- *Corresponding Author:
- R. K. Srivastava
SCM Shaikshik Sansthan, Sangipur, Pratapgarh-230 139, India
E-mail: rksalld@gmail.com
| Date of Submission | 31 January 2017 |
| Date of Revision | 10 October 2017 |
| Date of Acceptance | 23 May 2018 |
| Indian J Pharm Sci 2018;80(4):744-749 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
A sensitive, cost-effective, reproducible high-performance liquid chromatography method was developed and validated for quantitative determination of acetaldehyde in candesartan cilexetil using the concept of threshold of toxicological concern. Acetaldehyde is reacted with 2,4-dinitrophenylhydrazine to form a Schiff base product with an absorbing maximum at 364 nm. Effective chromatographic separation was achieved on an Inertsil ODS 3V, 250×4.6 mm, 5 µm column with a mobile phase of 40:60 v/v water and acetonitrile and at a flow rate of 1.0 ml/min. The column temperature was controlled at 25° and the injection volume was 30 µl. These conditions resolved the dinitrophenylhydrazine-acetaldehyde product with unreacted dinitrophenylhydrazine, the drug substances and related impurities, as well as diluent peak within 20 min. The retention time of dinitrophenylhydrazine-acetaldehyde product was approximately 10.6 min. The method was linear, accurate, precise, specific, rapid and found suitable for this analysis.
Keywords
Impurity, HPLC, TTC, DNPH, validation, ICH guidelines
Candesartan cilexetil (CC) is a non-peptide tetrazole derivative drug and official in European, British, Japanese and US Pharmacopoeia. Its molecular formula is C33H34N6O6 and molecular weight is 610.67. The drug is used mainly for the treatment of hypertension and commercially available in 4, 8, 16 and 32 mg of tablet dosage form either individually or in combination with other antihypertensive drugs [1-3].
Acetaldehyde (ACD) is a highly toxic, mutagenic and genotoxic carcinogen [4]. The exposure of ACD results in effects including irritation of the eyes, skin, and respiratory tract and may cause toxicity if inhaled, ingested, or absorbed through the skin. It is a skin and mucous membrane irritant, which causes a burning sensation of the nose, throat, and eyes. Large exposure of ACD may cause death due to respiratory paralysis [5]. The International Agency for Research on Cancer has listed ACD as a first group carcinogen and one of the most frequently found air toxin with a high risk of cancer [6,7]. In addition, ACD appears to DNA and cause abnormal muscle development as it binds to proteins. 1-Chloroethylcyclohexyl carbonate was used as a reagent in the esterification step of CC synthesis [8]. ACD was generated as a byproduct during this step and identified as a genotoxic impurity according to the guidelines [9,10]. The chemical structures of CC and ACD are presented in Figure 1.
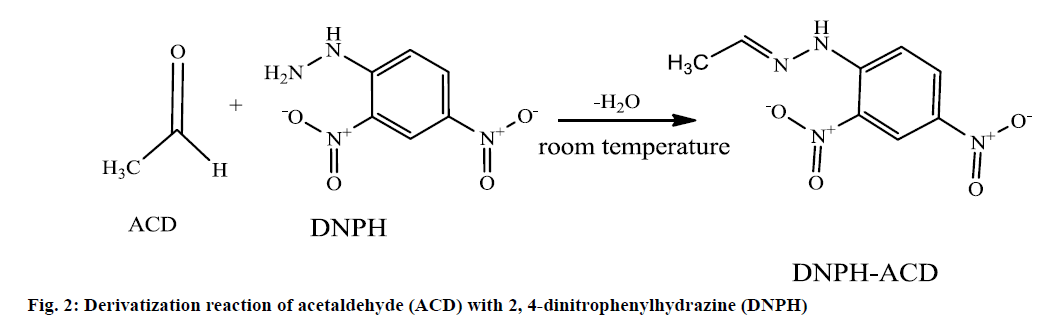
ACD has no chromophore and for this reason not detected by the UV detector. But due to its functional carbonyl group and low molecular weight ACD can be derivatized with several derivatization agents. The most widely used derivatization agent is 2,4-dinitrophenylhydrazine (DNPH). In acidic media, DNPH reacted with carbonyl group of ACD to form Schiff base derivatization product DNPH-ACD [11,12]. The derivatization reaction leads to an orange colored mixture of DNPH-ACD as shown in Figure 2.
Detailed literature survey revealed that the many high-performance liquid chromatography (HPLC) and gas chromatography (GC) methods have been reported for the determination of the CC individually or in combination with other drugs. However, very few HPLC methods are reported for the analysis of aldehyde using a derivatization reaction with DNPH in drug substances [13-15] and blood plasma [16]. To our knowledge no HPLC method is reported for quantitative determination of ACD in CC. In this communication, a validated HPLC method for quantitative determination of ACD in CC using a derivatization reaction was reported.
HPLC-grade water, orthophosphoric acid, carbon tetrachloride and acetonitrile were procured from Merck, Mumbai, India. AR grade DNPH (97 %) was purchased from Thomas Baker, Mumbai, India. ACD standard (99.9 %) was purchased from Sigma-Aldrich. All pure drug substances and impurities were procured from Macleods Pharmaceutical Ltd. The HPLC system consisted of Shimadzu model LC 2010 CHT, UV and photodiode array detector. The output signals were monitored and integrated using LC solution software. A Sartorius analytical balance and a Pico+ pH meter were used.
A reversed phase analytical column, Inertsil ODS 3V (250×4.6 mm, 5 μm) was used. Water and acetonitrile (40:60, v/v) was used as mobile phase and detection wavelength was set at 364 nm. The injection volume was 30 μl and flow rate was set at 1.0 ml/min. The run time was kept 20 min, the column temperature was maintained at 25° and water was used as the diluent.DNPH solution was prepared by adding 250 mg of DNPH standard to a 250 ml dry separating funnel containing 85 ml acetonitrile and shaking the funnel for 5 min to dissolve DNPH. Carbon tetrachloride (14 ml) and orthophosphoric acid (1 ml) were added to the funnel and 100 ml of HPLC-grade water was added at the end and allowed for complete separation of the two layers. The lower layer was discarded and the upper layer was used as a reagent solution. To 5 ml of DNPH solution, 3 ml of acetonitrile was added in a 10 ml volumetric flask and diluted to volume with water. This solution was allowed to stand at room temperature for 60 min and then used as blank solution.
To a 50 ml volumetric flask, 8.7 mg of ACD standard was added, dissolved in 10 ml of water and diluted to volume with water. This solution was labelled as 0.174 mg/ml. One milliliter aliquot of the 0.174 mg/ml solution was pipette out into a 50 ml volumetric flask and diluted to volume with water and this solution was labelled as 0.00348 mg/ml. To a 10 ml volumetric flask, 1 ml of 0.00348 mg/ml, 5 ml of DNPH solution, and 3 ml of acetonitrile were added and diluted to volume with water and this allows standing at room temperature for 60 min and used as derivatized standard solution.
To a 10 ml volumetric flask, 1 ml of ACD solution (0.00348 mg/ml), 5 ml of DNPH solution, and 3 ml of acetonitrile were added and this solution allowed to stand at room temperature for 10, 20, 30, 40, 60, 120, 180 min. At each interval, diluted reaction was analysed by HPLC and ACD was quantitatively converted to the derivatization product in 60 min.
Approximately 75 mg of CC was accurately weighed, transferred to a 10 ml volumetric flask, 5 ml of DNPH solution and 3 ml of acetonitrile were added and diluted to volume with water. This solution allowed to stand at room temperature for 60 min, filtered through a 0.45 μ nylon filter and the clear solution obtained was used for injection.
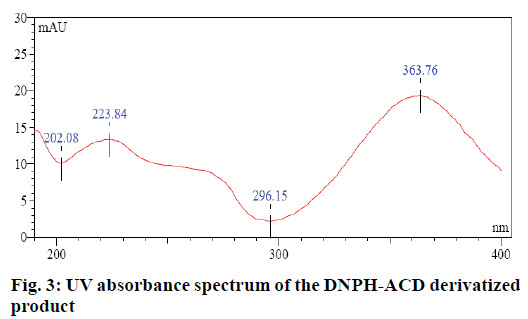
Evaluation limit for the genotoxic ACD impurity in CC was calculated based on threshold of toxicological concern (TTC) and the maximum daily dose of CC which was 32 mg. The maximum daily exposure target of genotoxic impurities is 1.5 μg/day per person. Hence the limit for ACD impurity was 1.5/0.0320=46.88 μg/g. The desired specificity of the method was achieved on an Inertsil ODS 3V (250×4.6 mm, 5μm) column with water and acetonitrile in the ratio (40:60, v/v) as mobile phase. Impurities were monitored using the detector at 364 nm (Figure 3).
In order to validate the developed HPLC method, validation characteristics such as specificity, detection limit, quantitation limit, linearity, precision, accuracy robustness and solution stability were considered as per ICH guideline [17]. The analyte ACD had excellent miscibility with water and the CC sample was insoluble. This ensured that the sample containing ACD as impurity at trace level could be detected and with a proper peak shape and without any interference from impurities and diluent peak.
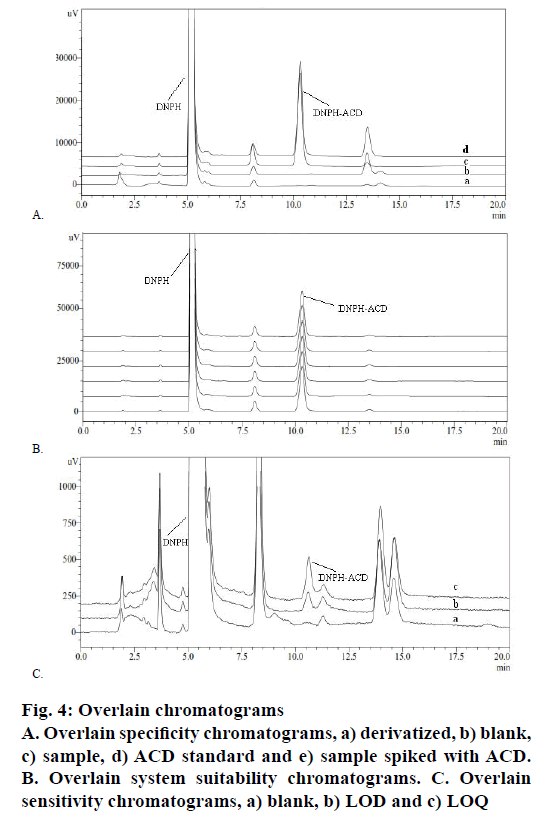
Specificity of the method for the estimation of the ACD impurity in CC in the present study was achieved by injecting separately derivatized solutions of blank, standard, sample and sample spiked with ACD standard individually. Figure 4A showed overlain chromatograms, which gave evidence that no interference with the DNPH-ACD derivatization product. The retention times of DNPH and DNPH-ACD have recorded at 5.3 and 10.6 min, while the CC peak was not detected due to being insoluble in the selected diluent.
According to USP, system suitability test is an integral part of liquid chromatographic methods to verify that the system is adequate for the analysis. Standard solution was prepared and 30 μl in six replicates was injected into the HPLC system. The obtained peak was calculated for the % RSD for six replicate injections of the standard was 0.46>5 %. The result was found to comply with USP requirements, which indicated that the chromatographic system is adequate for the intended analysis. Overlain chromatograms of replicate standard injection were presented in Figure 4B.
Sensitivity was determined by establishing limit of detection (LOD) and limit of quantification (LOQ) through signal-to-noise ratio of 3:1 and 10:1, respectively, by injecting a series of dilute solutions having a known concentration. LOD of the impurity is defined as the lowest concentration that can be detected and LOQ is the lowest concentration that can be quantified with acceptable precision and accuracy. The LOD and LOQ value for the impurity was found to be 0.20 and 0.58 μg/g, respectively. Precision study was carried out at LOQ by injecting six individual preparations of impurity and calculating % RSD i.e. 2.32>10 %. The representative chromatograms were shown in Figure 4C.
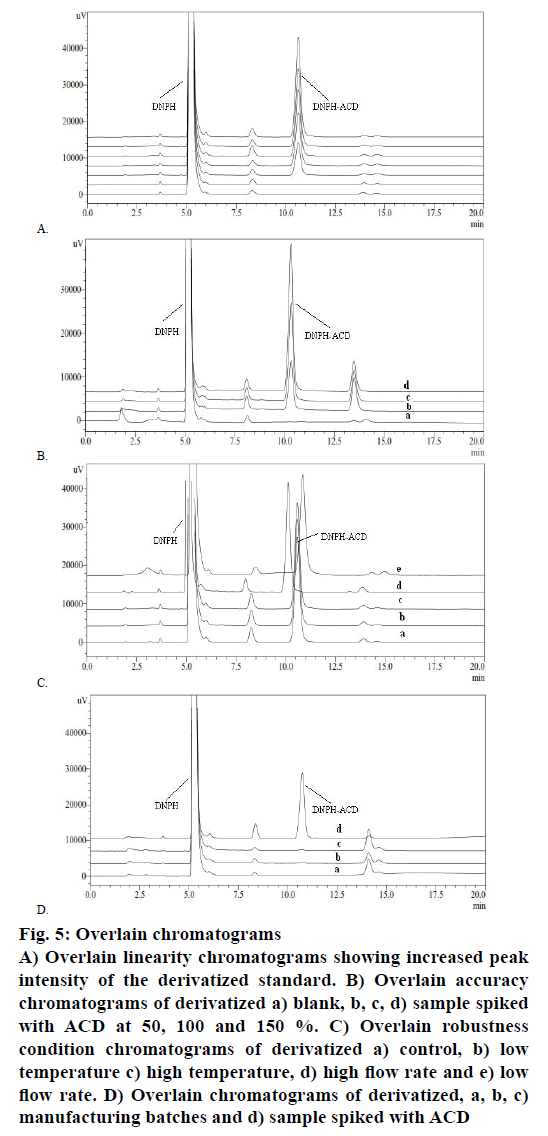
The method was found to exhibit good linearity (Figure 5A) in the 364 nm absorption with increasing concentration of ACD standard LOQ to 150 % of the evaluation limit. The result of correlation coefficient was observed (R2=0.9998<0.990). Accuracy and precision were validated on a CC sample spiked with ACD at three concentration levels covering the specified range with six replication for 46.88 μg/g ACD concentration and three replicates for 23.44 and 70.32 μg/g. The sample solution was prepared at a concentration of 7.5 mg/ml. The individual percent recoveries for all preparations were from 95.12-105.64 % and the % RSD of six replicate at 46.88 μg/g was 1.12>10 %. The representative chromatograms were shown in Figure 5B. Solution stability on the standard concentrations was tested for <2, 4, 12 and 24 h time point at laboratory temperature. Solution stability runs indicated that the DNPH-ACD adduct is stable up to 24 h at observed room temperature.
Figure 5: Overlain chromatograms
A) Overlain linearity chromatograms showing increased peak
intensity of the derivatized standard. B) Overlain accuracy
chromatograms of derivatized a) blank, b, c, d) sample spiked
with ACD at 50, 100 and 150 %. C) Overlain robustness
condition chromatograms of derivatized a) control, b) low
temperature c) high temperature, d) high flow rate and e) low
flow rate. D) Overlain chromatograms of derivatized, a, b, c)
manufacturing batches and d) sample spiked with ACD
To determine the robustness of the method, experimental conditions were deliberately altered and the system suitability result was evaluated. To study the effect of flow rate, it was changed by 0.2 units from 1.0 to 0.8 and 1.2 ml/min. The effect of column temperature was studied by changing 5° units from 25° to 20° and 30°. These results revealed that the deliberate changes in the method i.e. flow rate of mobile phase and column oven temperature have no impact on system suitability. The Overlain chromatograms of robustness condition were shown in Figure 5C. The developed and validated method was tested on three batches of manufactured CC. The samples were taken and subjected to the derivatization reaction. Figure 5D showed the overlain chromatograms of these experiments where the CC without ACD spiked was compared against spiked samples demonstrating that ACD was not detected. The summary of the validation result is presented in Table 1. The isocratic HPLC method developed for the quantitative determination of genotoxic ACD impurity in CC is linear, precise, accurate, rugged and robust.
| Parameter | Experiment | Results | |||||
|---|---|---|---|---|---|---|---|
| System suitability | The standard solution injected six replicate and measured the peak area response | % RSD of six replicate DNPH-ACD peak 0.46>5 % | |||||
| Specificity | Method blank, As such sample and spiked sample | No interference at retention time DNPH-ACD peak | |||||
| Limit of detection | 0.20 µg/g concentration of ACD standard | Signal to noise ratio 3.68 | |||||
| Limit of quantitation | 0.58 µg/g concentration of ACD standard and six replicated injections | Signal to noise ratio 14.65 and % RSD of peak is 2.32 | |||||
| Linearity | ACD standard at six levels with the concentration of 0.58, 23.44,37.50,46.88, 56.26, 70.32 µg/g of the evaluation limit | Correlation coefficient (R2): 0.9998, slope: 7201.78, intercept: –3427.39, residual sum of square: 61326262.6 | |||||
| Accuracy/ precision |
Six replicate sample solutions (7.5 mg/ml) were spiked with ACD at 46.88 µg/g, three replicate sample solutions (7.5 mg/ml) were spiked with ACD at 23.44 and 70.33 µg/g | Accuracy/ Precision |
46.88 µg/g (n=6) | 23.44 µg/g (n=3) |
70.33 µg/g (n=3) |
||
| Recovery Mean | 99.2 | 95.8 | 104.3 | ||||
| % RSD | 1.12 | 0.70 | 1.46 | ||||
| Solution stability | Standard solution was stored at room temperature for 24 h | The % RSD of DNPH-ACD peak at 24 h was less than 5.0 % | |||||
| Robustness | The peak DNPH-ACD should be well-resolved from unknown peak under robustness conditions % RSD of DNPH-ACD peak area of six replicate standard solutions not more than 5.0 % under robustness conditions |
Robustness | RT (min) | % RSD | |||
| Control | 10.6 | 1.14 | |||||
| 0.8 ml/min | 11.2 | 1.25 | |||||
| 1.2 ml/min | 9.8 | 1.65 | |||||
| 20° | 10.6 | 1.98 | |||||
| 30° | 10.6 | 1.23 | |||||
Table 1: Summary of method validation data
Satisfactory results were obtained from validation of the method as per ICH guideline. This method exhibited good performance in terms of sensitivity and specificity with no observed sample matrix and impurity interference and could be used for routine determination of the content of ACD in quality control laboratory.
Acknowledgements
The authors wish to thank the management of Macleods Pharmaceutical Ltd., for supporting this work.
Conflict of interests
There are no conflicts of interest.
References
- Pradhan KK, Mishra US, Pattnail S, Pand CK, Sahu KC. Development and Validation of a Stability-indicating UV Spectroscopic Method for Candesartan in Bulk and Formulations. Indian J Pharm Sci 2011;73:693-6.
- United States Pharmacopeia, USP39 (NF-34). Rockville, Maryland, United States: United States Pharmacopeial Convention; 2016. p. 2894-8.
- Husain A, Azim Md S, Mitra M, Bhasin PS. A review on Candesartan: Pharmacological and Pharmaceutical profile. J Appl Pharm Sci 2011;01:12-7.
- Seitz HK, Stickel F. Acetaldehyde as an underestimated risk factor for cancer development: role of genetics in ethanol metabolism. Genes Nutr 2010;5:121-8.
- Brooks PJ, Theruvathu JA. DNA adducts from acetaldehyde: implications for alcohol- related carcinogenesis. Alcohol 2005;35:187-93.
- List of IARC Group 1 carcinogens. Available from: https://en.wikipedia.org/wiki/List_of_IARC_Group_1_carcinogens.
- Osorio VM, Cardeal ZL. Analytical methods to assess carbonyl compounds in food and beverages. J Braz Chem Soc 2013;24:1711-8.
- Zupancic S. Process for the preparation of candesartan cilexetil. United States patent US20120029201A1. 2005.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk M7. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Multidisciplinary/M7/M7_Step_4.pdf.
- European Medicines Agency, Evaluation of Medicines for Human Use. Guideline on the Limits of Genotoxic Impurities. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002903.pdf.
- Fung K, Grosjean D. Determination of Nanogram Amounts of Carbonyls as 2,4-Dinitrophenylhydrazones by High-Performance Liquid Chromatography. Anal Chem 1981;53: 168-71.
- Kozutsumi D, Arita M, Kawashima A, Adachi M, Takami M. An Improved Method for Acetaldehyde Determination in Blood by High-Performance Liquid Chromatography and Solid-Phase Extraction. J Chromatogr Sci 2002;40:477-82.
- Velankar SJ, Lokhande R, Yadav R, Pawar R. HPLC method development and validation for determination of formaldehyde and acetaldehyde traces in drug substances. IJCPA 2016;3:1-6.
- Soman A, Qiu Y, Chan Li Q. HPLC-UV method development and validation for the determination of low level formaldehyde in a drug substances. J Chromatogr Sci 2008(46):461-5.
- Nageswari A, Krishna Reddy KVSR, Mukkanti K. Low-level quantitation of formaldehyde in drug substances by HPLC-UV. Chromatographia 2012;75:275-80.
- Guan X, Rubin E, Anni H. An optimized method for the measurement of acetaldehyde by high-performance liquid chromatography. Alcohol Clin Exp Res 2012;36:398-405.
- International Conference on Harmonization. Validation of analytical procedures, Text and Methodology, Q2 (R1). Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002662.pdf.