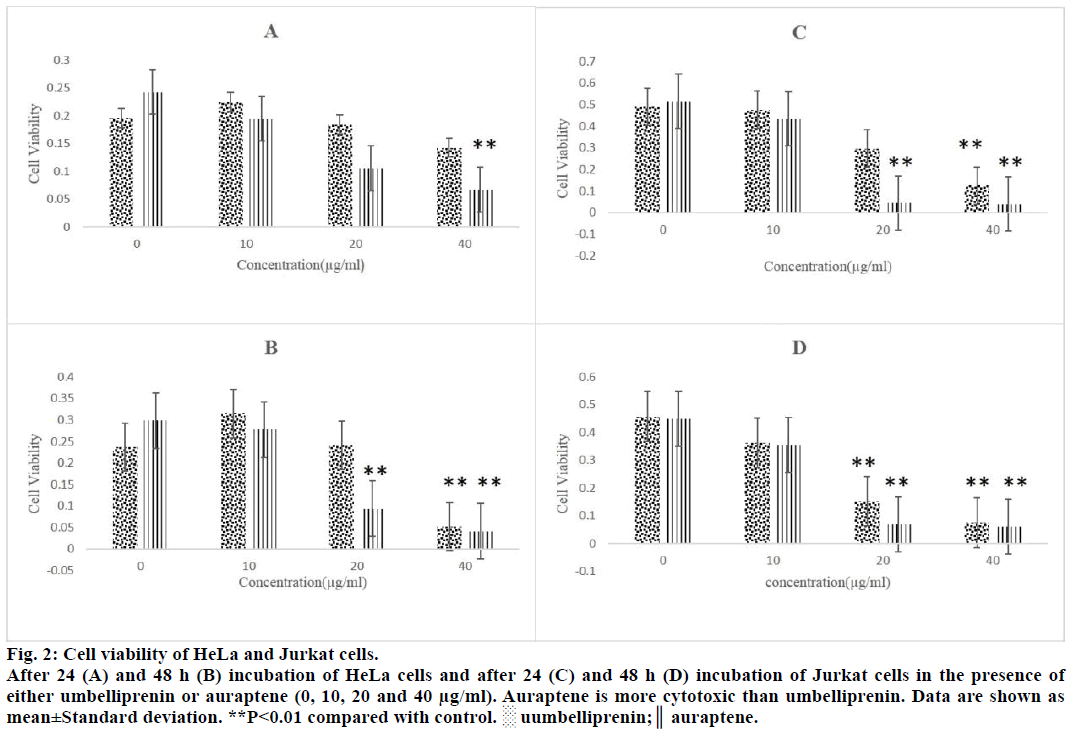
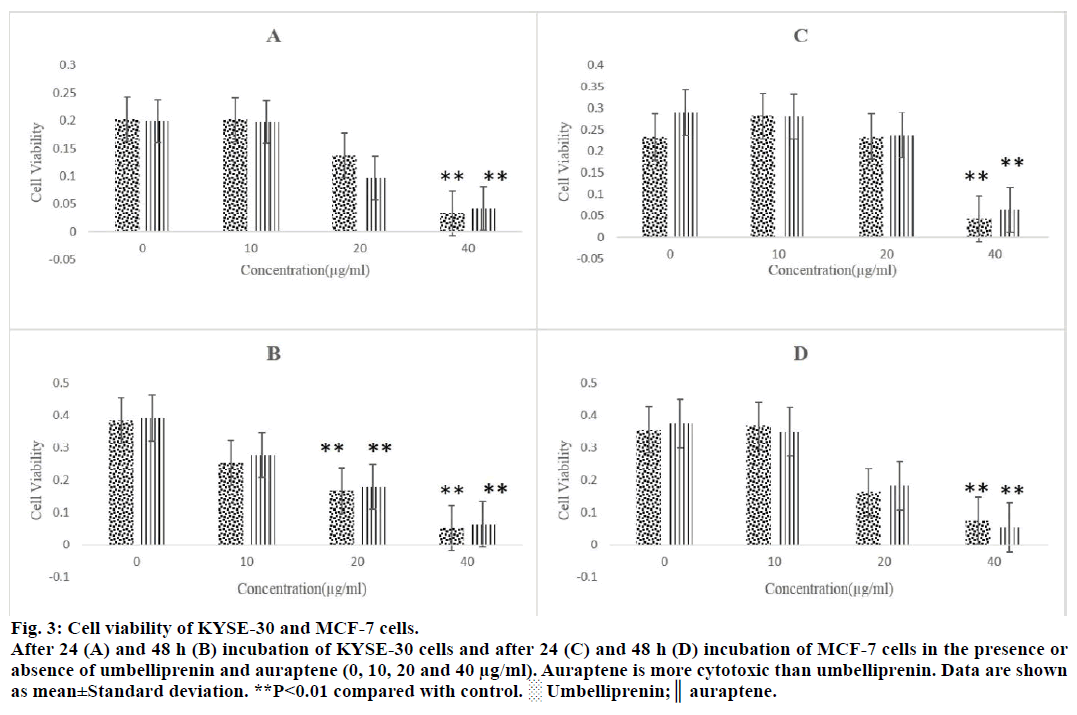
For comparison of cytotoxicity of auraptene and umbelliprenin, we incubated various concentrations (10, 20 and 40 μg/ml) of these compounds with HeLa (cervical cancer cell line), Jurkat (T cell leukaemia cell line), MCF-7 (breast cancer cell line) and KYSE- 30 (oesophageal carcinoma cell line) for 24 and 48 h. After these incubation periods the extent of cytotoxicity is estimated using the MTT assay. Auraptene was more cytotoxic than umbelliprenin against these cell lines (Figures 2 and 3). IC50 of these two compounds have been compared in Table 1. As shown in Table 1 cytotoxic effect is dose and time dependent. Moreover, auraptene is more potent than umbelliprenin in various concentrations and times (Table 1).
Figure 2: Cell viability of HeLa and Jurkat cells.
After 24 (A) and 48 h (B) incubation of HeLa cells and after 24 (C) and 48 h (D) incubation of Jurkat cells in the presence of
either umbelliprenin or auraptene (0, 10, 20 and 40 μg/ml). Auraptene is more cytotoxic than umbelliprenin. Data are shown as
mean±Standard deviation. **P<0.01 compared with control. ░ uumbelliprenin;║ auraptene.
Figure 3: Cell viability of KYSE-30 and MCF-7 cells.
After 24 (A) and 48 h (B) incubation of KYSE-30 cells and after 24 (C) and 48 h (D) incubation of MCF-7 cells in the presence or
absence of umbelliprenin and auraptene (0, 10, 20 and 40 μg/ml). Auraptene is more cytotoxic than umbelliprenin. Data are shown
as mean±Standard deviation. **P<0.01 compared with control. ░ Umbelliprenin;║ auraptene.
| Cell line | Compound | Time | IC50 |
|---|---|---|---|
| HeLa | Aur | 24 h | 13.33 |
| 48 h | 13.87 | ||
| Umb | 24 h | 20.22 | |
| 48 h | 27.26 | ||
| Jurkat | Aur | 24 h | 11.3 |
| 48 h | 11.49 | ||
| Umb | 24 h | 19.64 | |
| 48 h | 14.19 | ||
| KYSE-30 | Aur | 24 h | 11.75 |
| 48 h | 15.24 | ||
| Umb | 24 h | 29.51 | |
| 48 h | 24.32 | ||
| MCF-7 | Aur | 24 h | 29.66 |
| 48 h | 17.26 | ||
| Umb | 24 h | 29.76 | |
| 48 h | 15.10 |
Umbelliprenin and Auraptene (10, 20 and 40 µg/ml) were incubated with HeLa, Jurkat, MCF-7 and KYSE-30 cell lines for 24 and 48 h. Cytotoxicity was tested by MTT method. IC50 was calculated for each cell line and time of incubation. As it shows cytotoxic effect is dose and time dependent. Auraptene is more cytotoxic than umbelliprenin in various cell lines and times (time and IC50 have been indicated as h and µg/ml, respectively)
Table 1: Comparing the cytotoxicity of auraptene and umbelliprenin
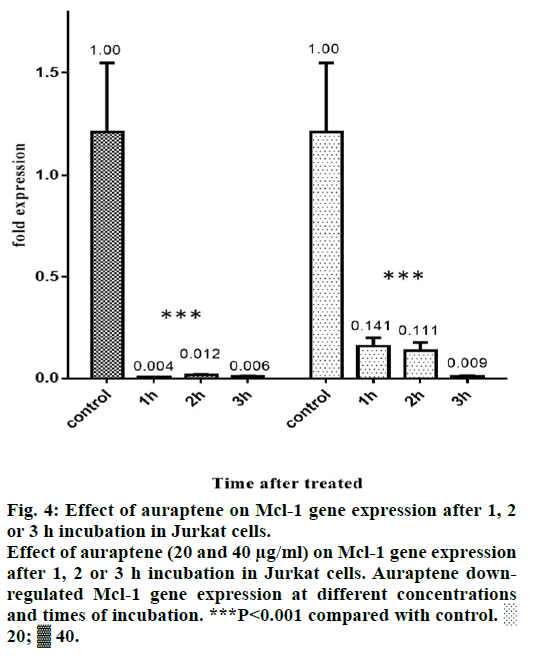
Mcl-1 protein has an obvious role in the chemoresistance of certain malignancies [22-24]. Forced Mcl-1 downregulation induce apoptosis in a number of cancer cell types [25]. Thus, Mcl-1 is a potential and attractive therapeutic target in a number of malignancies and is the focus of a number of studies reviewed [26]. In our study, we tested the effect of auraptene on Mcl-1 gene expression. We incubated Jurkat cells with 10, 20 and 40 μg/ml of auraptene for 1, 2 and 3 h. After that this effect was examined by real-time PCR method. 10 μg/ ml of auraptene did not have significant effect on Mcl- 1 gene expression. But auraptene down regulates Mcl- 1 gene in other concentrations and times of incubation (Figure 4).
Figure 4: Effect of auraptene on Mcl-1 gene expression after 1, 2
or 3 h incubation in Jurkat cells.
Effect of auraptene (20 and 40 μg/ml) on Mcl-1 gene expression
after 1, 2 or 3 h incubation in Jurkat cells. Auraptene downregulated
Mcl-1 gene expression at different concentrations
and times of incubation. ***P<0.001 compared with control. ░
20; ▓ 40.
Cancer is one of the most complicated diseases of this century. Natural products are very promising compounds in treatment of cancer. Among natural products, coumarins show cytotoxic effect in vitro and in vivo. Based on previous findings we decided to compare cytotoxic effect of two natural coumarin compounds (auraptene and umbelliprenin) in vitro.
Auraptene and umbelliprenin belongs to the class of natural terpenyloxy coumarins. Umbelliprenin has a different structure from auraptene by the presence of an acyclic sesquiterpenene group in place of the geranyl group at C7-OH of the 1,2-benzopyrone ring. The cytotoxicity of auraptene and umbelliprenin has been found to be related to the presence of the aliphatic sesquiterpenoid group linked at C7-OH. Cytotoxicity and apoptosis induction of auraptene and umbelliprenin on some of these cancerous cell lines were reported by other researchers [5,27,28]. Since, none of them compared the cytotoxicity of these two compounds together, we decided to do this. We showed that auraptene and umbelliprenin have doseand time-dependent cytotoxicity against these cell lines. In general, auraptene is more potent (has less IC50) than umbelliprenin in cytotoxic effects (Table 1). Umbelliprenin and auraptene have no cytotoxic effect on peripheral blood mononuclear cells (PBMCs) from healthy donors [5,29].
Induction of apoptosis by auraptene and umbelliprenin in Jurkat cells have previously shown [5,29]. These studies showed that umbelliprenin and auraptene, both of them, induced apoptosis in Jurkat cells by activation of caspase-8 (hallmark of extrinsic pathway of apoptosis) and caspase-9 (hallmark of intrinsic pathway of apoptosis) [5,29]. In our review article, we compared proapoptotic properties of umbelliprenin and auraptene. We notified that umbelliprenin promoted G1 cell cycle arrest but auraptene promoted G1/S cell cycle arrest in cancerous cell lines. Moreover, we noticed that umbelliprenin decreased Bcl-2 and Mcl-1 gene expression but auraptene decreased Bcl-XL gene expression (Table 1) [30].
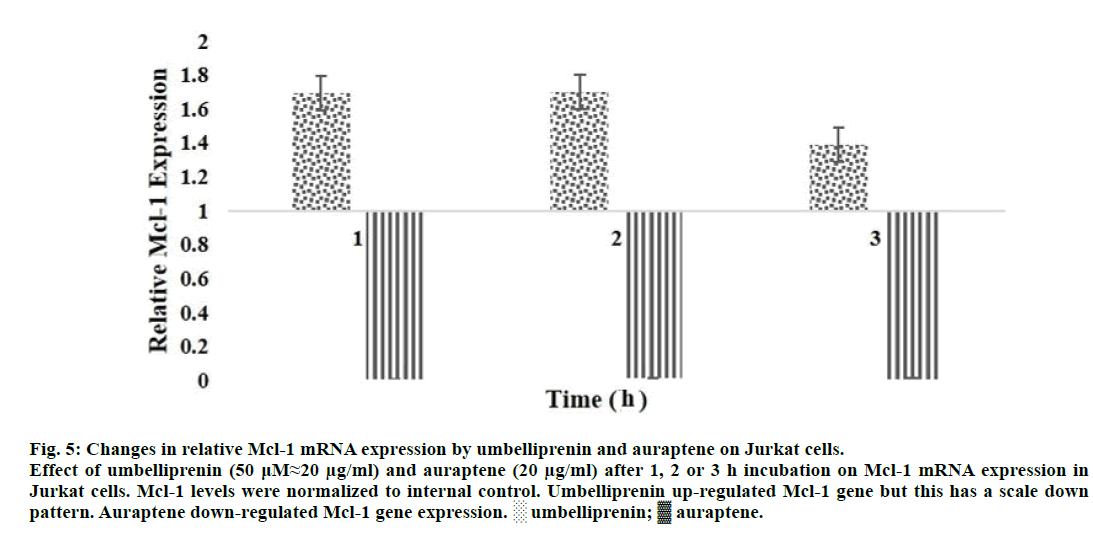
One of the most important proteins that were upregulated in leukemic cells like Jurkat is Mcl-1 protein. We previously showed that Mcl-1 gene was upregulated by umbelliprenin (50 μM≈20 μg/ml) from 1 to 3 h incubation in Jurkat cells [31]. In this study, we showed that Mcl-1 was down-regulated by auraptene (20 and 40 μg/ml) from 1 to 3 h incubation in Jurkat cells.
Umbelliprenin increased expression of Mcl-1 mRNA from 1 to 3 h incubation, but this increase has a scale down pattern [31]. Auraptene decreased expression of Mcl-1 mRNA for same incubation times. In Figure 5, we compared the extent of Mcl-1 mRNA changes by umbelliprenin (50 μM≈20 μg/ml) and auraptene (20 μg/ml) after 1, 2 and 3 h incubation.
Figure 5: Changes in relative Mcl-1 mRNA expression by umbelliprenin and auraptene on Jurkat cells.
Effect of umbelliprenin (50 μM≈20 μg/ml) and auraptene (20 μg/ml) after 1, 2 or 3 h incubation on Mcl-1 mRNA expression in
Jurkat cells. Mcl-1 levels were normalized to internal control. Umbelliprenin up-regulated Mcl-1 gene but this has a scale down
pattern. Auraptene down-regulated Mcl-1 gene expression. ░ umbelliprenin; ▓ auraptene.
Extent of Mcl-1 gene down-regulation by auraptene is different for various concentrations and times of incubation. This can be related to the different extent of apoptosis/necrosis induction by auraptene. As it shows in Figure 4, the extent of Mcl-1 gene down-regulation in 20 μg/ml is more than 40 μg/ml. This shows that auraptene induce apoptosis more than necrosis in 20 μg/ml concentration. In 40 μg/ml auraptene induce necrosis more than apoptosis. Our findings are consistent with those reported by June (Figure 3) [5]. More studies are needed to get further clarity. Finally, it may be concluded that umbelliprenin (but not auraptene) falls into the category of apoptosis inducing agents that initially up-regulates Mcl-1 gene.
It also appeared important to verify if auraptene and umbelliprenin promote anticoagulant effects and modulate immune functions. Anticoagulant effects may lead to side effects. However, auraptene and umbelliprenin may also contribute to decrease metastasis because interference with the fibrinolytic system affects angiogenesis. At least in one study, certain coumarins exhibiting anticoagulant effects inhibited metastasis in several animal models [32].
In a review regarding the structure-activity relationships and anticancer effects of natural and synthetic coumarins, the author reported that coumarins may enhance lymphocyte response to mitogen and induce immune-induced antitumor effects. The author further suggested that the effects of auraptene (and possibly umbelliprenin) would partly be due to an enhancement of immune function [32]. However, this stimulatory effect needed to be demonstrated.
In conclusion, we showed that umbelliprenin and auraptene have cytotoxic effects against some cancer cell lines. In this regard, auraptene was more cytotoxic than umbelliprenin against these cell lines. This study also demonstrated that auraptene down-regulated Mcl- 1 mRNA expression in a pattern that was different from umbelliprenin
Since, auraptene has a shorter length 7-prenyloxy chain than umbelliprenin, it has more favourable pharmacokinetic properties than umbelliprenin. Moreover, auraptene is more cytotoxic than umbelliprenin against cancerous cell lines. In conclusion, auraptene is more attractive compound for future studies (for example in vivo studies) than umbelliprenin.
Acknowledgements
The authors wish to thank Professor Mehrdad Iranshahi (Biotechnology Research Center and School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran) for providing preparations of umbelliprenin and auraptene.
Conflicts of interest
There are no conflict of mentioned.
Financial support and sponsorship
Nil.
References
- Qi F, Li A, Inagaki Y, Gao J, Li J, Kokudo N, et al. Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Bio Sci Trends 2010;4:297-307.
- Wang Z, Wang N, Chen J, Shen J. Emerging glycolysis targeting and drug discovery from chinese medicine in cancer therapy. Evid Based Complement Alternat Med 2012:873175.
- Safarzadeh E, Shotorbani SS, Baradaran B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv Pharm Bull 2014;4:421-7.
- Yang HL, Chen CS, Chang WH, Lu FJ, Lai YC, Chen CC, et al. Growth inhibition and induction of apoptosis in MCF-7 breast cancer cells by Antrodia camphorata. Cancer Lett 2006;231:215-27.
- Jun DY. Apoptogenic activity of auraptene of Zanthoxylum schinifolium toward human acute leukemia Jurkat T cells is associated with ER stress-mediated caspase-8 activation that stimulates mitochondria-dependent or -independent caspase cascade. Carcinogen 2007;28:1303-13.
- Ujval AK, Jochen HM. Anti-apoptotic BCL-2 family proteins in acute neural injury. Front Cell Neurosci 2014;8:281.
- Placzek WJ, Wei J, Kitada S, Zhai D, Reed JC, Pellecchia M. A survey of the antiapoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death Dis, 2010;1:e40.
- Quinn BA, Dash R, Azab B, Sarkar S, Das SK, Kumar S, et al. Targeting Mcl-1 for the therapy of cancer. Expert Opin Investig Drugs 2011;20:1397-411.
- Kostova I, Bhatia S, Grigorov P, Balkansky S, Parmar VS, Prasad AK, et al. Coumarins as antioxidants. Curr Med Chem 2011;18:3929-51.
- Alipour M, Khoobi M, Emami S, Fallah-Benakohal S, Ghasemi-Niri SF, Abdollahi M, et al. Antinociceptive properties of new coumarin derivatives bearing substituted 3,4-dihydro-2H-benzothiazines. Daru 2014;22:9
- Atmaca M, Bilgin HM, Obay BD, Diken H, Kelle M, Kale E. The hepatoprotective effect of coumarin and coumarin derivates on carbon tetrachloride-induced hepatic injury by antioxidative activities in rats. J Physiol Biochem 2011;67:569-76.
- Peng XM, Damu GL, Zhou C. Current developments of coumarin compounds in medicinal chemistry. Curr Pharm Des 2013;19:3884-930.
- Curini M, Epifano F, Maltese F, Marcotullio MC, Gonzales SP, Rodriguez JC. Synthesis of collinin, an antiviral coumarin. Aust J Chem 2003;56:59-60.
- Emami S, Foroumadi A, Faramarzi MA, Samadi N. Synthesis and antibacterial activity of quinolone-based compounds containing a coumarin moiety. Arch Pharm (Weinheim) 2008;341:42-8.
- Ostrov DA, Hernández PJA, Corsino PE, Finton KA, Le N, Rowe TC. Discovery of novel DNA gyrase inhibitors by high-throughput virtual screening. Antimicrob Agents Chemother 2007;51:3688-98.
- Manvar A, Bavishi A, Radadiya A, Patel J, Vora V, Dodia N, et al. Diversity oriented design of various hydrazides and their in vitro evaluation against Mycobacterium tuberculosis H37Rv strains. Bioorg Med Chem Lett 2011;21:4728-31.
- Nair RV, Fisher EP, Safe SH, Cortez C, Harvey RG, DiGiovanni J. Novel coumarins as potential anticarcinogenic agents. Carcinog 1991;12:65-9.
- Sashidhara KV, Kumar A, Chatterjee M, Rao KB, Singh S, Verma AK, et al. Discovery and synthesis of novel 3-phenylcoumarin derivatives as antidepressant agents. Bioorg Med Chem Lett 2011;21:1937-41.
- Yuce B, Danis O, Ogan A, Sener G, Bulut M, Yarat A. Antioxidative and lipid lowering effects of 7,8-dihydroxy-3-(4-methylphenyl) coumarin in hyperlipidemic rats. Arzneimittelforschung 2009;59:129-34.
- Razavi SF, Khoobi M, Nadri H, Sakhteman A, Moradi A, Emami S, et al. Synthesis and evaluation of 4-substituted coumarins as novel acetylcholinesterase inhibitors. Eur J Med Chem 2013;64:252-9.
- Shakeri A, Iranshahy M, Iranshahi M. Biological properties and molecular targets of umbelliprenin-a mini-review. J Asian Nat Prod Res 2014;16:884-9.
- Fu JR, Liu WL, Zhou JF, Sun HY, Zheng M, Huang M, et al. The effects of Mcl-1 gene on ATRA-resistant HL-60 cell. Zhonghua Xue Ye Xue Za Zhi 2005;6:352-4.
- Song L, Coppola D, Livingston S, Cress D, Haura EB. Mcl-1 regulates survival and sensitivity to diverse apoptotic stimuli in human non-small cell lung cancer cells. Cancer Biol Ther 2005;4:267-76.
- Thallinger C, Wolschek MF, Maierhofer H, Skvara H, Pehamberger H, Monia BP, et al. Mcl-1 is a novel therapeutic target for human sarcoma: synergistic inhibition of human sarcoma xenotransplants by a combination of mcl-1 antisense oligonucleotides with low-dose cyclophosphamide. Clin Cancer Res 2004;10:4185-91.
- Moulding DA, Giles RV, Spiller DG, White MR, Tidd DM, Edwards SW. Apoptosis is rapidly triggered by antisense depletion of MCL-1 in differentiating U937 cells. Blood 2000;96:1756-63.
- Akgul C. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cell Mol Life Sci 2009;66:1326-36.
- Krishnan P, Kleiner-Hancock H. Effects of Auraptene on IGF-1 Stimulated Cell Cycle Progression in the Human Breast Cancer Cell Line, MCF-7. Int J Breast Cancer 2012:502092.
- Gholami O, Jeddi-Tehrani M, Iranshahi M, Zarnani AH, Ziai SA. Umbelliprenin from Ferula szowitsiana activates both intrinsic and extrinsic pathways of apoptosis in Jurkat T-CLL cell line. Iran J Pharm Res 2013;12:371-6.
- Ziai SA, Gholami O, Iranshahi M, Zamani AH, Jeddi-Tehrani M. Umbelliprenin Induces Apoptosis in CLL Cell Lines. Iran J Pharm Res 2012;11653-9.
- Gholami O, Shamsara J. Comparison of the cytotoxic effects of umbelliprenin and auraptene. Int J Pharm Pharm Sci 2016;8:1-4.
- Gholami O, Jeddi-Tehrani M, Iranshahi M, Zarnani AH, Ziai SA. Mcl-1 is up regulated by prenylated coumarin, umbelliprenin in jurkat cells. Iran J Pharm Res 2014;13:1387-92.
- Kostova I. Synthetic and natural coumarins as cytotoxic agents. Curr Med Chem Anticancer Agents 2005;5:29-46.