- Corresponding Author:
- A. Srinatha
Department of Pharmaceutics, National College of Pharmacy, Shimoga-577 201, India
E-mail: asrinatha@yahoo.com
| Date of Submission | 17 March 2011 |
| Date of Revision | 04 December 2011 |
| Date of Acceptance | 10 December 2011 |
| Indian J Pharm Sci, 2011, 73 (6): 641-648 |
Abstract
The present paper describes development of a polysaccharide based compression coated tablets of secnidazole for colon delivery. Core tablet containing secnidazole was compression coated with various proportions of guar gum, xanthan gum and chitosan, either alone or in combinations. Drug release studies were performed in simulated gastric fluid (SGF) for 2 h followed by simulated intestinal fluid (SIF, pH 7.4) up to 24 h. Secnidazole release from the prepared formulations was dependent on the type and concentration of polymer used in the formulation. Tablets coating containing either guar gum or xanthan gum showed ~30-40% drug release in 8 h. Further, in vitro dissolution studies of selected formulations performed in the dissolution media with rat caecal contents showed 54.48±0.24 - 60.42±0.16% of drug release. Formulations with single polymer in coating layer were unsuitable for targeting secnidazole release to colon region. Combination of chitosan with guar gum or xanthan gum exhibited control over secnidazole release.
Keywords
Chitosan, colon targeted drug delivery, compression coating, guar gum, secnidazole, xanthan gum
Entamoeba histolytica is a single celled protozoa responsible for causing amoebic colitis by infecting the lower part of intestine [1]. Such conditions of large intestinal amoebiasis and giardiasis are treated with certain nitroimidazoles like secnidazole, tinidazole, ornidazole and metronidazole which are drugs of choice [2]. Secnidazole, is the latest nitroimidazole derivative used in treatment of colonic infections. However, oral administration of secnidazole is associated with certain adverse effects like dizziness, neurological disturbance, headache and gastrointestinal disturbance like nausea, anorexia and abdominal pain [3,4]. In view of severe adverse effects, especially in the abdominal region, it is beneficial to deliver the drug to lower part of gastrointestinal tract. This not only avoids adverse effects of secnidazole but also deliver the drug at the required site for effective treatment conditions like amoebiasis, giardiasis and tricomoniasis.
Site-specific drug delivery to colonic region may be achieved by different approaches. Majority of methods widely explored includes covalently linked drug to a carrier, pH-sensitive polymer either as coating or matrix, time dependent release systems and the use of colonic bacteria degradation dependent systems [5]. Enteric coated systems are widely reported for colonic drug delivery. However, this system is limited by the fact that the pH difference between small intestine and colonic region is very narrow. Therefore, judicious selection of polymer is the necessity to avoid drug dumping in small intestine. The other system, time dependent release systems are designed considering the normal gastrointestinal transit time. Any variation in the gastrointestinal transit time is not sensed by the formulation and similar to pH dependent systems drug may be dumped in small intestine. Considering the limitations of pH dependent and time dependent systems, microbial degradation dependent (colonic bacteria) matrix tablets is the most suitable alternative approach [5,6]. It has been reported in the literature that polysaccharides are good carriers for developing microbial degradation dependent colon targeted systems [7-14].
The purpose of this research was to develop compression coated matrix tablets of secnidazole for colonic delivery based on the principle of microbial degradation. Further, it was aimed to investigate the effect of combinations of selected polysaccharide polymers (guar gum, xanthan gum and chitosan) on release profile of secnidazole.
Materials and Methods
Secnidazole was obtained as gift sample from Magnus Pharma Pvt. Ltd, Nepal. Sodium starch glycolate was obtained as gift sample from Wockhardt research centre, Aurangabad. Guar gum (GG) and microcrystalline cellulose were purchased from SD fine chemicals, Mumbai. Xanthan gum (XG) and Chitosan (Mw 45 kDa, 87% DD) were purchased from Himedia laboratories Ltd, Mumbai. All other chemicals used were of analytical grade.
Preparation of fast disintegrating core tablets
The core tablets of secnidazole were prepared by direct compression technique using the composition given in Table 1. Secnidazole, sodium starch glycolate, microcrystalline cellulose, magnesium stearate and talc were thoroughly mixed in a polybag and passed through sieve no #80 (179 μm) to ensure complete mixing. Sodium starch glycolate was incorporated in the formulation as a superdisintegrant. The mixture was compressed in to tablet on a single station tablet punching machine (M/s Cadmach, India) using 9.5 mm round, flat-faced punches.
| Ingredients | Quantity (mg) |
|---|---|
| Secnidazole | 100 |
| Microcrystalline cellulose | 25 |
| Sodium starch glycolate | 20 |
| Magnesium stearate | 2.5 |
| Talc | 2.5 |
| Total weight | 150 |
Table 1: Composition Of Secnidazole Core Tablet
Preparation of compression coated tablets
The composition of compression coating material is shown in Table 2. All the ingredients of each coating layer were weighed accurately and mixed in a polybag. 40% of total weight (250 mg) of coating mixture was placed in the die cavity of single station tablet punching machine, the core tablet was placed on it at centre, remaining 60% of coating mixture was added to the die cavity and tablets were compressed using 12.6 mm flat punches. The total weight of the compression coated tablet was about 400 mg.
| Formulation | Guar gum (mg) | Xanthan gum (mg) | Chitosan (mg) | Microcrystalline | Magnesium | Talc (mg) |
|---|---|---|---|---|---|---|
| cellulose (mg) | stearate (mg) | |||||
| F1 | 200 | 0 | 0 | 45 | 3 | 2 |
| F2 | 0 | 200 | 0 | 45 | 3 | 2 |
| F3 | 0 | 0 | 200 | 45 | 3 | 2 |
| F4 | 133.33 | 0 | 66.66 | 45 | 3 | 2 |
| F5 | 150 | 0 | 50 | 45 | 3 | 2 |
| F6 | 166.66 | 0 | 33.33 | 45 | 3 | 2 |
| F7 | 183.33 | 0 | 16.66 | 45 | 3 | 2 |
| F8 | 0 | 133.33 | 66.66 | 45 | 3 | 2 |
| F9 | 0 | 150 | 50 | 45 | 3 | 2 |
| F10 | 0 | 166.66 | 33.33 | 45 | 3 | 2 |
| F11 | 0 | 183.33 | 16.66 | 45 | 3 | 2 |
| F12 | 125 | 25 | 50 | 45 | 3 | 2 |
| F13 | 100 | 50 | 50 | 45 | 3 | 2 |
| F14 | 75 | 75 | 50 | 45 | 3 | 2 |
| F15 | 50 | 100 | 50 | 45 | 3 | 2 |
Table 2: Composition Of Compression Coating Mixture
Evaluation of tablets
The thickness and diameter of the tablets was determined by using dial thickness apparatus and vernier callipers respectively. Tablet hardness of all the formulations was determined by using Monsanto hardness tester. All trials were done on five tablets from each formulations in triplicate and average values were recorded.
Both core and compression coated tablets were subjected to friability test using friabilator. Ten tablets were weighed (W0) and placed inside the Roche friabilator. The instrument was operated for 4 min at 25 rpm. The resulting tablets after 100 falls from a height of six inches were collected; weighed (Wt) and percentage loss was calculated using following equation: Friability (%)=(Wo-Wt)/Wo×100.
The weight variation study of the prepared formulations was performed as per the standard procedure following Indian Pharmacopoeia [15].
Determination of drug content
Tablets from each formulation of compression coated and the core tablets were powdered and transferred in to 100 ml volumetric flask. Initially, 50 ml of phosphate buffer (pH 7.4) was added and allowed to rotate in a rotary shaker for 24 h at 100 rpm and volume was made up with phosphate buffer (pH 7.4). The amount of secnidazole present in the solution was estimated by using UV spectrophotometer (UV 1601, Shimadzu, Japan) at 320 nm against a suitable blank.
Swelling index
Tablets from each formulation was randomly selected, weighed individually (W1) and placed separately in a wire basket which was placed in a 100 ml beaker containing 0.1 N HCl for first 2 h and later phosphate buffer (pH 7.4) (24 h). After 2, 4, 6, 8 and 24 h the tablets were removed from wire basket and excess water was removed using filter paper. The swollen tablets were reweighed (W2) and swelling index of each tablet was calculated using the below equation. Swelling Index (%)= (W2-W1)/W1×100
Preparation of 2% rat caecal content
Rat caecal content was prepared using the previously described method [1]. Briefly, male Wistar rats (150-200 gm) on normal diet were used and asphyxiated using carbon dioxide. Caecal content were collected by dissection at the abdominal region and immediately transferred into phosphate buffer (pH 7.4) to prepare a final suspension at a concentration of 2% (w/v). Constant supply of CO2 was maintained throughout the experiment to maintain the anaerobic condition of colon.
In vitro drug release study
Dissolution studies were carried out in a USP basket type apparatus with a basket speed of 100 rpm. During the dissolution studies 900 ml of simulated gastric fluid (SGF; 0.1N HCl, pH 1.2) maintained at 37±1° was used as dissolution medium for the initial 2 h, followed by simulated intestinal fluid (SIF, phosphate buffer, pH 7.4) for remaining durations (i.e., 24 h). An aliquot was withdrawn at regular time intervals and was replenished with fresh dissolution medium to maintain sink condition. The aliquots were assayed on UV-spectrophotometer at 277 nm and 320 nm in SGF and SIF, respectively.
Drug release studies were also performed in presence of rat caecal content to evaluate the effect of microbial degradation on drug release from the prepared tablets. The experimental procedure for dissolution studies in presence of rat caecal content was same as described above but with a modification that 2% w/v rat caecal contents was added to phosphate buffer (pH 7.4), simulating colonic fluid (SCF).
Release kinetics
In vitro release data were fit to first order, zero order and Higuchi equations to analyse the kinetics of drug release from the tablets. Further, the dissolution data were fit to the following Korsmeyer-Peppas equation [16], to analyse drug release mechanism. Mt/M∞=Ktn, Where Mt/Mα is the fraction of drug released at time t, K is kinetic constant and ‘n’ is release exponent that characterize the drug transport.
Stability studies
Stability of the formulations was assessed by storing formulations F1, F5 and F11 at 40±2°/75±5% RH for 6 months. At the end of study period, formulations were evaluated for physical change, drug content and in vitro drug release.
Statistical analysis
The dissolution data collected during the experiments were statistically analysed using Student's t-test. A value of P<0.05 was considered statistically significant.
Results and Discussion
The present study was aimed at developing oral colon targeted formulations for secnidazole using different polysaccharides. Further, it was aimed to identify the most suitable polysaccharide, either alone or in combinations, for colonic delivery of secnidazole based on microbial degradation. It is a prerequisite for the colon drug delivery system that it should remain intact without releasing the loaded drug in stomach but on reaching the colon should completely be depleted of the drug. Hence, attempts were made to formulate compression coated tablets using guar gum, xanthan gum and chitosan, either alone or in combination based on bacterial degradation concept.
Results of drug content, hardness, thickness, diameter and friability of core tablet and the compression coated formulations are shown in Table 3. Hardness of the prepared formulations was in the range of 5.67±0.15 to 6.23±0.15 kg/cm2 indicating that tablets with sufficient hardness could be prepared using the selected polymers. However, hardness of secnidazole core tablet was 2.8±0.01 kg/cm2. The friability of all formulations was in the range of 0.86±0.04 to 0.98±0.03% indicating that the tablets could withstand the usual mechanical stress during handling. In case of core tablets the percentage friability was high and was found to be 3.06±0.12%. The disintegration time was measured for core tablet and was found to disintegrate within 60 s. The faster disintegration was desired as the core tablet is expected to completely release the drug in the colon region as soon as the compression coating was digested by the colonic microbes. The faster disintegration of the tablets could be explained for two reasons: first, it was a result of sodium starch glycolate which was incorporated in the formulation as a superdisintegrant. Sodium starch glycolate rapidly uptake dissolution fluid followed by a rapid and enormous swelling causing faster disintegration of tablets [17,18]. Secondly, the hardness of the tablet was very low indicating that core tablet is loosely compressed. The average weight of prepared formulations was in the range of 396.67±3.51 to 402.33±2.08 mg and in case of core tablets it was 152.67±3.51 mg. The results of weight variation test indicated that percentage deviation of tablet weights was within the acceptable limits as per Indian Pharmacopoeia [15]. The drug content of core tablets and compression coated tablets was in a range of 98.39±0.58 to 101.17±1.18%.
| Formulation | Thickness (mm) | Diameter (mm) | Hardness (Kg/cm2) | Tablet Weight (mg) | Friability (%) | Drug Content (%) |
|---|---|---|---|---|---|---|
| Core tablet | 2.08±0.02 | 9.50±0.01 | 2.80±0.01 | 152.67±3.51 | 3.06±0.12 | 101.09±0.90 |
| F1 | 2.68±0.04 | 12.6±0.01 | 6.13±0.23 | 397.67±1.53 | 0.92±0.05 | 99.94±1.19 |
| F2 | 2.68±0.01 | 12.6±0.01 | 6.23±0.15 | 399.33±3.21 | 0.91±0.07 | 100.17±1.23 |
| F3 | 2.70±0.05 | 12.6±0.03 | 5.93±0.15 | 400.33±3.21 | 0.98±0.03 | 98.39±0.58 |
| F4 | 2.64±0.04 | 12.6±0.01 | 5.97±0.21 | 404.33±1.15 | 0.91±0.07 | 99.44±0.24 |
| F5 | 2.59±0.03 | 12.6±0.11 | 5.77±0.15 | 400.33±3.05 | 0.88±0.06 | 98.78±1.28 |
| F6 | 2.77±0.02 | 12.6±0.02 | 6.00±0.1 | 400.00±1.00 | 0.92±0.04 | 100.70±2.04 |
| F7 | 2.60±0.03 | 12.6±0.01 | 6.07±0.11 | 402.33±2.52 | 0.94±0.06 | 99.42±2.21 |
| F8 | 2.66±0.01 | 12.6±0.03 | 6.00±0.20 | 401.00±3.00 | 0.93±0.06 | 99.55±0.48 |
| F9 | 2.69±0.01 | 12.6±0.01 | 5.67±0.15 | 401.67±1.53 | 0.91±0.02 | 99.13±1.33 |
| F10 | 2.59±0.02 | 12.6±0.01 | 5.87±0.15 | 398.67±2.52 | 0.86±0.04 | 100.13±1.60 |
| F11 | 2.61±0.03 | 12.6±0.01 | 6.17±0.21 | 402.33±2.08 | 0.93±0.02 | 100.09±1.64 |
| F12 | 2.64±0.08 | 12.6±0.02 | 6.03±0.21 | 396.67±3.51 | 0.93±0.05 | 98.63±0.74 |
| F13 | 2.62±0.02 | 12.6±0.02 | 5.93±0.30 | 399.67±1.53 | 0.89±0.04 | 99.78±1.49 |
| F14 | 2.58±0.02 | 12.6±0.01 | 6.07±0.11 | 396.00±4.36 | 0.95±0.08 | 99.66±1.57 |
| F15 | 2.60±0.03 | 12.6±0.01 | 5.97±0.15 | 398.00±2.64 | 0.88±0.06 | 101.17±1.18 |
All values are expressed as mean ±SD, n=3
Table 3: Physico-Chemical Properties Of The Prepared Tablets
Swelling study was performed for all formulations for duration of 24 h in simulating gastric and intestinal fluid. For first 2 h, swelling studies were conducted in 0.1N HCl (pH 1.2) and later in phosphate buffer (pH 7.4). The swelling behaviour was dependent upon the polymer or their combinations in coating material. In case of the formulations with guar gum (F1), xanthan gum (F2) and chitosan (F3) as coating polymers, the swelling index after 24 h was 478.09±14.02%, 1089.01±9.07% and 149.33±11.91%, respectively (Table 4). However, in formulations with combination of guar gum and chitosan as coating polymers, the swelling index for formulations F4, F5, F6 and F7 was 243.33±4.95%, 168.12±4.87%, 154.2±29.16% and 140.15±10.23%, respectively, after 2 h in SGF, and 388.79±8.11%, 394.05±14.96%, 422.62±21.55%.and 470.01±13.44%, respectively, in SIF after 24 h. It is clear from the results of the swelling studies that as chitosan proportion in the coating layer increases, the swelling index also increases in SGF, but swelling decreases in SIF. The result is expected since the amine group of chitosan is protonated in acidic fluids causing substantial swelling, while it is contrary in alkaline fluids. In case of combination of xanthan gum and chitosan, the swelling index for the formulations F8, F9, F10 and F11 in SGF was 262.53±6.06%, 150.42±6.30%, 141.46±4.35% and 127.52±5.59%, respectively, and in SIF after 24 h was 737.65±8.45%, 850.19±2.94%, 959.44±12.24% and 1009.18±10.75%, respectively. Similar to swelling pattern of guar gum with chitosan, formulations with a combination of xanthan gum and chitosan exhibited a unique pattern of pH depended and chitosan dependent swelling pattern. Chitosan showed a predominant effect on swelling of the prepared formulations in SGF, while the subsequent swelling was controlled by the amount of other hydrophilic polymer present in the coating mixture. Swelling index increased with increase in concentration of chitosan in SGF and the rest of swelling was influenced by the amount of either xanthan gum or guar gum in coating layer. Amongst guar gum and xanthan gum, the latter exhibited highest swelling as evidence by formulation F11. This is further supported by the swelling index of formulation F2 which contained xanthan gum. In case of combination of all three polymers, the swelling index for the formulations F12, F13, F14 and F15 after 24 h was 386.47±7.27%, 416.04±10.43%, 576.68±5.57% and 472.10±7.03%, respectively. The data indicates that the formulation F14 containing equal proportion of guar gum and xanthan gum (each at 75 mg) was more swollen compared with other formulations.
| Formulation | Time | ||
|---|---|---|---|
| 2 h | 8h | 24 h | |
| F1 | 131.60±10.99 | 261.04±8.04 | 478.09±14.02 |
| F2 | 135.21±4.83 | 866.96±6.76 | 1089.01±9.07 |
| F3 | 262.76±12.04 | 216.08±4.51 | 149.33±11.91 |
| F4 | 243.33±4.95 | 342.73±4.57 | 388.79±8.11 |
| F5 | 168.12±4.87 | 279.21±11.36 | 394.05±14.95 |
| F6 | 154.22±9.16 | 273.15±3.63 | 422.62±21.55 |
| F7 | 140.15±10.23 | 268.13±6.70 | 470.01±13.45 |
| F8 | 262.53±6.06 | 404.96±12.26 | 737.65±8.45 |
| F9 | 150.42±6.31 | 559.52±1.95 | 850.19±2.94 |
| F10 | 141.47±4.35 | 595.68±6.53 | 959.45±12.24 |
| F11 | 127.52±5.59 | 779.56±5.72 | 1009.18±10.75 |
| F12 | 187.04±1.87 | 300.22±4.74 | 386.48±7.27 |
| F13 | 198.36±4.99 | 308.81±6.40 | 416.04±10.43 |
| F14 | 248.74±9.03 | 528.1±5.18 | 577.68±5.57 |
| F15 | 195.69±11.21 | 396.37±3.27 | 472.10±7.03 |
All values are expressed as mean ±SD, n=3
Table 4: Swelling Index (%) Of Compression Coated Tablets
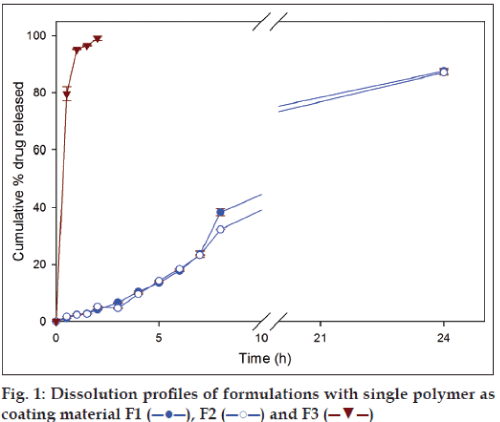
In case of formulations with single polymer, the amount of drug released from the formulation F1 (GG) and F2 (XG) after 8 h were 38.39±1.23% and 32.29±0.1%, respectively, and at 24 h 87.59±0.92% and 87.07±0.91%, respectively (fig. 1). Although, at the end of 8 h higher amount of drug was released from formulation containing guar gum (F1), at the end of 24 h there was no significant difference in amount of secnidazole released. Among the two polymers, guar gum and xanthan gum, the latter requires a higher duration (≈ 50 h) to completely swell in comparison to guar gum (24 h) [1]. Therefore, guar gum has shown a higher release in the initial periods of drug release studies due to faster imbibition of dissolution medium by the polymeric matrix. Formulation containing chitosan as coating polymer (F3) released 99.13±0.79% of secnidazole during the first 2 h of dissolution studies. The observed result is not a surprise as it is known that amine groups of chitosan undergo protonation in acidic environment causing higher swelling and subsequent dissolution of chitosan. The results indicate that chitosan alone is not suitable as a coating material for colon targeting as it leads to drug dumping in stomach.
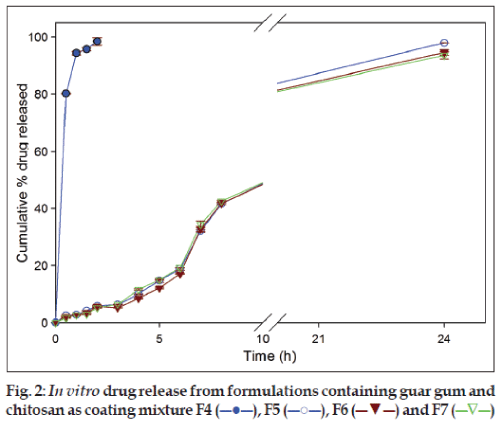
Formulations (F5, F6 and F7) with a mixture of guar gum and chitosan as coating layer, released 5.86±0.25%, 5.86±0.07% and 5.14±0.17% of drug, respectively, in SGF and 41.61±0.16%, 41.79±0.45% and 42.60±0.31%, respectively, in SIF after 8 h. At the end of dissolution studies (24 h) 97.89±0.62%, 94.59±0.93% and 93.57±1.24% of secnidazole was released from respective formulations (fig. 2). However, formulation F4 released 98.43±1.35% of secnidazole in SGF itself and was considered not suitable for suitable for colonic delivery. This faster release of secnidazole was probably due to the presence of chitosan at a higher amount (66.66 mg) which caused faster dissolution of chitosan matrix.
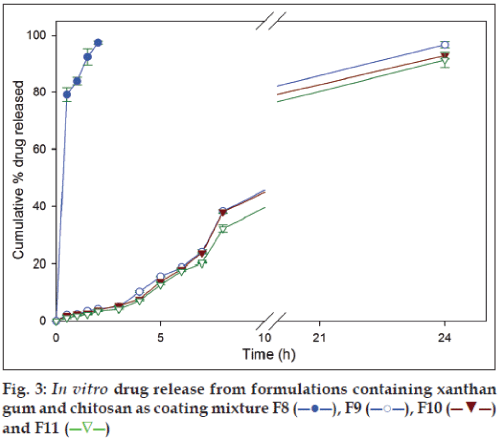
In the formulations with xanthan gum and chitosan coating mixtures (F9, F10 and F11), 4.17±0.11%, 3.95±0.15% and 3.51±0.15% of secnidazole was released in SGF, respectively, and 37.42±0.60%, 38.03±0.59% and 32.13±1.36%, respectively, in SIF after 8 h. At the end of 24 h dissolution studies, same set of formulations released 96.25±1.81%, 92.83±1.20% and 91.25±2.72% of secnidazole (fig. 3). Similar to the observations in formulations with guar gum and chitosan coating mixtures, formulation with 66.66 mg of chitosan (F8) released 97.45±0.61% of secnidazole in SGF itself indicating its inefficiency in controlling drug release in SGF. It can be noted that other polymers added in coating mixture (i.e., guar gum and xanthan gum) had a negligible influence on controlling drug release in SGF.
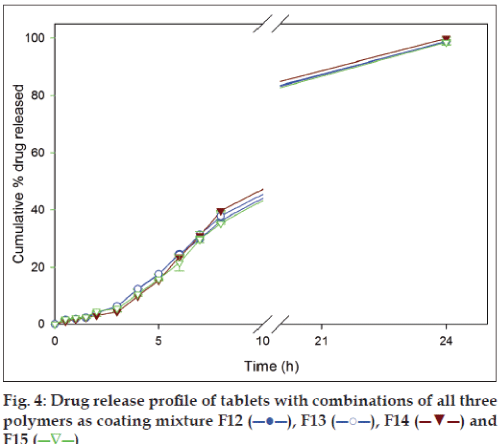
Dissolution studies were also conducted for formulations with mixture of all three polymers as coating materials to study their influence. Chitosan in all the combinations was maintained at 50 mg as the amount was enough to provide the desired release profile for 24 h. The amount of drug released from the formulation F12, F13, F14 and F15 in SGF after 2 h were 3.49±0.05%, 4.12±0.07%, 3.06±0.29% and 4.56±0.15%, respectively, and in SIF after 8 h were 31.57±0.45%, 37.83±1.13%, 39.69±0.14% and 35.34±0.32%, respectively. With continuation of dissolution and at the end of 24 h the same set of formulations gave 95.95±1.37%, 98.83±0.06%, 99.95±0.28% and 98.56±0.78% drug release, respectively (fig. 4). The drug release profiles of formulations F12-F15 (fig. 4) indicated that mixture of all three polymers released smaller amount of drug (<10%) in SGF in comparison to SIF.
The release of the drug embedded in a polymeric matrix generally is either by erosion of outermost gel layer or dissolution of drug by GI fluid and further diffusion through the gelled polymeric barrier [19]. In the present study, polysaccharides, either alone or in combinations were studied for compression coating with an aim to achieve drug release in colon region. The in vitro drug release studies indicated that a combination of polymers is desirable rather than a single polymer for modulating drug release profile. This is possibly because that tablets on imbibing dissolution fluid form gels and the gel consistency controls the polymeric matrix erosion which governs the drug release. Looser gels are more susceptible for matrix erosion which leads to faster drug release [20]. In the present study use of a single polysaccharide polymeric matrix may have produced a gel on swelling with loose consistency. In contrary, a combination of polysaccharide matrix might have produced stronger gel on swelling which resulted in a better control over drug release. However, further studies are necessary to determine whether gel strength of swollen matrix alone is responsible or other factors which may be responsible for the observed drug release pattern.
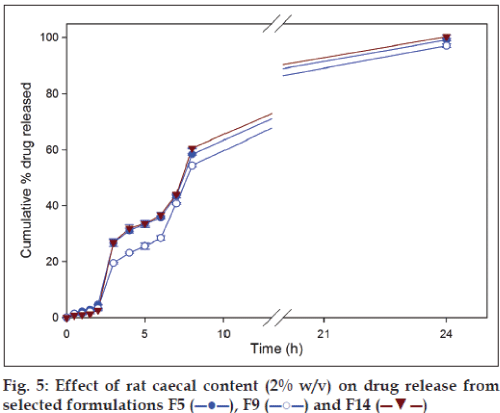
Glycosidases and polysaccharidases are colonic enzymes identified to cause hydrolysis of di- and tri-oligosaccharides, and polysaccharides, respectively. Both these enzymes are in the human colon produced by anaerobic bacteria [21]. Rat cecum is believed to have the same microbial content as that of the human and rats being readily available are the common species used during microbial degradation studies [22]. Microbial load in the colon is 1011-1012 CFU/ml [14] and 2% w/v rat caecal content is believed to mimic the desired microbial load in the dissolution fluid. On the basis of drug release data, formulation F5, F9 and F14 were selected to carryout dissolution studies in the presence of rat caecal contents. In vitro drug dissolution studies in presence of rat caecal content simulates an in vivo condition wherein the drug release is modulated by the microbial degradation of the polymer matrix. A significantly (P<0.05) higher amount of drug release in presence of rat caecal contents (simulated colonic fluid) was observed visà- vis simulated intestinal fluid (fig. 5). Inclusion of rat caecal contents to the dissolution fluid, mimicking colonic environment, releases glycosidases which act upon polysaccharide polymers like chitosan, xanthan gum and guar gum causing complete drug release due to degradation [14].
On the basis of the drug release profiles of formulations F5, F9 and F14, they were selected for analyzing the stability of the formulations. Formulations were stored at 40±2°/75±5% RH for 6 months. At the end of storage period, formulations were evaluated for any physical modifications and assayed for drug content and in vitro dissolution. No significant changes either in physical appearance or drug content were observed. When the dissolution study was conducted in the simulated physiological environment as described under experimental sections, no significant (P>0.05) change in dissolution pattern was observed in the secnidazole release from any of the tested formulations.
The dissolution data were treated with first order, zero order and Higuchi equations for analyzing the kinetics and mechanism of drug release. All formulations except F3, F4 and F8 showed the linearity with respect to zero order (Table 5), while F3, F4 and F8 followed first-order release. In presence of rat caecal content (2% w/v) in dissolution medium (SCF), the tested formulations F5, F9 and F14 showed linearity with respect to first order equation (Table 5).
| Formulation | R2 | n | ||
|---|---|---|---|---|
| Zero- order | First- order | Higuchi model | ||
| F1 | 0.9746 | 0.9183 | 0.8507 | 1.131 |
| F2 | 0.9896 | 0.9512 | 0.8498 | 1.186 |
| F3 | 0.6525 | 0.9655 | 0.8906 | 0.492 |
| F4 | 0.6448 | 0.9467 | 0.8854 | 0.481 |
| F5 | 0.9749 | 0.9223 | 0.8509 | 1.154 |
| F6 | 0.9634 | 0.9404 | 0.8416 | 1.251 |
| F7 | 0.9673 | 0.9515 | 0.8651 | 1.236 |
| F8 | 0.6746 | 0.9533 | 0.9004 | 0.483 |
| F9 | 0.9847 | 0.9227 | 0.8402 | 1.174 |
| F10 | 0.9787 | 0.9374 | 0.8364 | 1.184 |
| F11 | 0.9851 | 0.9327 | 0.8236 | 1.279 |
| F12 | 0.9873 | 0.9174 | 0.8283 | 1.103 |
| F13 | 0.9891 | 0.9150 | 0.8730 | 1.302 |
| F14 | 0.9820 | 0.8858 | 0.8578 | 1.395 |
| F15 | 0.9901 | 0.9113 | 0.8547 | 1.246 |
| F5* | 0.8968 | 0.9528 | 0.9342 | 1.405 |
| F9* | 0.9285 | 0.9683 | 0.9192 | 1.431 |
| F14* | 0.8845 | 0.9586 | 0.9245 | 1.614 |
* Data obtained for dissolution studies performed in presence of rat cecal contents (2% w/v)
Table 5: Regression And Diffusion Co-Efficient Of Dissolution Studies
The mechanism of drug release was analysed by plotting drug release data according to Korsmeyer- Peppas equation. The ‘n’ value (diffusional exponent) indicates the mechanism of drug release. For a tablet system, the drug release is considered to follow Fickian diffusion if n<0.45 while 0.45<n<0.89, the drug release is considered to be by anomalous (non-Fickian) transport. ‘n’ value of 0.89 indicates of zero-order release and n>0.89 indicates a super case-II transport. Except for F3, F4 and F8, the ‘n’ values were in the range of 1.10-1.61, indicating a super case-ІІ transport. This value indicated that the drug release from the prepared matrix systems was due to both diffusion and polymeric chain relaxation. The ‘n’ values for formulation F3, F4 and F8 were found in the range of 0.481-0.492 which describes that drug release was following anomalous transport.
Secnidazole, is a drug with therapeutic applications in amoebiasis and its therapeutic index is increased with proper and complete drug availability in colon region. The drug release from the developed formulations indicates that drug release can be modulated to the desired release profile by judicious selection of the polymer and its amount in the formulation. It can be concluded from the studies that compression coating with a mixture of chitosan (50 mg) with guar gum or xanthan gum at equal proportion (each at 75 mg) is most likely to provide targeted delivery of secnidazole to the colon. Based on the drug release studies, it appears that a single polysaccharide (guar gum or xanthan gum) may not be suitable for compression coating for efficient targeting to the colon. Further in vivo degradation studies of the formulations would help in optimization of the formulation.
References
- Mundargi CR, Patil SA, Agnihotri SA, Aminabhavi TM. Development of polysaccharide-based colon targeted drug delivery systems for the treatment of amoebiasis. Drug DevInd Pharm 2007;33:255-64.
- Montovani PA, Pinto AM, Santos D, Prado W, Vieira DL, Manfio JL. Bioavailability of two oral formulations of secnidazole in healthy volunteers. Braz J Pharm Sci 2009;45:687-92.
- Gardner TB, Hill DR. Treatment of giardiasis. ClinMicrobiol Rev2001;14:114-28.
- Farooqui NA, Smith AA, Sharma HK, Manavalan R. Analytical method development and validation of secnidazole tablets by RP-HPLC. J Pharm Sci Res 2010;2:412-6.
- Ugurlu T, Turkoglu M, Gurer US, Akarsu BG. Colonic delivery of compression coated nisin tablets using pectin/HPMC polymer mixture. Eur J Pharm Biopharm 2007;67:202-10.
- Krishnaiah YS, Bhaskar Reddy PR, Satyanarayana V, Karthikeyan RS. Studies on the development of oral colon targeted drug delivery systems for metronidazole in the treatment of amoebiasis. Int J Pharm 2002;236:43-55.
- Amrutkar JR, Gattani SG. Chitosan-chondroitin sulphate based matrix tablets for colon specific delivery of indomethacin. AAPS PharmSciTech 2009;10:670-7.
- Sinha VR, Kumria R. Polysaccharides in colon-specific drug delivery. Int J Pharm 2001;224:19-38.
- Milojevic S, Newton JM, Cummings JH. Amylose, the new perspective in oral drug delivery to the human large intestine. STP Pharm Sci 1995;5:47-53.
- Vervoot L, Kinget R. In vitro degradation by colonic bacteria of inulin HP incorporated in Eudragit RS films. Int J Pharm 1996;129:185-90.
- Krishnaiah YS, Satyanarayana V, Dinesh Kumar B, Karthikeyan RS. In vitro drug release studies on guar gum-based colon targeted oral drug delivery systems of 5- fluorouracil. Eur J Pharm Sci 002;16:185- 92.
- Krishnaiah YS, Satyanarayana S, Rama Prasad YV, NarasimhaRao S. Evaluation of guar gum as a compression coat for drug targeting to colon. Int J Pharm 1998;171:137-46.
- Kinage K, Nandgude T, Bhise K, Deshmukh P. Studies on development of oral colon targeted drug delivery system of locust bean and xanthan gums. Int J Green Pharm 2007;1:33-6.
- Moore WE, Holdeman LV. Discussion of current bacteriological investigations of the relationship between intestinal flora, diet and colon cancer. Cancer Res 1975;35:3418-20.
- Indian Pharmacopoeia, Vol. 1. New Delhi: Controller of Publication, Government of India, Ministry of Health and Family Welfare; 1996. p. 736.
- Korsmeyer RW, Gurney R, Doelker EM, Buri P, Peppas NA. Mechanism of solute release from porous hydrophilic matrices. Int J Pharm 1983;15:25-35.
- Thilbert R, Hancock BC. Direct visualization of superdisintegrant hydration using environmental scanning electron microscopy. J Pharm Sci 1996;85:1255-8.
- Khan KA, Rhodes CT. Water-sorption properties of tablet disintegrants. J Pharm Sci 1975;64:447-51.
- Feely LC, Davis SS. Influence of polymeric excipients on drug release from hydroxypropyl methyl cellulose matrices. Int J Pharm 1988;44: 131-9.
- Marcos BP, Gutierrez C, Gomez-Amoza JL, Martinez-Pacheco R, Souto C, Concheiro A. Usefulness of certain varieties of carbomer in the formulation of hydrophilic furosemide matrices. Int J Pharm 1991;67:113-9.
- Hovgaard L, Bronsted H. Current applications of polysaccharides in colon targeting. Crit Rev Ther Drug Carrier Syst 1996;13:185-223.
- Hawksworth G, Drasar BS, Hill MJ. Intestinal bacteria and the hydrolysis of glycosidic bonds. J Med Microbiol 1971;4:451-9.