- Corresponding Author:
- Y. M. Jagtap*
Department of Pharmaceutics, STES’s Sinhgad College of Pharmacy, Vadgaon (Bk.), Pune-411 041, India
E-mail: yogeshjagtap2007@rediffmail.com
| Date of Submission | 17 October 2011 |
| Date of Revision | 16 November 2012 |
| Date of Acceptance | 21 November 2012 |
| Indian J Pharm Sci., 2012, 74 (6): 512-520 |
Abstract
Floating microspheres have emerged as a potential candidate for gastroretentive drug delivery system. For developing a desired intragastric floatation system employing these microspheres, it is necessary to select an appropriate balance between buoyancy and drug releasing rate. These properties mainly depend on the polymers used in the formulation of the microspheres. Hence it is necessory to study the effect of these polymer concentrations on the various physicochemical properties of the microspheres. Floating microspheres were prepared by emulsion solvent evaporation technique utilising different polymers such as ethyl cellulose, Eudragit® RS and Eudragit® RL by dissolving them in a mixture of dichloromethane and methanol. Release modifiers studied were hydroxypropyl methylcellulose K4M, hydroxypropyl methylcellulose E50 LV and Eudragit® EPO. Prepared microspheres were analysed for particle size, surface morphology, entrapment efficiency, buoyancy, differential scanning calorimetry and in-vitro drug release. Ethyl cellulose and Eudragit® EPO resulted microspheres with high percentage yield, excellent spherical shape but had very less buoyancies with a high cumulative drug release. Ethyl cellulose microspheres prepared using hydroxypropyl methylcellulose K4M showed more sustained drug release and high buoyancies than that of the microspheres formulated with the hydroxypropyl methylcellulose E50 LV. Amongst these hydroxypropyl methylcellulose E50 LV showed good balance between buoyancy and the drug release.
Keywords
Buoyancy, in-vitro drug release, release modifiers, solvent emulsion diffusion technique
Floating microspheres are gastroretentive drug delivery systems based on nonefferevescent approach [1]. Floating microspheres are in strict sense, spherical empty particles without core. These are free flowing particles; size ranging between 1 to 1000 µm. Kawashima et al. [2] had developed noneffervescent hollow polycarbonate microspheres by using an emulsion solvent diffusion technique. This gastrointestinal transit-controlled preparation is designed to float on gastric juice with a specific density of less than 1. This property results in delayed transit through the stomach. The drug is released slowly at desired rate resulting in increased gastric retention with reduced fluctuations in plasma drug concentration.
Ethyl cellulose (EC) and various types of Eudragit® are the most commonly used polymers for the preparation of the floating microspheres by emulsion solvent diffusion technique. The drug release from the microspheres consists of only EC or Eudragit® is very less [3,4]. According to Lee et al. [5], many drugs are not released in significant amount from these microparticles at the pH of gastric fluids. So there is a need of some hydrophilic polymers to be added into the formulation. These polymers cause rapid ingress of the dissolution medium into the microspheres facilitating more drug releases.
Srivastava et al. [6] prepared microspheres using a gradually increasing EC concentration in combination with a fixed concentration of hydroxypropyl methylcellulose (HPMC) to assess the effect of polymer concentration on the size of microspheres and found that mean particle size of the microspheres significantly increased with increasing EC. Baykara and Kiliçarslan, have studied the effect of the drug-to-polymer ratio on the properties of verapamil hydrochloride loaded microspheres. They found that the drug dissolution profile could be slowed down by increasing polymer amount in the formulations, and that particle size, surface characteristics of microspheres, and dissolution rate of verapamil hydrochloride could be modified through the variation of drug-to-polymer ratio [7].
The objective of the present investigation was to study the effect of the various polymers as release modifiers and their concentrations on the drug release and other physicochemical properties of the floating microspheres. The polymers employed in the preparation of the floating microspheres were EC, Eudragit® RS and Eudragit® RL. The release modifiers employed in the study were HPMC K4M, HPMC E50 LV and Eudragit® EPO.
Simvastatin was selected as a model drug for the preparation of the microspheres. It is a HMG-CoA reductase inhibitor used extensively in the treatment of hyperlipidemia. It is administered in the form of tablet on once a daily basis having systemic bioavailability of only 5%. It is having short half-life of only 2 h and hence necessitate frequent administration [8]. Floating drug delivery system will be able to prolong the gastric retention of microspheres, thereby improving oral bioavailability of simvastatin.
Materials and Methods
Simvastatin was obtained as a gift sample from Alkem Pharmaceuticals Ltd., Mumbai, India. EC was purchased from Analab Fine Chemicals, Mumbai, India. HPMC K4M and HPMC E50 LV were obtained as a gift sample from Colorcon Asia Ltd., Goa, India. Eudragit® EPO, Eudragit® RS and Eudragit® RL were obtained as gift samples from Evonic Degussa India Pvt. Ltd., Mumbai. Polyvinyl alcohol, methanol and dichloromethane were purchased from Merck Specialties Pvt. Ltd., Mumbai, India. All other chemicals were of analytical reagent grade and were used as received.
Preparation of drug-loaded floating microspheres
The floating microspheres were prepared by emulsion solvent diffusion technique [1]. Weighed amount of polymers (Table 1) were dissolved in 15 ml of dichloromethane:methanol mixture (1:1). Drug was then added to polymer solution and mixed for 15 min. This solution was added in a thin stream to 200 ml aqueous 0.75% polyvinyl alcohol containing 0.02% v/v of Tween 80, while stirring using a mechanical stirrer (Dolphin, Mumbai, India). Stirring was continued for 2 h at 500 rpm at room temperature until solvents evaporated completely. The formulated microspheres were filtered by using filter paper. The residual solvent was washed 4-5 times with 50 ml portions of distilled water. The product was dried overnight at temperature of 45°.
| Formulations | Drug:Polymer | EC:HPMC | EC:HPMC | EC:Eudragit® | Eudragit® RSPO:HPMC | Eudragit® RLPO:HPMC | |
|---|---|---|---|---|---|---|---|
| (D:P) ratio | K4M | E50 | LV | EPO | K4M | K4M | |
| F1 | 1:1 | 10:0 | - | - | - | - | |
| F2 | 1:1 | 9:1 | - | - | - | - | |
| F3 | 1:1 | 8:2 | - | - | - | - | |
| F4 | 1:1 | 7:3 | - | - | - | - | |
| F5 | 1:1 | 6:4 | - | - | - | - | |
| F6 | 1:1 | - | 9:1 | - | - | - | |
| F7 | 1:1 | - | 8:2 | - | - | - | |
| F8 | 1:1 | - | 7:3 | - | - | - | |
| F9 | 1:1 | - | 6:4 | - | - | - | |
| F10 | 1:1 | - | - | 9:1 | - | - | |
| F11 | 1:1 | - | - | 8:2 | - | - | |
| F12 | 1:1 | - | - | 7:3 | - | - | |
| F13 | 1:1 | - | - | 6:4 | - | - | |
| F14 | 1:2 | 10:0 | - | - | - | - | |
| F15 | 1:2 | 9:1 | - | - | - | - | |
| F16 | 1:2 | 8:2 | - | - | - | - | |
| F17 | 1:2 | 7:3 | - | - | - | - | |
| F18 | 1:2 | 6:4 | - | - | - | - | |
| F19 | 1:2 | - | 9:1 | - | - | - | |
| F20 | 1:2 | - | 8:2 | - | - | - | |
| F21 | 1:2 | - | 7:3 | - | - | - | |
| F22 | 1:2 | - | 6:4 | - | - | - | |
| F23 | 1:2 | - | - | 9:1 | - | - | |
| F24 | 1:2 | - | - | 8:2 | - | - | |
| F25 | 1:2 | - | - | 7:3 | - | - | |
| F26 | 1:2 | - | - | 6:4 | - | - | |
| F27 | 1:3 | 10:0 | - | - | - | - | |
| F28 | 1:3 | 9:1 | - | - | - | - | |
| F29 | 1:2 | - | - | - | 10:0 | - | |
| F30 | 1:2 | - | - | - | - | 10:0 | |
All experiments were performed in triplicate (n=3). EC=Ethyl cellulose, HPMC=Hydroxy propyl methyl cellulose
Table 1: Formulation Showing Various Proportions of the Drug and Polymers
Yields of microspheres
The practical yield of microspheres of various batches were calculated using the weight of final product after drying with respect to the initial total weight of the drug and polymer used for preparation of microspheres. Yields were calculated as per the formula, %Yield=(weight of dried hollow microspheres)/(weight of drug taken+total polymer weight)x100...(1).
Drug content and encapsulation efficiency of microspheres
Predetermined amount of microspheres (25 mg) containing drug was dissolved in methanol (25 ml) by ultrasonication (Model USB 40, Spectralab, India). The solution was filtered through 0.45 µm Whatman filter paper and 0.5 ml was transferred to 10 ml volumetric flask. The volume was made up to the mark with methanol. Absorbance was determined by UV spectrophotometer (Shimadzu, V-1800, Japan) and the drug content was calculated according to the equation, % drug content=(weight of drug in microspheres/weight of microspheres recovered)×100...(2). The percentage entrapment efficiency (%EE) was calculated by the equation, %EE=(calculated drug concentration/theoretical drug content)×100...(3).
Surface morphology
The external morphology of the microspheres was studied by scanning electron microscope (SEM, JEOL JSM-6360A, Japan). The microspheres were attached to solid metal specimen stubs, which had a circular face. The upper surface of microspheres was then coated under vacuum with a platinum film. The metal stubs with their coated microspheres were placed in the specimen chamber. The field was scanned at various magnifications (×250 to ×2000) for examination of microspheres.
Particle size analysis
The particle size is measured using an optical microscope (LM-52-1704, Lawrence and Mayo, India), and the mean particle size is calculated by measuring 200-300 particles with the help of a calibrated ocular micrometer. The average particle size of the microspheres was expressed as diameter.
Micromeritic properties
The microspheres were characterised for micromeritics such as tapped density, angle of repose (?) and compressibility index [9].
To determine tapped bulk density 1 g of simvastatin microspheres was introduced into a 10 ml measuring cylinder. Initial volume was measured as bulk volume; the cylinder was placed on bulk density apparatus. The tapping was continued until no further change in volume was noted. Tapped densities were calculated by using the equation, Tapped density= (Mass of microspheres/Volume of microspheres after tapping)...(4)
Compressibility index has been proposed as an indirect measure of bulk density, size and shape, surface area, moisture content and cohesiveness of materials. The following formula was used to calculate compressibility index: %Compressibility index=(1-(V/V0))×100...(5), where V and V0 are the volumes of the sample after and before the standard tapping, respectively.
The angle of repose has been used to characterize the flow properties of solids. Angle of repose of simvastatin microspheres was determined by fixed funnel method. The powder was poured from a funnel on vibration free base. The powder was poured with help of a spatula until a hip of particular height was formed. The angle of repose was determined by measuring the height and diameter of the cone of powder and calculated from the following equation, tan ?=h/r...(6), where, h is the height of heap of powder and r is the radius of the base of the powder cone.
Floating behaviour
The floating test on the microspheres was carried out using the dissolution type II apparatus specified in the USP XXII (Electrolab, TDT-08L, USA). The microspheres were spread over the surface of the dispersing medium (900 ml) which is agitated by a paddle rotated at 100 rpm. Dissolution medium (pH 1.2) containing Tween 20 (0.02%, w/v) was used as dispersing medium to simulate gastric fluid. After agitation for a previously determined interval, the hollow microspheres that floated over the surface of medium and those that settled to the bottom of the flask were recovered separately. After drying, each fraction of the hollow microspheres was weighed. The buoyancy of the hollow microspheres was represented by the equation [10]: Buoyancy(%)=(Qf/ (Qf+Qs))×100...(7), where, Qf and Qs are the weights of the floating and the settled hollow microspheres, respectively.
In vitro drug release study
In vitro dissolution studies can be carried out in a USP XXII paddle type dissolution apparatus. Microspheres equivalent to the drug dose are introduced into 900 ml of the dissolution medium stirred at 100 rpm at 37±0.5°. The dissolution medium was 0.1 N HCl (pH 1.2) containing 0.2% Tween 20 to maintain the sink condition. Aliquots of 10 ml were withdrawn at an interval of 1, 2, 4, 6 and 8 h. The equivalent volume was replaced with dissolution medium to maintain the sink condition. The withdrawn samples were filtered through 0.45 µm syringe filter. The samples were analysed spectrophotometrically at 239 nm to determine the drug concentration.
Differential scanning calorimetry
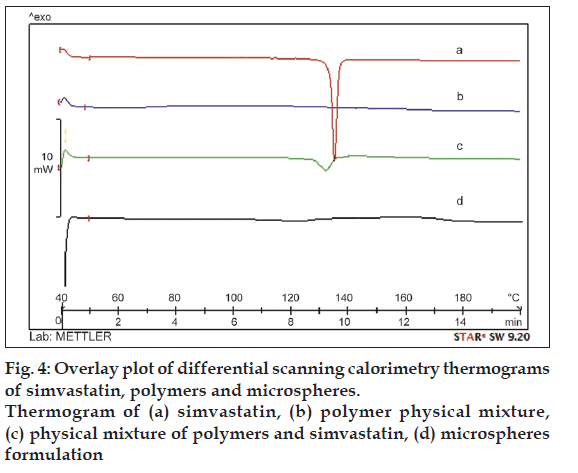
Thermal analysis was performed using a differential scanning calorimeter (differential scanning calorimetry (DSC) 1, Stare System, Mettler Toledo, USA) equipped with a computerized data station. The sample of pure drug, physical mixture of drug and polymers, mixture of polymers and microspheres were weighed and heated at a scanning rate of 10°/min between 40 and 200° and 40 ml/min of nitrogen flow with an empty aluminium crucible as reference pan.
Results and Discussion
The floating microspheres were prepared by emulsion solvent diffusion technique as shown in Table 1 [1]. Water insoluble polymers show higher solubility in dichloromethane than methanol. However, methanol has higher solubility in water. As soon as the polymer solution was added to the aqueous medium, the methanol diffuses rapidly from the droplets of the polymer solution. Simultaneous diffusion of water inside the sphere further decreased the methanol concentration, and hence the polymer precipitated resulting in the formation of microspheres. Dichloromethane remaining as the central core diffused slowly due to its low water solubility. Due to the poor miscibility, water could not effectively invade the dichloromethane rich core. Therefore, the diffusion of dichloromethane began late, after the initial solidification, and formed a central hollow structure. During the diffusion of the solvents, the polymer was pulled outward as a result of the dragging force of the solvents and thus the central void space emerged. The central cavity produced by the solvents was gradually filled with water due to the reduced internal pressure. Water escaped out of the cavity during the drying process ultimately forming hollow microspheres [11]. The microspheres were formed when prepared with EC, but the microspheres were not formed with the batches F29 and F30 containing, Eudragit® RS and Eudragit® RL, respectively with the given experimental condition. This could be due to inability of the polymers to form a film leading to formation of nonfloating particles.
The percentage yield of formed microspheres is shown in Table 2. The product yield for microspheres was found to be in the range of 58.66±1.15% to 90.27±1.56%. The product yield depended upon the agglomeration and sticking of polymer to blades of stirrer and to the wall of the beaker during microsphere formation. The product yield was also found to be dependent on the choice of the polymer. The yield of the microspheres containing HPMC polymers was found to be decreased with increase in the concentration of the HPMC polymers (F1 to F9 and F14 to F22) whereas the yield of the formulations containing Eudragit® EPO was found to be independent of the concentration (F10 to F13 and F23 to F26). This may be due to migration of HPMC into continuous phase forming agglomerates accompanied with sticking of the polymer to the stirrer blade and beaker surface.
| Formulations | % Yield | Particle size (µm) | Tapped density (g/ml) | Angle of repose (?) | Compressibility index |
|---|---|---|---|---|---|
| F1 | 84.75±1.26 | 150.54±31.35 | 0.162±0.011 | 33.70±1.50 | 22.84±0.45 |
| F2 | 72.00±1.21 | 140.77±25.78 | 0.160±0.010 | 33.44±1.03 | 22.50±0.58 |
| F3 | 69.91±1.06 | 131.34±22.57 | 0.163±0.013 | 34.56±0.93 | 22.66±0.65 |
| F4 | 65.00±0.94 | 125.19±11.02 | 0.165±0.009 | 35.12±1.25 | 23.03±0.84 |
| F5 | 63.33±0.99 | 120.81±17.23 | 0.158±0.011 | 35.46±1.56 | 24.05±0.45 |
| F6 | 70.00±1.00 | 150.93±21.24 | 0.156±0.010 | 33.10±1.53 | 23.07±0.49 |
| F7 | 66.00±0.98 | 145.30±19.98 | 0.156±0.011 | 33.98±0.96 | 22.43±0.55 |
| F8 | 61.33±0.87 | 133.04±28.34 | 0.159±0.012 | 34.98±1.21 | 23.27±0.64 |
| F9 | 58.66±1.15 | 126.73±21.75 | 0.158±0.011 | 35.11±2.02 | 23.41±0.67 |
| F10 | 80.33±1.24 | 400.32±23.23 | 0.161±0.009 | 32.44±1.54 | 23.60±0.44 |
| F11 | 79.66±1.01 | 351.61±30.09 | 0.162±0.009 | 31.87±0.55 | 23.45±0.74 |
| F12 | 78.00±0.87 | 410.88±37.57 | 0.160±0.011 | 31.34±0.077 | 22.36±0.66 |
| F13 | 80.66±1.49 | 423.19±40.39 | 0.161±0.011 | 30.23±0.53 | 23.60±0.41 |
| F14 | 90.27±1.56 | 201.11±25.98 | 0.162±0.009 | 35.20±0.76 | 25.52±0.63 |
| F15 | 78.33±0.92 | 183.37±32.67 | 0.161±0.011 | 35.34±1.15 | 25.74±0.89 |
| F16 | 76.66±1.19 | 160.45±19.83 | 0.167±0.016 | 34.00±1.08 | 25.00±0.78 |
| F17 | 72.11±0.99 | 151.39±29.12 | 0.168±0.014 | 34.87±0.99 | 23.31±0.90 |
| F18 | 65.66±0.83 | 145.66±33.29 | 0.166±0.015 | 35.46±1.30 | 24.09±0.88 |
| F19 | 77.66±0.99 | 169.84±29.60 | 0.164±0.010 | 33.67±1.51 | 24.09±0.67 |
| F20 | 75.33±1.08 | 152.12±28.43 | 0.163±0.011 | 34.23±0.93 | 25.14±0.91 |
| F21 | 71.11±1.32 | 141.32±32.49 | 0.166±0.009 | 33.12±1.05 | 25.00±0.74 |
| F22 | 64.44±1.22 | 132.04±22.51 | 0.166±0.010 | 34.55±1.16 | 24.24±0.56 |
| F23 | 75.00±1.54 | 480.87±54.20 | 0.167±0.012 | 30.12±0.57 | 24.39±0.80 |
| F24 | 77.71±1.34 | 470.58±34.36 | 0.164±0.011 | 31.89±0.93 | 22.36±0.49 |
| F25 | 74.44±1.11 | 494.20±41.55 | 0.165±0.012 | 30.43±1.12 | 25.90±0.66 |
| F26 | 72.55±1.15 | 450.43±26.90 | 0.164±0.011 | 30.99±0.85 | 23.78±0.71 |
| F27 | 80.70±1.11 | 200.43±26.90 | 0.165±0.011 | 30.30±0.92 | 25.08±0.45 |
| F28 | 74.25±0.85 | 190.23±31.07 | 0.169±0.012 | 28.44±0.51 | 24.26±0.34 |
Mean±SD (n=3), SD=Standard deviation
Table 2: Yield, Particle Size, Drug Content and Micromeritics Properties
It has been observed that the EE of the microspheres containing HPMC polymers has been increased with an increase in the concentration of the polymers in the formulation (Table 3). This may be due to an increase in the entrapment of drug in the swollen or gel structure of HPMC. The entrapment efficiency of the Eudragit® was found to be unaffected by increase in the concentration of the Eudragit® as it was unable to form gel like structure. It was observed that the percent drug content was found to decrease with the increase in the drug-to-polymer ratio.
| Formulations | Drug content | EE % | Buoyancy | Drug release |
|---|---|---|---|---|
| % | % | in 8 h % | ||
| F1 | 23.38±1.23 | 46.76±0.32 92.89±1.03 | 42.27±2.14 | |
| F2 | 28.22±1.17 | 54.52±0.38 85.12±2.12 | 48.73±2.56 | |
| F3 | 34.60±1.03 | 69.20±0.45 79.54±3.76 | 71.15±2.01 | |
| F4 | 34.20±0.91 | 68.40±0.34 70.23±3.34 | 93.82±3.11 | |
| F5 | 43.15±1.13 | 86.32±0.23 56.37±4.56 | 100.6±0.34 | |
| F6 | 20.30±0.99 | 73.50±0.33 82.98±3.44 | 53.96±2.71 | |
| F7 | 35.50±1.17 | 71.00±0.35 78.04±3.78 | 76.33±2.01 | |
| F8 | 38.08±0.84 | 76.16±0.43 68.59±3.34 | 100.2±0.89 | |
| F9 | 39.46±0.81 | 78.92±0.39 57.64±4.09 | 101.1±0.56 | |
| F10 | 33.32±0.97 | 66.64±0.29 50.76±5.98 | 80.85±2.31 | |
| F11 | 32.10±1.19 | 64.20±0.26 41.96±4.01 | 87.02±1.67 | |
| F12 | 37.58±0.97 | 76.76±0.41 30.92±5.76 | 96.40±1.49 | |
| F13 | 30.80±0.94 | 61.56±0.39 15.10±6.98 | 99.01±1.21 | |
| F14 | 23.04±1.11 | 69.14±0.44 96.22±2.13 | 32.12±1.56 | |
| F15 | 23.58±1.06 | 70.76±0.37 91.02±4.01 | 38.82±2.42 | |
| F16 | 26.73±1.18 | 80.22±0.26 86.22±3.11 | 45.15±2.10 | |
| F17 | 24.03±1.06 | 72.14±0.21 75.63±4.00 | 65.46±1.34 | |
| F18 | 25.28±0.93 | 76.80±0.29 60.89±4.12 | 98.46±2.07 | |
| F19 | 22.21±0.80 | 68.18±0.30 85.61±2.43 | 53.82±2.23 | |
| F20 | 24.73±0.99 | 74.22±0.34 81.90±3.19 | 67.60±1.45 | |
| F21 | 28.30±0.96 | 84.95±0.30 70.17±3.29 | 85.92±1.03 | |
| F22 | 24.11±1.14 | 72.38±0.48 62.32±4.17 | 100.1±0.45 | |
| F23 | 25.42±0.89 | 76.31±0.27 75.29±2.83 | 71.45±2.51 | |
| F24 | 20.60±0.97 | 61.83±0.29 66.41±3.02 | 90.60±1.29 | |
| F25 | 25.72±0.95 | 77.19±0.31 50.88±4.76 | 98.36±2.13 | |
| F26 | 21.80±1.13 | 65.54±0.38 | 25.21±6.32 | 101.5±0.23 |
| F27 | 18.73±0.96 | 75.16±051 | 96.21±2.46 | 27.37±1.34 |
| F28 | 17.80±1.03 | 74.25±0.26 | 94.54±3.23 | 35.25±2.10 |
Mean±SD (n=3). SD=Standard deviation, EE=Encapsulation efficiency , The values are release obtained after 8h.
Table 3: Drug Content, Encapsulation Efficiency, Buoyancy and Drug Release
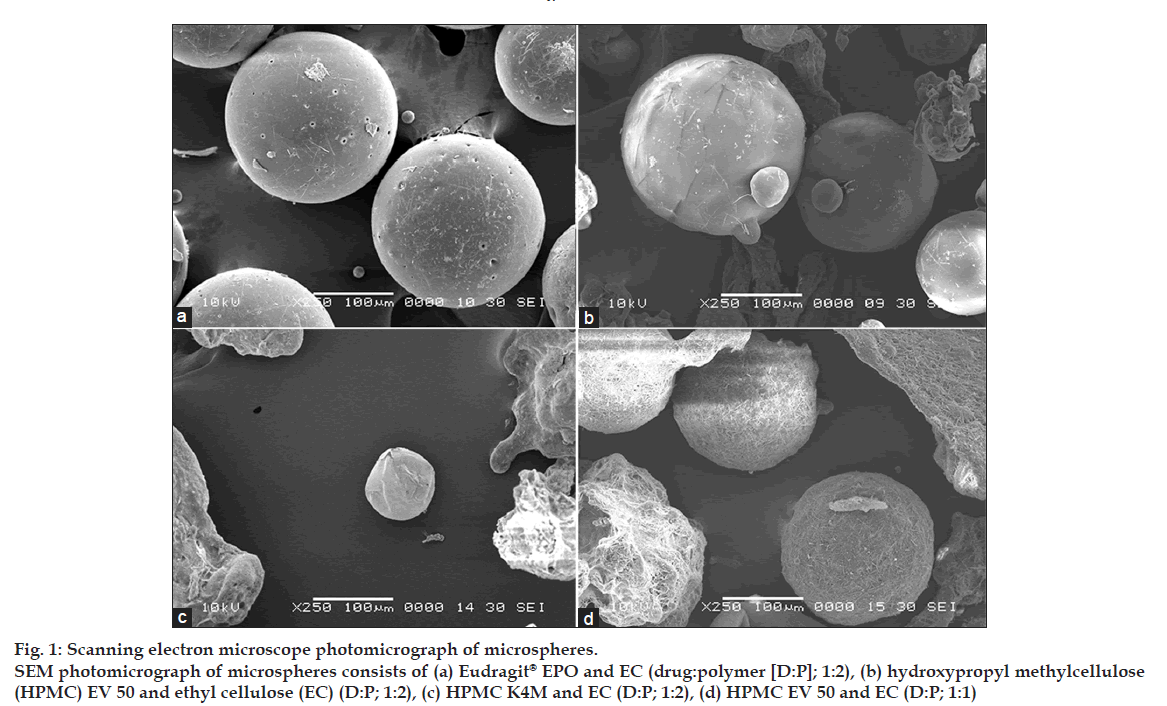
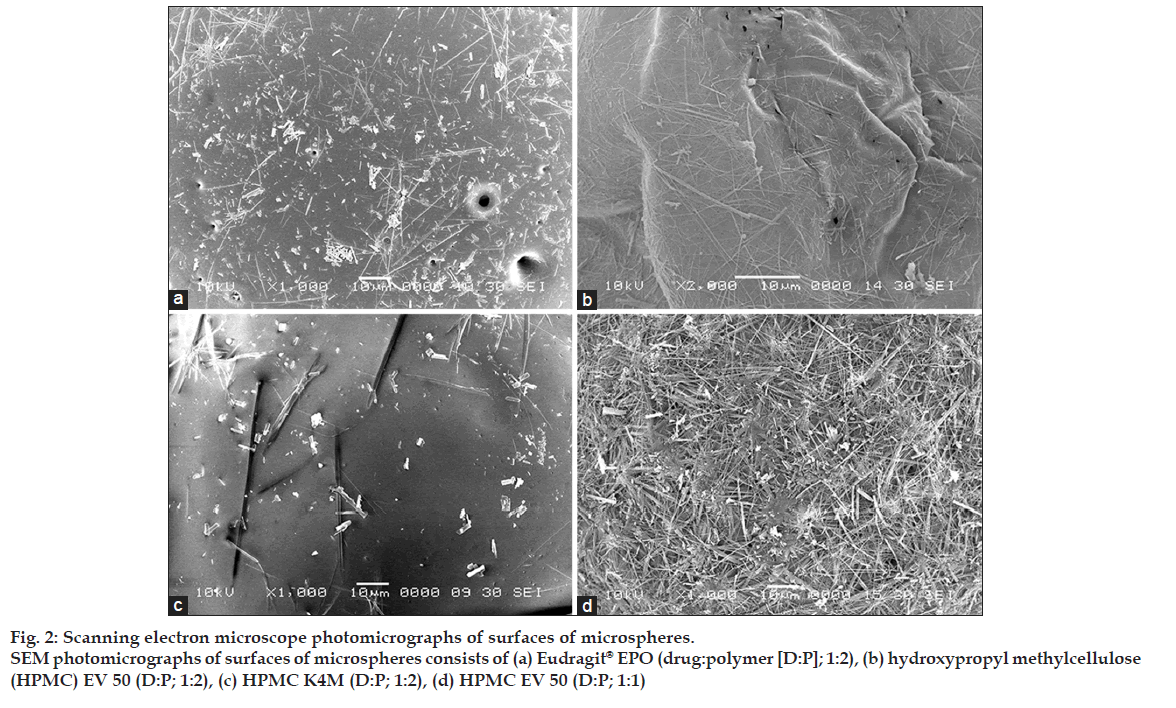
Shape and surface morphological examination of microspheres were done by scanning electron microscope (SEM). Fig. 1 shows the SEM photograph of the whole microsphere and fig. 2 shows the nature of the surface of the microspheres. From the figures, it may be concluded that the surface of the microspheres containing high drug polymer concentration (fig. 2a-c) is smoother than that of the microspheres of the low drug polymer concentration (fig. 2d). This may be due to rapid diffusion of the solvents from the formulations having low drug polymer concentration. The smoothest surface was found with the HPMC K4M (fig. 2c) as HPMC K4M is having highest viscosity it leads to slower diffusion of the solvent. The microspheres containing Eudragit® EPO show pores on the surface due to traces of solvent evaporation [9].
Figure 1: Scanning electron microscope photomicrograph of microspheres.
SEM photomicrograph of microspheres consists of (a) Eudragit® EPO and EC (drug:polymer [D:P]; 1:2), (b) hydroxypropyl methylcellulose
(HPMC) EV 50 and ethyl cellulose (EC) (D:P; 1:2), (c) HPMC K4M and EC (D:P; 1:2), (d) HPMC EV 50 and EC (D:P; 1:1)
Figure 2: Scanning electron microscope photomicrographs of surfaces of microspheres.
SEM photomicrographs of surfaces of microspheres consists of (a) Eudragit® EPO (drug:polymer [D:P]; 1:2), (b) hydroxypropyl methylcellulose
(HPMC) EV 50 (D:P; 1:2), (c) HPMC K4M (D:P; 1:2), (d) HPMC EV 50 (D:P; 1:1)
The particle size analysis revealed that the mean diameter of the microspheres containing Eudragit® EPO is greater than that of HPMC polymers (Table 2). Mean diameter of microspheres containing HPMC polymers was found to be in the range of 120.81±17.23 to 201.11±25.98 µm whereas that of Eudragit® EPO was found in the range of 351.61±30.09 to 494.20±41.55 µm.
The flow properties of the microspheres are expressed in terms of Carr?s index and angle of repose. The values of the angle of repose was in the range of 28-35, which indicates good to passable flow properties, whereas the Carr?s index for all formulations was in the range of 22-25, which indicated fair flow properties [9]. This suggests that the microspheres can be easily handled during processing.
The % buoyancies of the microspheres were found to decrease with an increase in the HPMC or Eudragit® EPO concentration (Table 3). This may be due to rapid penetration of the dissolution medium in the microspheres, as HPMC is water swellable polymer and Eudragit® is soluble in dissolution medium pH 1.2. The maximum decrease in the buoyancy with an increase in the polymer concentration was found with the Eudragit® EPO (drug: polymer; 1:1), which is 15.10±6.98%; this may be due to the rapid ingress of the dissolution medium into the microspheres due to rapid dissolution of the Eudragit® EPO creating more porous structure. The buoyancies were found to be higher with high polymer concentration; highest 96.22±2.13% with drug-to-polymer ratio (1:3) and lowest 15.10±6.98% with drug-to-polymer ratio (1:1). HPMC K4M having high viscosity form a strong barrier to the ingress of dissolution medium, hence have higher percentage of buoyancies than that of HPMC E50 LV. The buoyancy decreases with time as the drug diffused out from the microspheres, small pores were formed in the system which allowed surrounding medium to enter and fill up the void spaces, thereby increasing weight [12].
The results of in vitro dissolution studies are depicted in Table 3. It was observed that the release of the microspheres was found to increase with decrease in the drug-to-polymer ratio from 1:3 to 1:1. The formulations containing Eudragit® EPO shows a high percentage of cumulative release with an increase in the Eudragit proportion in the formulation, this may be due to rapid ingress of dissolution medium into the microspheres. The formulations F10 to F13 and F23 to F26 represent the batches containing Eudragit® EPO. The release of the drug from the batch F10 to F13 increased from 80.85±2.31% to 99.01±1.21% in 8 h. These formulations have very less % buoyancies (50.76±5.98 to 15.10±6.98%). The formulations F23 to F26 shows more sustained release than that of earlier formulations of Eudragit® EPO ranging from 71.45±2.51 to 101.5±0.23%. The formulations containing HPMC K4M and HPMC E50 LV are represented as F2 to F9 for drug polymer ratio 1:1. It has been found that for the same ratio of the EC: HPMC, the percentage release was higher for the HPMC E50 LV as compared to HPMC K4M. This may due to low viscosity of the HPMC E50 LV facilitating penetration of dissolution medium into the microspheres [5]. The formulations containing drug-to-polymer ratio 1:2 show wide range of percent drug release from 32.12±1.56 to 100.1±0.45%. The formulation F15 to F18, which contains HPMC K4M shows more sustained drug release. The formulations F19 to F22 show drug release from 53.82±2.23 to 100.1±0.45% within 8 h. The F21 formulation containing HPMC E50 LV in the ratio of 7:3 (EC:HPMC E50 LV) shows 85.92±1.03% of drug release in 8 h and also has good % buoyancy (70.17±3.29%). Considering these facts F21 formulation was considered as a best formulation. The dissolution profiles of the formulations are given in fig. 3.
In order to determine the physical state of the drug in the microspheres the DSC study was carried out. DSC thermogram of the pure drug and microspheres formulation are shown in fig. 4. DSC thermogram of pure simvastatin exhibited a single sharp endothermic peak at 139° corresponding to its melting transition temperature (fig. 4a). This peak was also observed in the thermogram of the physical mixture of simvastatin and polymer, which is broadened; less intense as compared to simvastatin alone and shifted slightly (fig. 4c). This may be possibly due to fact that presence of EC and HPMC in the physical mixture depresses the intensity of endothermic peak of simvastatin, broadens its melting point and was uniformly dispersed at the molecular level in the microspheres.
Floating microspheres were prepared by emulsion solvent diffusion method. The microspheres were formed only with EC. Microspheres were not formed with Eudragit® RS and Eudragit® RS with given set of experimental conditions. Effects of HPMC K4M, HPMC E50 LV and Eudragit® EPO as the release modifiers were studied to find out the effect on buoyancy and the drug release. Eudragit® EPO gave microspheres with high percentage yield, excellent spherical shape but had very less buoyancies with high cumulative drug release. The microspheres formed with HPMC K4M showed more sustained drug release and higher buoyancies than that of the microspheres formed with the HPMC E50 LV. HPMC polymers showed better control over the drug release pattern than that of the Eudragit® EPO. There was an inverse relationship between the buoyancy of microspheres and the level of drug release from the microspheres; as the buoyancy decreased more microspheres sank to the bottom leading to more drug release. Thus, it was found that the factors influencing the buoyancy of microsphere are polymer and release modifiers ratio in the formulation. Furthermore, drug released from microspheres was also affected by the polymer and release modifiers ratio.
Acknowledgements
The authors wish to thank to All India Council of Technical Education (AICTE), New Delhi, for financial support. The authors gratefully acknowledge Alkem Pharmaceuticals Ltd., Mumbai and Signet Chemical Corporation Pvt. Ltd., Mumbai, for providing the drug and the polymers as a gift samples.
References
- Singh BN, Kim KH. Floating drug delivery systems: An approach to oral controlled drug delivery via gastric retention. J Control Release 2000;63:235-59.
- Kawashima Y, Niwa T, Takeuchi H, Hino T, Itoh Y. Hollow microspheres for use as a floating controlled drug delivery system in the stomach. J Pharm Sci 1992;81:135-40.
- Sato Y, Kawashima Y, Takeuchi H, Yamamoto H. in vitro and in vivo evaluation of riboflavin-containing microballoons for a floating controlled drug delivery system in healthy humans. Int J Pharm 2004;275:97-107.
- Sato Y, Kawashima Y, Takeuchi H, Yamamoto H. in vitro evaluation of floating and drug releasing behaviors of hollow microspheres (microballoons) prepared by the emulsion solvent diffusion method. Eur J Pharm Biopharm 2004;57:235-43.
- Lee JH, Park TG, Choi HK. Development of oral drug delivery system using floating microspheres. J Microencapsul 1999;16:715-29.
- Srivastava AK, Ridhurkar DN, Wadhwa S. Floating microspheres of cimetidine: Formulation, characterization and in vitro evaluation. Acta Pharm 2005;55:277-85.
- Kiliçarslan M, Baykara T. The effect of the drug/polymer ratio on the properties of the verapamil HCl loaded microspheres. Int J Pharm 2003;252:99-109.
- Moffat AC, Osselton MD, Widdop B, editors. Clarke?s Analysis of Drugs and Poisons. 3rd ed., electronic version. London: Pharmaceutical Press; 2005.
- Sinko PJ. Micrometrics. In: Martin A, editor. Martin?s Physical Pharmacy and Pharmaceutical Science. 5th ed. Baltimore: Lippincott Williams and Wilkins; 2006. p. 553-9.
- Kawashima Y, Niwa T, Takeuchi H, Hino T, Itoh Y. Preparation of multiple unit hollow microspheres (microballoons) with acrylic resin containing tranilast and their drug release characteristics (in vitro) and floating behavior (in vivo). J Control Release 1991;16:279-90.
- Nepal PR, Chun MK, Choi HK. Preparation of floating microspheres for fish farming. Int J Pharm 2007;341:85-90.
- Porwal A, Swami G, Saraf S. Preparation and evaluation of sustained release microballoons of propranolol. Daru 2011;19:193-201.
- Butoescu N, Jordan O, Burdet P, Stadelmann P, Petri-Fink A, Hofmann H, et al. Dexamethasone-containing biodegradable uperparamagnetic microparticles for intra-articular administration: Physicochemical and magnetic properties, in vitro and in vivo drug release. Eur J Pharm Biopharm 2009;72:529-38.
 F2
F2  F3
F3  F4
F4  F5
F5  F6
F6  F7
F7  F8
F8  , F9
, F9  F10
F10  F11
F11