- *Corresponding Author:
- B. J. Chacko
Department of Pharmacy, Prime College of Pharmacy, Palakkad-678 551, India
E-mail: junachacko25@gmail.com
| Date of Submission | 06 July 2016 |
| Date of Revision | 07 April 2017 |
| Date of Acceptance | 03 January 2018 |
| Indian J Pharm Sci 2018;80(2): 215-222 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Targeting of drugs to brain is one of the most challenging issues for pharmaceutical research as blood-brain barrier acts as an insurmountable obstacle for the passage of systemically delivered therapeutics and the brain extracellular matrix attributes to poor distribution of locally delivered drugs. Amongst various invasive or non-invasive methods to warrant blood-brain barrier, nanoparticle is one of promising ways to administer central nervous system drugs. The concept of nanoparticle-based drug targeting make a tremendous progress and gigantic era to overcome the above limitations with improved drug efficacy and reduced drug toxicity. In recent years, new strategies of surfactant coating of biodegradable polymeric nanoparticles, which differ from conventional methodologies of brain targeting, have emerged at the forefront of medical science. The non-ionic surfactant, polysorbate 80 as a coating material promises an unparalleled opportunity for enhancement of brain targeting of colloidal particles. The aim of this review is to evaluate the potential application of surfactant coated nanoparticles as drug carrier system for various central nervous system diseases.
Keywords
Blood-brain barrier, polysorbate 80, brain targeting, nanoparticles, surfactant, polymers
Drug targeting remains as a challenge for treating various diseases, as their entry into target is restricted by various defensive barriers [1-3]. Most of biological barriers especially blood-brain barrier (BBB) are complex structures composed of highly organized populations of individual cells [4,5]. The ultimate goal of targeted treatment is to deliver the drug at the right place, at the right concentration for the right period of time while eliminating or reducing the accumulation of the drug at any non-target sites [6-8]. An effective targeted drug delivery system requires four key elements namely retain [9], evade [10], target and release [11]. For formulations intended for intravenous administration, this means efficient drug loading into some type of delivery vehicle, sufficient residence in the circulation to reach intended sites of the body [12-14], retention by specific characteristics within intended sites (i.e., targeting), and drug release at the intended site within a time that allows for effective function of the drug [15]. So if targeted drug delivery has been achieved, the treatment should have been better with much less side effects [16,17]. The BBB is a dynamic barrier formed at the level of the endothelial cells of the cerebral capillaries [18-20] and impedes drug transport into the brain [21,22]. The existence of BBB and the idea of these revolutionary therapies were inspired by a visit of one of the giants in science, Paul Ehrlich in the late 19th century [23-25].
The BBB endothelial cells are characterized by the absence of fenestrations, more extensive tight continuous circumferential junctions, lack of lymphatic drainage and absence of major histocompatibility complex antigens [26] and expression of various transporters including GLUT1, LAT1, efflux transporters such as p-glycoprotein (P-gp) [27] and multidrug resistance related proteins [28,29]. Due to the unique properties of the BBB, paracellular transport of hydrophilic drugs is virtually absent and transcellular transport by passive diffusion is only available to molecules which fulfil certain criteria such as: 1) molecular weight is less than 500 Da; 2) compounds are unionised; 3) log P value of the drug is close to 2; 4) cumulative number of hydrogen bonds is not more than 10 [30]. The BBB inhibits the transport of 98 % of all small molecule drugs and 100 % of large-molecule pharmaceutics into the brain [31,32]. Moreover, even after successful endothelial cell absorption, active efflux mechanisms (ATP-binding cassette transporter) may pump these molecules back into the blood stream [33]. So therapeutic strategies to a variety of central nervous system (CNS) active drugs are thus limited.
Various drug targeting strategies to brain
Various invasive and non-invasive strategies are employed to circumvent BBB and facilitate the delivery of the active ingredient into brain [34-37]. The long back employed non-invasive methods involving temporary opening of BBB by osmotic opening or direct administration of drugs by convection enhanced delivery [34,38] or craniotomy-based drug delivery including intra-ventricular and intracerebral implantation of depot formulations such as the Gliadel® wafer, which is polifeprosan 20 with carmustine implant (FDA approved) indicated for patients with newly diagnosed or recurrent highgrade malignant glioma in addition to radiation and/or surgery [39]. Despite extensive research in clinical trials, the craniotomy-based drug delivery relies on diffusion from the local depot sites and it depends heavily on the infused drug concentration and molecular size of the drug [40]. Indeed, the diffusional distance that can be reached in the brain using such systems does not exceed 5 mm whereas target beyond this distance remain unattainable [41]. As a consequence, it is essential to instil a very high concentration into the brain to generate an adequate concentration gradient to distribute drug a significant distance into the tissue. However for many chemotherapeutic agents, this source concentration is likely to be toxic to normal brain tissue, leading to significant side effects [42]. The opening of the tight junctions by osmotic pressure, however, is a very invasive procedure that also enables the entry of unwanted substances into the brain [42-44]. Obviously, all invasive techniques are associated with a high risk of complications, such as intracranial infections or brain oedema. In addition to, these procedures are rather expensive and require considerable expertise for administration [42]. A number of non-invasive approaches that have been made to enhance CNS drug delivery include manipulation of drug (chemical modification of active ingredient to pharmacologically inactive and lipophilic prodrugs) or delivery via endogenous transporters (carrier-mediated transporters, receptormediated transporters) or inhibition of active efflux transporters as well as administration of drug by IM route [35-37]. However, in the latter case the drug is delivered intranasally bypassing the bloodstream and transported along olfactory sensory neurons and the concentration of drugs in the cerebrospinal fluid and olfactory lobes do not exceed nano molar levels with bioavailability ranging from 0.01 to 0.1 % [34]. Even if the above mentioned techniques offer capabilities of molecules to cross the BBB and some interesting results, they also appear to have certain drawbacks such as loss of the therapeutic effect after linking with lipophilic moiety or ligand or intolerable adverse side effects after P-gp inhibition [45-47].
Molecular Trojan horses are genetically engineered proteins or peptide, a second peptide or peptidomimetic monoclonal antibody that binds a specific receptor on the BBB and cross the BBB via endogenous receptormediated transport processes. Molecular Trojan horses provide a non-invasive delivery of large molecule therapeutics to the human brain. The Trojan horse enables receptor-mediated delivery of the fusion protein across the BBB so that the protein drug can enter the brain and exert the desired pharmacological effect [48,49].
Solute carrier (SLC) transporters are widely used nowadays, which consists of a group of more than 300 membrane bound proteins. These proteins facilitate the transport of a wide range of substrates across various biological membranes, which have important roles in physiological processes. It ease the cellular uptake of nutrients and the absorption of drugs and other xenobiotics [50]. A large number of SLC transporters demonstrate enriched expression in the human BBB, and the drug transporters with imperative roles in renal and hepatic drug disposition were expressed at similar or higher levels in the BBB. SLC transporters in the BBB play a vital role in maintaining CNS homeostasis of neurotransmitters, amino acids, vitamins and various other essential nutrients. These transporters may be targeted to achieve CNS delivery of pharmaceuticals for the treatment of neurodegenerative and other CNS diseases [51].
Over the past few decades, substantial research efforts have demonstrated that colloidal carriers particularly nanoparticles and liposomes placed an interesting improvement for effective systemic and local delivery of therapeutics into CNS [52,53]. Out of these treatment outcomes biodegradable, biocompatible polymeric nanoparticle made a tremendous progress due to their low toxicity, enhanced bioavailability, controlled release, huge specific surface area and self-regulation of drug release [54-56]. Nanoparticles are actually solid colloidal particles ranging in size from 10 to 1000 nm. They consist of macromolecular materials in which the active principle (drug or biologically active material) is dissolved, entrapped, encapsulated and/or to which the active principle is adsorbed or covalently attached, have now found entry into the relevant specialised encyclopaedias [23,57]. The main benefits of using nanoparticles as drug carriers include enhancing solubility and reducing degradation of therapeutic agent and prolonging the residence time by increasing the contact time between drug and target [58]. Owing to preferential accumulation at the target site, the therapeutic agent might also show reduced toxicity and improvement of efficacy [59,60].
An exciting development of universal ‘magic bullet’ nanoparticle-based drug delivery system to brain [61], the key objectives to be considered are size of particles [62], surface properties as well as discharge of drugs [63] or the active ingredient to accomplish highest efficacy [64]. The nanoparticle sizes range from 10-100 nm is preferred for targeted delivery in brain, however the nanoparticle sizes less than 230 nm with a polydispersity of 0.10 were also used for brain delivery [65]. As shown by a number of studies, surface modification of the nanoparticles enables their entry into the brain after intravenous administration by evading BBB and protective mechanism [66-68]. Another important advantage of this technology is that it does not require any modification of the drug molecule for the brain delivery, which is achieved by masking the unfavourable physicochemical characteristics of the incorporated molecule [44].
Surfactant-based approach to prolong brain retention of polymeric nanoparticles
There is an extensive body of evidence to demonstrate that surfactant-coated nanoparticles are highly efficient to target therapeutic concentrations of molecule of interest to brain whilst remarkably reducing reticuloendothelial system (RES) uptake. A study observed that nanoparticles coated with certain surfactants including polysorbate 80 (Tween 80, P80) were taken up by brain capillary endothelial cells in tissue cultures [22,23]. Kreuter et al. first reported the transport of hexapeptide dalargin bound to polybutylcyanoacrylate (PBCA) nanoparticles, coated with P80 in mice model and exert its analgesic effect [69]. Later they again investigated the influence of different surfactants on drug delivery across BBB by measuring the analgesic effect of dalargin by tail flick test in mice after intravenous injection of surfactantcoated PBCA nanoparticles. Only nanoparticles that had been coated with polysorbate 20, 40, 60 and 80 yielded a significant effect. The highest effect was observed with P80 [70-72]. Other drugs that have successfully been transported into the brain using this carrier include the dipeptide kytorphin, loperamide, tubocurarine, the NMDA receptor antagonist MRZ 2/576, and doxorubicin (DOX) [73,74]. It has been also reported that intravenously injected DOX-loaded P80-coated nanoparticles were able to lead to a 40 % cure in rats with intracranially transplanted glioblastomas 101/8 [75-77]. Gelperina along her group showed that very significant brain concentrations of DOX, 65-fold above detection limit, could be achieved when the drug was bound to the P80-coated PBCA nanoparticles, whereas the concentrations of all control formulations remained below the detection limit [78].
Benvegnu et al. study make obvious that haloperidol-loaded polysorbate-coated polymeric poly(-caprolactone) nanocapsules decreases its adverse motor side effects as well as oxidative damages in extrapyramidal brain region in relation to free haloperidol in rats model of pseudo-psychosis [56]. Similarly, P80 (poly(oxyethylene)-sorbitan-20- monooleate) can be used to delay opsonization. Lipidcore nanocapsules stabilized with P80 showed efficient delivery of drugs to the brain [60].
The study reported by Sun et al. confirmed that even partial coverage of P80 coating play a crucial role for interaction between nanoparticles and brain micro-vessel endothelial cells and entry into CNS on targeting of nanoparticles to brain [79]. Further in 2008, Wilson et al. demonstrated that the brain concentration of intravenously injected tacrine and rivastigmine can be enhanced over 4.07 and 3.82 fold by binding to PBCA nanoparticles coated with 1 % nonionic surfactant P80 whilst reducing accumulation of drugs in liver and spleen [80].
Another study reported the antitumor effects of P80- coated gemcitabine PBCA nanoparticles in vitro and the pharmacodynamics effects in vivo on C6 glioma cells of a brain tumor model. Various preparations (saline, gemcitabine alone or 1 % P80-coated gemcitabine PBCA nanoparticles) were injected into the brain tumor model, which was produced after inoculating C6 glioma cells into Sprague Dawley rats for 14 d, it was shown that 1 % P80-coated gemcitabine PBCA nanoparticles could significantly extend the survival time compared with the saline control [81].
A study compared the brain tissue concentration and achieved following intravenous delivery of pure bacoside-A solution and P80-coated PLGA nanoparticles. They demonstrated that in Wistar rats in vivo study when compared to pure drug solution (2.56±1.23 μg/g tissue), higher brain concentration of bacoside-A (23.94±1.74 μg/g tissue) suggesting a significant role of surface-coated nanoparticles on brain targeting [82].
Gelperina et al. [78] study evaluated the acute toxicity of DOX associated with P80-coated nanoparticle in healthy rats and to establish a therapeutic dose range for this formulation in rats with intracranially implanted 101/8 glioblastoma. The presence of P80 in the formulations was not associated with changes in toxicity compared with free or nanoparticulate drug. The results in tumour-bearing rats were similar to those in healthy rats.
These results demonstrate that the toxicity of DOX bound to nanoparticle was similar or even lower than that of free DOX. The critical concentration of P80 coating on nanoparticle for maximum translocation of blood to brain in most of studies was found to be (1 % w/w) [78]. In some studies it was proved that up to (2 % w/w) P80 concentration is effective or brain targeting of nanoparticles [83,84]. In most of the studies, for coating 1 % w/v of P80 was added to nanoparticle suspensions and incubated for 30 min with constant stirring and finally lyophilized [85,86].
The targeted polymeric nanoparticle possess adequate stability on storage. For systemic delivery, the nanoparticle coated with P80 or PEG linked nanoparticles for targeting should also: 1) be stable in blood, 2) avoid the RES, and 3) have prolonged circulation times [87]. To summarise these extensive experimental findings, the nanoparticles over-coated by non-ionic surfactants (especially P80) were capable of transporting the loaded drugs across BBB after administration, which supplied tools delivering drugs to brain.
Mechanism of P80-coated nanoparticle-mediated drug transport to the brain
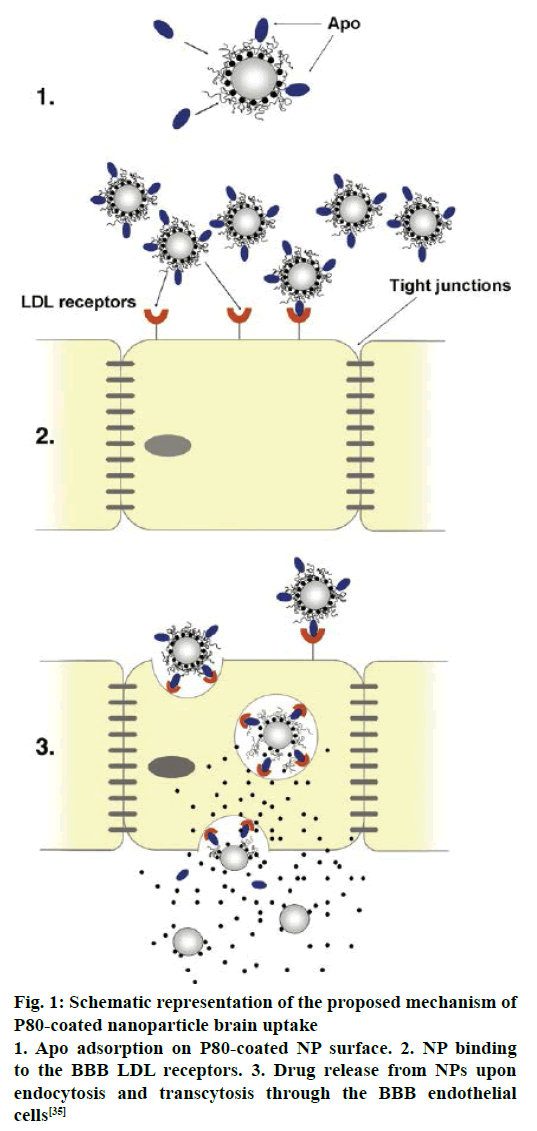
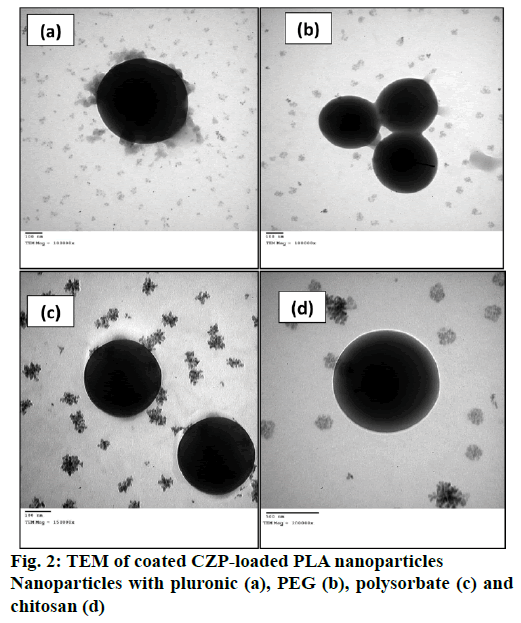
Several mechanisms have been proposed for the transport of nanoparticles coated with P80 across the BBB [88-90]. An increased retention of nanoparticle in the brain blood capillaries and binding to endothelial cell lining could provide a drug concentration gradient and thus enhanced drug transport across BBB by passive diffusion [91]. P80 plays a crucial role as an anchor between nanoparticles and the Apo lipoprotein, especially ApoE and ApoB, which get adsorbed from blood over the P80-coated nanoparticles [44]. Thus the nano carriers would mimic as lipoprotein particles and could interact with and then be taken up by the brain capillary endothelial cells via receptor-mediated endocytosis. After this the drug may be released in these cells and diffuse into the brain interior or the particles may be transcytosed. Inhibition of drug efflux transporters especially P-gp at the BBB is also an approach that warrants improved internalization of P-gp substrates into brain [18]. A general surfactant effect characterized by a solubilisation of the endothelial cell membrane lipids that would lead to membrane fluidization and destabilization and enhanced drug permeability through the BBB [88,91]. It is a successful strategy for prevention or delay of opsonin adsorption and enhancement of circulation life times. P80 further act as a lead substance to circumvent the mononuclear phagocyte system (MPS), consisting mainly of monocytes and macrophages (e.g. Kuppfer liver cells, spleen red pulp macrophages), is an additional step towards elevated plasma and brain level of the drug [41]. The schematic representation of the proposed mechanism of P80-coated nanoparticle brain uptake are shown in Figure 1 and TEM of coated CZP-loaded PLA nanoparticles with pluronic, PEG, polysorbate and chitosan are depicted in Figure 2.
Figure 1: Schematic representation of the proposed mechanism of P80-coated nanoparticle brain uptake
1. Apo adsorption on P80-coated NP surface. 2. NP binding to the BBB LDL receptors. 3. Drug release from NPs upon endocytosis and transcytosis through the BBB endothelial cells [35]
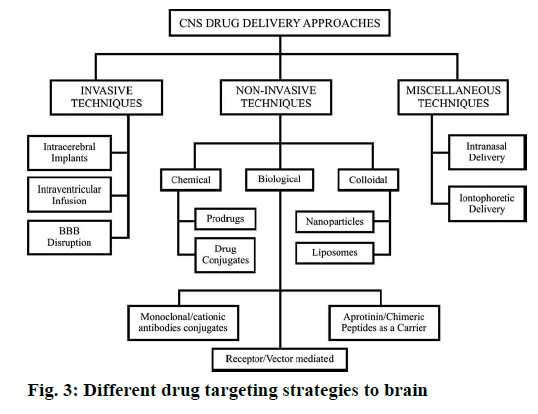
The biodistribution of polysorbate nanoparticles revealed that after intravenous injection, particles with an average size below 7 μm are generally taken up by the RES, particularly by the Kupffer cells of the liver [4]. Wilson et al. [80] investigated the biodistribution of P80- coated tacrine loaded PBCA nanoparticle for brain targeting. The concentration of drug in brain, liver, lungs, spleen and kidneys was analysed after 1 h of post injection of IV injection. A higher concentration of drug tacrine was observed in liver, spleen and lungs with the nanoparticles in comparison to the free drug. The accumulation of drug tacrine in the liver and spleen was reduced, but kidneys get increase when nanoparticles were coated with 1 % P80. In the brain a significant increase in tacrine concentration was observed in the case of poly(n-butylcyanoacrylate) nanoparticles coated with 1 % P80 compared to the uncoated nanoparticles and the free drug [41]. It is similar to other reported evidences. Although all these mechanisms are expected to work together to overcome BBB the endocytosis mediated transport is the most acceptable one. Different drug targeting strategies to brain are presented in Figure 3. The various brain disorders and the molecules patented to treat those disorders are presented in Table 1.
| Brain Disorder | Molecules patented brain disorder or under investigation | |
|---|---|---|
| Drugs | Peptides | |
| Cerebral ischemia/stroke | Deferoxamine, trientine, ebselen, heregulin, cobalt, tetrathiomolybdate, remacemide, nicergoline, hydergine, lubeluzole |
Growth factors, brain-derived neurotrophic factor, interleukin- 1, tumour necrosis factor-α, vasoactive intestinal peptide, leuenkephalin, pyruvate, N-acetyl cysteine amide, lactate |
| Brain tumors | Procarbazine, lomustine, vincristine, temozolomide, dexamethasone |
Epidermal growth factor receptor antisense, RNA interference |
| Pain | Morphine, frovatriptan, cannabinoids, oxycodone, levallorphan, fentanyl, alfentanil |
Interleukin-1, enkephalins, botulinum toxin, dynorphin, β- endorphin |
| Alzheimer’s disease | Ambenonium, edrophonium, neostigmine, tacrine, rivastigmine, pyridostigmine, galantamine |
Peptide T, interleukin-1 |
| Parkinsonism | Selegiline, rimantadine, amantadine, levodopa, taolcapone, entacapone, pramipexole, ropinirole |
Glial-derived neurotrophic factor, interleukin-1, tyrosine hydroxylase |
Table 1: Brain Disorders and Molecules Patented for its Treatment
Taking all data into account the non-ionic surfactant over-coated nanoparticle-based non-invasive administration by intravenous injection or infusion of drug-loaded nanoparticles enables the brain delivery of agents, including low molecular drugs, macromolecules, and new biological entities, that cannot independently permeate the BBB in therapeutically effective concentrations. Binding to the particles also may offer clinical advantages such as decreased drug dose, reduced drug side effects, increased drug viability, noninvasive routes of administration and improved patient quality of life. The nanoparticles may be especially helpful for the treatment of the disseminated and very aggressive brain tumours by fine-tuning polysorbate concentration but further clinical trials are required.
Acknowledgements
The authors would like to thank the Principal, Prime College of Pharmacy and the Principal, Grace College of Pharmacy for the constant support and encouragements to carry out research works.
Conflict of interest
The authors report no declarations of interest.
References
- Ahmed F, Ali MJ, Kondapi AK. Carboplatin loaded protein nanoparticles exhibit improve anti-proliferative activity in retinoblastoma cells. Int J Biol Macromol 2014;70:572-82.
- Albrecht C, Knaapen AM, Becker A, Hohr D, Haberzettl P, Van Schooten FJ, et al. The crucial role of particle surface reactivity in respirable quartz induced reactive oxygen/nitrogen species formation and APE/Ref-1 induction in rat lung. Respir Res 2005;6:129.
- Alyautdin RN, Petrov VE, Langer K, Berthold A, Kharkevich DA, Kreuter J. Delivery of loperamide across the blood-brain barrier with polysorbate 80-coated polybutyl cyanoacrylate nanoparticles. J Pharm Res 1997;14(3):325-8.
- Campbell A, Oldham M, Becaria A, Bondy SC, Meacher D, Sioutas C, et al. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology 2005;26(1):133-40.
- Radomski A, Jurasz P, Alonso-Escolano D, Drews M, Morandi M, Malinski T, et al. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br J Pharmacol 2005;146(6):882-93.
- Kwon IK, Lee SC, Han B, Park K. Analysis on the current status of targeted drug delivery to tumors. J Control Release 2012;164(2):108-14.
- Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomedicine 2013;9:1-14.
- Barenholz Y. Liposome application: problems and prospects. Curr Opin Colloid Interface Sci 2011;6:66-77.
- Euliss LE, DuPont JA, Gratton S, DeSimone J. Imparting size, shape, and composition control of materials for nanomedicine. Chem Soc Rev 2006;35:1095-104.
- Lasic DD. Novel applications of liposomes. Trends Biotechnol 1998;16:307-21.
- Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release 2011;153(3):198-205.
- Hong M, Zhu S, Jiang Y, Tang G, Pei Y. Efficient tumor targeting of hydroxycamptothecin loaded PEGylated niosomes modified with transferrin. J Control Release 2009;133:96-102.
- Zensi A, Begley D, Pontikis C, Legros C, Mihoreanu L, Wagner S, et al. Albumin nanoparticles targeted with ApoE enter the CNS by transcytosis and are delivered to neurons. J Control Release 2009;137:78-86.
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Res 1986;46:6387-92.
- Lia YP, Yuan Y, Zhang XY, Gub ZH, Zhoub ZH, Yuanb WF, et al. PEGylated PLGA nanoparticles as protein carriers: synthesis, preparation and biodistribution in rats. J Control Release 2001;71:203-11.
- Petrak K, Goddard P. Transport of macromolecules across the capillary walls. Adv Drug Deliv Rev 1989;3(2):191-214.
- Nidhi M, Narayan PY, Vineet KR, Priyan S, Kuldeeo SY, Sanoyg J, et al. Efficient hepatic delivery of drugs: Novel strategies and their significance. Biomed Res Int 2013;2013:382184.
- Chen Y, Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Adv Drug Deliv Rev 2012;64(7):640-65.
- John SD. The concept of a blood-brain barrier. J Neurol Neurosurg Psychiatry 1980;43(4):374-5.
- Saito Y, Wright EM. Regulation of bicarbonate transport across the brush border membrane of the bull-frog choroid plexus. J Physiol 1984;350:327-42.
- Gonzalez ML, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol 2003;81:1-4.
- Matter K, Balda MS. Holey barrier: claudins and the regulation of brain endothelial permeability. J Cell Biol 2003;161:459-60.
- Kreuter J. Nanoparticles-a historical perspective. Int J Pharm 2007;331(1):1-10.
- Parascandola J. The theoretical basis of Paul Ehrlich's chemotherapy. J Hist Med Allied Sci 1981;36:19-43.
- Kristiansen JE. Dyes, antipsychotic drugs, and antimicrobial activity. Fragments of a development, with special reference to the influence of Paul Ehrlich. Dan Med Bull 1989;36:178-85.
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 2004;16(1):1-13.
- Stefano P, Chiara C. How stem cells speak with host immune cells in inflammatory brain diseases. Glia 2013;61(9):1379-401.
- Aileen JA, Daniel LH, Mitra JH, Harvey P, Christopher JS, Brian JC. Achieving stable human stem cell engraftment and survival in the CNS: is the future of regenerative medicine immunodeficient? Regen Med 2011;6(3):367-6.
- David WB, Israel A, Kourilsky P. The regulation and expression of MHC class I genes. Immunol Today 1990;11:286-92.
- Roney C, Kulkarni P, Arora V, Antich P, Bonte F, Wu A, et al. Targeted nanoparticles for drug delivery through the blood-brain barrier for Alzheimer's disease. J Control Release 2005;108(2-3):193-14.
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis 2010;37(1):1325.
- Dauchy S, Dutheil F, Weaver RJ, Chassoux F, Daumas DC, Couraud PO, et al. ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood-brain barrier. J Neurochem 2008;107(6):1518-8.
- Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med 2013;19:1584-96.
- White E, Bienemann A, Taylor H, Hopkins K, Cameron A, Gill S. A phase I trial of carboplatin administered by convection-enhanced delivery to patients with recurrent/progressive glioblastoma multiforme. Contemp Clin Trials 2012;33(2):320-31.
- Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev 2007;59(6):454-77.
- Pardridge WM. Vector-mediated drug delivery to the brain. Adv Drug Deliv Rev 1999;36(2):299-321.
- Bhaskar S, Tian F, Stoeger T, Kreyling W, De La FJM, Grazu V, et al. Multifunctional Nanocarriers for diagnostics, drug delivery and targeted treatment across blood–brain barrier: perspectives on tracking and neuroimaging. Part Fibre Toxicol 2010;7:3.
- Huwyler J, Wu D, Pardridge WM. Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci USA 1996;93(24):14164-9.
- http://www.fda.gov/ohrms/dockets/ac/01/briefing/3815b2_05_FDA.pdf (Accessed 28 December 2016).
- Kipp JE. The role of solid nanoparticle technonogy in the parental delivery of poorly water-soluble drugs. Int J Pharm 2004;284:109-122.
- Wilson B, Samanta MK, Santhi K, Kumar KP, Paramakrishnan N, Suresh B. Targeted delivery of tacrine into the brain with polysorbate 80-coated poly(n-butylcyanoacrylate) nanoparticles. Eur J Pharma Biopharm 2008;70(1):75-84.
- Carty CL, Gehring U, Cyrys J, Bischof W, Heinrich J. Seasonal variability of endotoxin in ambient fine particulate matter. J Environ Monit 2003;5(6):953-8.
- Calderon-Garciduenas L, Reed W, Maronpot RR, Henriquez-Rolden C, Delgado-Chavez R, Calderon-Garciduenas A, et al. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol 2004;32(6):650-8.
- Garcia-Garcia E, Andrieux K, Gil S, Couvreur P. Colloidal carriers and blood-brain barrier (BBB) translocation: a way to deliver drugs to the brain? Int J Pharm 2005;298(2):274-92.
- Verma RK, Mishra B, Garg S. Osmotically controlled oral drug delivery. Drug Dev Ind Pharm 2000;26(7):695-08.
- Hoare TR, Kohane DS. Hydrogels in drug delivery: progress and challenges. Polymer 2008;49(8):1993-2007.
- Fisher O, Kim T, Dietz SR, Peppas NA. Enhanced core hydrophobicity, functionalization and cell penetration of polybasic nanomatrices. Pharm Res 2009;26(1):51-60.
- Pardridge WM. Molecular Trojan horses for blood-brain barrier drug delivery. Curr Opin Pharmacol 2006;6(5):494-500.
- Wu D, Pardridge WM. Neuroprotection with noninvasive neurotrophin delivery to the brain. Proc Natl Acad Sci USA 1999;96:254-59
- Lawrence L, Sook WY, Richard BK, Kathleen MG. SLC transporters as therapeutic targets: emerging opportunities. Nat Rev Drug Discov 2015;14:543-60.
- Geier EG, Chen EC, Webb A, Papp AC, Yee SY, Sadee W, et al. Profiling solute carrier transporters in the human blood-brain barrier. Clin Pharmacol Ther 2013;94(6):636-39.
- Rani R, Dilbaghi N, Dhingra D, Kumar S. Optimization and evaluation of bioactive drug-loaded polymeric nanoparticles for drug delivery. Int J Biol Macromol 2015;78:173-9.
- Vauthier C, Dubernet C, Chauvierre C, Brigger I, Couvreur P. Drug delivery to resistant tumors: the potential of poly (alkyl cyanoacrylate) nanoparticles. J Control Release 2003;93(2):151-60.
- Tada H, Higuchi H, Wanatabe TM, Ohuchi N. In vivo real-time tracking of single quantum dots conjugated with monoclonal anti-HER2 antibody in tumors of mice. Cancer Res 2007;67(3):1138-44.
- Lu J, Liong M, Zink JI, Tamanoi F. Mesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugs. Small 2007;3(8):1341-6.
- Benvegnu DM, Barcelos RC, Boufleur N, Pase CS, Reckziegel P, Flores FC, et al. Haloperidol-loaded polysorbate-coated polymeric nanocapsules decrease its adverse motor side effects and oxidative stress markers in rats. Neurochem Int 2012;61(5):623-31.
- Cimini A, Gentile R, Angelucci F, Benedetti E, Pitari G, Giordano A, et al. Neuroprotective effects of prxi over-expression in an in vitro human Alzheimer’s disease model. J Cell Biochem 2013;114(3):708-15.
- Dawson TM. Unraveling the role of defective genes in Parkinson’s disease. Parkinsonism Relat Disord 2007;13(3):S248-9.
- Mattis VB, Svendsen CN. Induced pluripotent stem cells: A new revolution for clinical neurology? Lancet Neurol 2011;10(4):383-94.
- Bender EA, Adorne MD, Colome LM, Abdalla DS, Guterres SS, Pohlmann AR. Hemocompatibility of poly (ε-caprolactone) lipid-core nanocapsules stabilized with polysorbate 80-lecithin and uncoated or coated with chitosan. Int J Pharm 2012;426(1-2):271-9.
- Hunter AC, Elsom J, Wibroe PP, Moghimi SM. Polymeric particulate technologies for oral drug delivery and targeting: a pathophysiological perspective. Nanomed 2012;8(1):S5-20.
- De Jong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomed 2008;3(2):133-49.
- Wong BS, Yoong SL, Jagusiak A, Panczyk T, Ho HK, Ang WH. Carbon nanotubes for delivery of small molecule drugs. Adv Drug Deliv Rev 2013;65(15):1964-2015.
- Chakraborty C, Pal S, Doss GP, Wen ZH, Lin CS. Nanoparticles as ‘smart’ pharmaceutical delivery. Front Biosci 2013;18:1030-50.
- Kunzmann A, Andersson B, Thurnherr T, Krug H, Scheynius A, Fadeel B. Toxicology of engineered nanomaterials: Focus on biocompatibility, biodistribution and biodegradation. Biochim Biophys Acta 2011;1810 (3):361-73.
- Chauvierre C, Leclerc L, Labarre D, Appel M, Marden MC, Couvreur P, et al. Enhancing the tolerance of poly-(isobutylcyanoacrylate) nanoparticles with a modular surface design. Int J Pharm 2007;338(1-2):327-32.
- Kumar R, Roy I, Ohulchanskky TY, Vathy LA, Bergey EJ, Sajjad M, et al. In vivo biodistribution and clearance studies using multimodal organically modified silica nanoparticles. ACS Nano 2010;4(2):699-708.
- Gao K, Jiang X. Influence of particle size on transport of methotrexate across blood brain barrier by polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Int J Pharm 2006;310(1-2):213-9.
- Kreuter J, Alyautdin RN, Kharkevich DA, Ivanov AA. Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles). Brain Res 1995;674(1):171-4.
- Wu MP, Tamada JA, Brem H, Langer R. In vivo versus In vitro degradation of controlled-release polymers for intracranial surgical therapy. J Biomed Mater Res 1994;28(3):387-95.
- Tosi G, Costantino L, Rivasi F, Ruozi B, Leo E, Vergoni AV, et al. Targeting the central nervous system: in vivo experiments with peptide-derivatized nanoparticles loaded with Loperamide and Rhodamine-123. J Control Release 2007;122(1):1-9.
- Kreuter J, Kharkevich DV, Alyautdin RN. Influence of the type of surfactant on the analgesic effects induced by the peptide dalargin after its delivery across the blood-brain barrier using surfactant-coated nanoparticles. J Control Release 1997;49(1):81-7.
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008;57(2):178-01.
- Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 2013;36(3):437-49.
- Jaruszewski KM, Ramakrishnan S, Poduslo JF, Kandimalla KK. Chitosan enhances the stability and targeting of immuno-nanovehicles to cerebrovascular deposits of Alzheimer’s disease amyloid protein. Nanomed 2012;8(2):250-60
- Sundaramoorthi C, Palanisamy S, Kalaivani M, Dhivya MM, Kalaiselvan V, Rajasekaran A. Biosynthesis of silver nanoparticles from Aspergillus niger and evaluation of its wound healing activity in experimental rat model. Int J Pharmtech Res 2009;1(4):1523-9.
- Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev 2001;47(1):65-81.
- Gelperina SE, Khalansky AS, Skidan IN, Smirnova ZS, Bobruskin AI, Severin SE, et al. Toxicological studies of doxorubicin bound to polysorbate 80-coated poly(butyl cyanoacrylate) nanoparticles in healthy rats and rats with intracranial glioblastoma. Toxicol Lett 2002;126(2):131-41.
- Sun W, Xie C, Wang H, Hu Y. Specific role of polysorbate 80 coating on the targeting of nanoparticles to the brain. Biomaterials 2004;25(15):3065-71.
- Wilson B, Samanta MK, Santhi K, Kumar KP, Paramakrishnan N, Suresh B. Poly (n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer's disease. Brain Res 2008;1200:159-68.
- Wang CX, Huang LS, Hou LB, Jiang L, Yan ZT, Wang YL, et al. Antitumor effects of polysorbate-80 coated gemcitabine polybutylcyanoacrylate nanoparticles in vitro and its pharmacodynamics in vivo on C6 glioma cells of a brain tumor model. Brain Res 2009;1261:91-9.
- Jose S, Sowmya S, Cinu TA, Aleykutty NA, Thomas S, Souto EB. Surface modified PLGA nanoparticles for brain targeting of Bacoside-A. Eur J Pharm Sci 2014;63:29-35.
- Sheetal S, Anil KB, Rakesh KS, Tanima B, Amarnath M. Pharmacoscintigraphic evaluation of polysorbate80-coated chitosan nanoparticles for brain targeting. Am J Drug Deliv 2005;3(3):205-12.
- Das D, Lin S. Double-coated poly (butylcynanoacrylate) nanoparticulate delivery systems for brain targeting of dalargin via oral administration. J Pharm Sci 2005;94(6):1343-53.
- Josephine LJ, Vijaya C, Barnabas W. Albumin nanoparticles coated with polysorbate 80 as a novel drug carrier for the delivery of antiretroviral drug- Efavirenz. Int J Pharm Investig 2014;4(3):142-48.
- Sun D, Xue A, Zhang B, Lou H, Shi H, Zhang X. Polysorbate 80-coated PLGA nanoparticles improve the permeability of acetylpuerarin and enhance its brain-protective effects in rats. J Pharm Pharmacol 2015;67(12):1650-62.
- Jose S, Juna BC, Cinu TA, Jyoti H, Aleykutty NA. Carboplatin loaded Surface modified PLGA nanoparticles: Optimization, characterization, and in vivo brain targeting studies. Colloids Surf B Biointerfaces 2016;142:307-14.
- Wang H, Jia Y, Hu W, Jiang H, Zhang J, Zhang L. Effect of preparation conditions on the size and encapsulation properties of mPEG-PLGA nanoparticles simultaneously loaded with vincristine sulfate and curcumin. Pharm Dev Technol 2013;18(3):694-70.
- Mayur B, Zaved AK. Poly(n-butylcyanoacrylate) nanoparticles for oral delivery of quercetin: preparation, characterization, and pharmacokinetics and biodistribution studies in Wistar rats. Int J Nanomed 2015;10:3921-35.
- Wohlfart S, Gelperina S, Kreuter J. Transport of drugs across the blood-brain barrier by nanoparticles. J Control. Release 2012;161(2):264-73.
- Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev 2012;64:213-22.