- *Corresponding Author:
- Gabriela Delgado
Immunotoxicology Research Group, Pharmacy Department, Universidad Nacional de Colombia. Bogotá, Colombia. A.A. 14490, Bogotá-Colombia
E-mail: lgdelgadoml@unal.edu.co
| Date of Submission | 09 July 2013 |
| Date of Revision | 08 March 2014 |
| Date of Acceptance | 15 March 2014 |
| Indian J Pharm Sci 2014; 76(3):188-197 |
Abstract
Traditional medicine has provided a number of therapeutic solutions for the control of infectious agents, cancers, and other diseases. After screening a wide variety of Colombian plant extracts, we have identified promising antileishmanial activity in ethanol extracts from Ocotea macrophylla (Lauraceae) and Zanthoxyllum monophyllum (Rutaceae). In this study, we evaluated the in vitro activity of two ethanol extracts, one from Ocotea macrophylla and the other from Zanthoxyllum monophyllum and one alkaloid fraction of ethanol extract of Zanthoxyllum monophyllum, on peritoneal macrophages isolated from golden Syrian hamsters (Mesocricetus auratus) infected with Leishmania panamensis and Leishmania major promastigotes. All of the extracts studied displayed promising (≤2) selectivity indices (S/I), the most significant of which were for ethanol extract of Zanthoxyllum monophyllum against Leishmania panamensis (S/I=12) and alkaloid fraction of ethanol extract of Zanthoxyllum monophyllum against Leishmania major (S/I=11). These results support the use of ethanol extracts and alkaloid fractions isolated from Ocotea macrophylla and Zanthoxyllum monophyllum, respectively; as therapeutic options for cutaneous leishmaniasis.
Keywords
Leishmaniasis, hamster macrophages, natural products, Rutaceae, Lauraceae
Leishmania, a protozoan parasite belonging to the Trypanosomatidae family, is the causative agent of leishmaniasis. It has a complex life cycle with two basic stages: An intracellular stage within a vertebrate host, known as the amastigote form, and an extracellular stage within an invertebrate vector, known as the promastigote form [1]. Leishmaniasis is endemic in multiple regions of the American continent. In the Andean and Amazonian regions (Venezuela, Colombia, Ecuador, Peru, and Bolivia), infection is predominantly caused by Leishmania Viannia [2] and results in cutaneous lesions. A small number of cases progress to mucocutaneous leishmaniasis [3]. In the old world, localized cutaneous leishmaniasis is often a result of L. major infection [4]. Cutaneous leishmaniasis is characterized by the formation of skin papules, nodes, or ulcerations.
Currently, leishmaniasis is endemic to 88 countries, 72 of which are developing countries, and 90% of cases present with cutaneous symptoms. In 2011 in Colombia, 8.023 cases were reported, of which 147 cases were mucocutaneous leishmaniasis, and 18 cases were visceral leishmaniasis [5]. There is currently no vaccine for controlling leishmaniasis, and the available treatments are toxic and costly [6]. Another confounding issue is the appearance of drug-resistant parasite strains [3]. These drawbacks demonstrate the urgent need to explore new therapeutic agents in the treatment of leishmaniasis [7].
The exploitation of plants is a popular and widely practiced method for the control of a variety of parasitic infections, including leishmaniasis [8]. While specific compounds isolated from plants have been identified as antileishmanial agents, a large proportion of them have not been adequately studied. Further analysis of these compounds could help validate their everyday use as ethno botanical resources [7,9].
The immunotoxicology research group has previously reported the in vitro antileishmanial activity of ethanol extracts from Ocotea macrophylla and Zanthoxyllum monophyllum. They demonstrated cytotoxic activity on a cell line of J774 murine macrophages and inhibitory activity on L. panamensis and L. braziliensis promastigotes. This activity showed a selectivity index [10] that warranted further study in a model closer to humans. While the murine model has clarified many aspects of Leishmaniasis immunopathology [11], it has become clear that other experimental animal models with greater similarity to humans need to be considered.
The golden hamster, for example, is an appropriate model for cutaneous leishmaniasis caused by parasites of the Viannia subgenus. Hamsters that are infected with Leishmania (Viannia) spp develop a chronic cutaneous lesion [12] similar to that developed by susceptible humans. This pathology is more similar to human infection than that observed in mice or rats for a number of pathogens, including L. (Viannia) spp [13]. Here we confirm the in vitro cytotoxic activity of plant-derived extracts on hamster peritoneal macrophages. We also show antileishmanial activity of these extracts on L. panamensis and L. major promastigotes and intracellular amastigotes.
Materials and Methods
Peritoneal macrophages
The macrophages were harvested from the peritoneal cavities of golden Syrian hamsters that had received an intraperitoneal (ip) injection of sterile 0.4% thioglycolate (Sigma Chemical Co, St Louis, USA) [14]. Three days after the thioglycolate injection, each animal received an additional ip injection of 15 ml of RPMI 1640 culture medium (RPMI, Gibco BRL-Life Technologies Inc., Grand Island, NY, USA). After a soft abdominal massage, the peritoneal fluid was aseptically extracted into vials containing sodium heparin.
The laboratory animal treatment was carried out according to Act 84 of the Republic of Colombia, Chapter VI, from 1989 and Resolution 594 from July 11th, 1996, which refer to the use of live animals in experiments and research. The care and use of laboratory animals was performed under the conditions established by the CCAC (Committee on Care and Use of Laboratory Animals, US) and according to the Standard Operating Procedures established and implemented by the Biotery at the National University of Colombia. All protocols and procedures were approved by the Science Department Ethics Committee (Universidad Nacional de Colombia), and these followed all of the international standards.
The procedure described here was presented and endorsed by the Institutional Ethics Committee. The cells were grown on polystyrene petri dishes (BD Bioscience, Falcon) in RPMI supplemented with inactivated (cRPMI) fetal bovine serum (FBS) (Microgen Ltd. Bogota Colombia). The macrophages were allowed to adhere over a 24-h period, and then the cells were washed, and the non-viable and/or nonadherent cells were discarded. This step also eliminated erythrocytes or platelets that might have been present. The adherent peritoneal macrophages were removed from the petri dish using a cell scraper (Techno Plastic Products AG, Switzerland). The cells were either added to 96-well plates (Techno Plastic Products AG, Switzerland) at 2×104 cells/well in 100 μl of cRPMI for cytotoxicity assays or in in 24-well plates in 400 μl cRPMI for amastigote assays [14].
L. panamensis and L. major parasites
L. panamensis (MHOM/CO/87/UA140, donated by the Universidad de Antioquia, Colombia) and L. major (Friedlin V1 strain, donated by the Universidad Autónoma de Madrid, Spain) promastigotes were grown in 25 cm2 flasks (Techno Plastic Products AG, Switzerland) in RPMI, supplemented with 10% FBS and 1% L-glutamine, at 27° in ambient gasification and humidity conditions. The samples were kept in 5 to 6 mL of medium for 7 days, at which point they were moved to 15 ml tubes, and the media was replaced. A 50 μl aliquot of the parasite culture was mixed with 50 μl of saline solution containing 2% Giemsa and 2% formol (to immobilize the parasites for counting) for parasite quantification.
Extracts source
The ethanol extracts were obtained from Lauraceae and Rutaceae plants from the Natural Plant Products Research Group at the National University of Colombia Chemistry Department. The O. macrophylla plants were collected in the town of Nocaima (Cundinamarca), and the Z. monophyllum plants were collected in San Bernardo (Cundinamarca). A sample of each plant species may be found in the National Herbarium at the Natural Sciences Institute of the National University of Colombia under collection numbers COL517191 and COL517520, respectively. Upon collection, the plants were stored and ultimately macerated in 96% ethanol to obtain the extracts. In the case of O. macrophylla, the leaves were used for maceration, and in the case of Z. monophyllum, the bark was used. Once obtained, the extracts were dissolved in dimethylsulfoxide (DMSO) and were later diluted with RPMI to ensure a final DMSO concentration of <1%.
Three different ethanol extracts were used in this study, one derived from O. macrophylla (EEOM) and two derived from Z. monophyllum (AFEEZM and EEZM). In detail, the crude extract derived of leaves of O. macrophylla (EEOM) was obtained by maceration in 96% ethanol to room temperature.
For Z. monophyllum plants, the dry bark and ground was used to obtaining the crude extract (EEZM) by maceration in 96% ethanol to room temperature and distillation by reduced pressure. This extract was performed in acid base extraction of alkaloids, then it was solubilized in a mixture H2O-Et2O (1:1) with aid of ultrasound; later it was acidified with 2N HCl to pH2. The aqueous solution was separated, the pH was adjusted to 9 with NH4OH and successively partitioned with CHCl3 and mixture of CHCl3-EtOH (80:20). Evaporating the solvent of the extract yielded the alkaloidal fraction, which was subjected to flash chromatography to identify the purified fractions that have predominantly bernerine, jathrorrhizina, 3beta-glucositosterol and monophyllidina [15,16].
Cytotoxicity assays
Macrophages were cultured in 96-well plates (TRP, Trasadingen, Switzerland) for 24-h at 37° and 5% CO2. After 24-h, the cells were exposed to the 3 extracts at 6 different concentrations, as previously described by Sánchez-Suarez et al. 2011 [10]. AFEEZM at 450, 400, 350, 300, 250, and 200 μg/ml; EEOM at 150, 125, 100, 75, 50, and 25 μg/ml; and EEZM at 150, 100, 75, 50, 25, and 12.5 μg/ml. The assays were each performed in triplicate three separate time.
The following were used as control molecules with antileishmanial activity: pentamidine isethionate (Pentacarinat®, Sanofi-Aventis, France) at 6 different concentrations beginning at 50 μg/ml and serially diluted down three-fold [17]; and sodium stibogluconate (Laboratorios Ryan, Colombia) at 8 different concentrations beginning at 2000 μg/ml and serially diluted down two-fold.
Pentamidine isethionate was used as a antileishmanial control in the promastigote assays, because does not need be metabolized and act directly on the parasite inhibiting polyamine transport, which disrupts the balance of concentrations between intracellular and extracellular polyamines, thereby promoting the delay or detention of parasite replication [18]. In this sense pentamidine has antileishmanial activity over promastigotes and amastigotes.
On the other hand, antileishmanial activity of sodium stibogluconate only is reached after intracellular metabolism of this pro-drug into the toxic trivalent forms for the intracellular amastigotes [19]. For this reasons, two different drugs is used like an experimental control of parasitocide activity over each one parasite forms.
After 72-h of culture to determine cellular viability, 100 μl of resazurin was added to the cells for a final concentration of 44 μM [20]. Four hours later, the reduction of resazurin to resorufin (a fluorescent compound) was evaluated in a Tecan GENios Microplate Reader (Tecan Austria GmbH, Grodig Austria), with an excitation of 535 nm and emission of 590 nm. The data were analyzed using Magellan 4 software (Tecan Austria GmbH, Grodig, Austria). The corresponding absorbance values were obtained using the spectrofluorometric reading (arbitrary fluorescence units, AFU) for each extract concentration evaluated. Percent cell viability was obtained using the following formula: % viability=(AFU for macrophages exposed to extracts or positive control/control macrophage AFU)×100, and the mortality % was calculated using the formula: % mortality=100−% viability. Graph Pad Prism 5.00 was used to determine the 50% lethal concentration (LC50). All of the assays were performed in triplicate, and the described results represent the mean of two independent experiments.
Assays for antileishmanial activity on promastigotes
L. panamensis and L. major promastigotes, which had pre-incubated with 100 μl of plant extract or RPMI, were plated in triplicate into 96-well flat-bottom dishes at 2×105 per well at a final volume of 200 μl. Pentamidine (PTM) was used as a positive control for parasiticidal treatment in this assay. Unlike pro-drugs, such as meglumine antimoniate or sodium stibogluconate, PTM does not need to be metabolized and acts directly on the parasite [18].
The parasites were incubated for 72-h, after which 50 μl of resazurin-containing RPMI (final resazurin concentration of 44 μM) was added. After 36-h, the reduction of resazurin to resorufin was evaluated in a Tecan GENios Microplate Reader. The data were analyzed using the Magellan4 software. The measurement of the 50% effective concentration (EC50) was performed using the same statistical principle as the macrophage cytotoxicity assay described in the previous section.
Assays for antileishmanial activity on internalized amastigotes
The peritoneal macrophages were infected with stationary-phase promastigotes [21] at a MOI of 1:40 (cells:parasites) and were incubated at 37° for 24-h, after which the wells were washed to remove noninternalized parasites, and the cells were incubated for an additional 24-h to stabilize the infection. Then, 4 different extract concentrations were added to the cells. Due to the low cell availability and the complexity of the assay, only 4 concentrations of extracts were used for the amastigote assay. The chosen concentrations fell within the range identified in the LC50 cytotoxicity and EC50 efficacy assays. In almost every case, we began with 80 μg/ml and performed two-fold serial dilutions. For the treatment of L. major with EEZM, we began with 40 μg/ml and performed two-fold serial dilutions. The cells were then incubated for an additional 48-h.
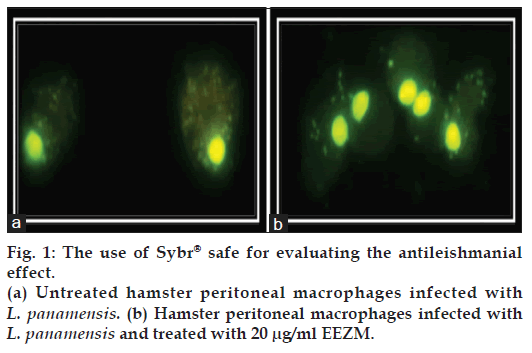
The efficacy of the extracts was compared to sodium stibogluconate (STB) [22], a first-line drug used in the treatment of cutaneous leishmaniasis [22]. To determine the EC50, 100 cells were counted per well to estimate the rate of infection. A Giemsa tincture (polychromatic coloration) is routinely used to evaluate Leishmania infection. A second reading is often performed using a conventional microscope in what is known as the gold standard for the intracellular identification of Leishmania. However, in this study we chose to evaluate the infection rate by staining with SYBR® Safe due to its benefits over Giemsa staining [23]. Fig. 1 is a representative image of the results obtained with SYBR® Safe staining; infected and untreated macrophages exhibited a high density of cytoplasmic parasites (A), while the treated cells contained fewer parasites in their cytoplasm (B). The images obtained through this technique are more defined, and thus, observer bias is reduced. The EC50, the concentration at which a 50% reduction in the number of infected cells is observed, was calculated from the results obtained by this technique. As such, the rate of infection for untreated macrophages is considered to be 100%. Once the LC50 and EC50 were calculated, the corresponding selectivity indexes (S/I) were established as follows: S/I=LC50/EC50.
Results
In vitro cytotoxicity of EEOM, AFEEZM, and EEZM on hamster peritoneal macrophages
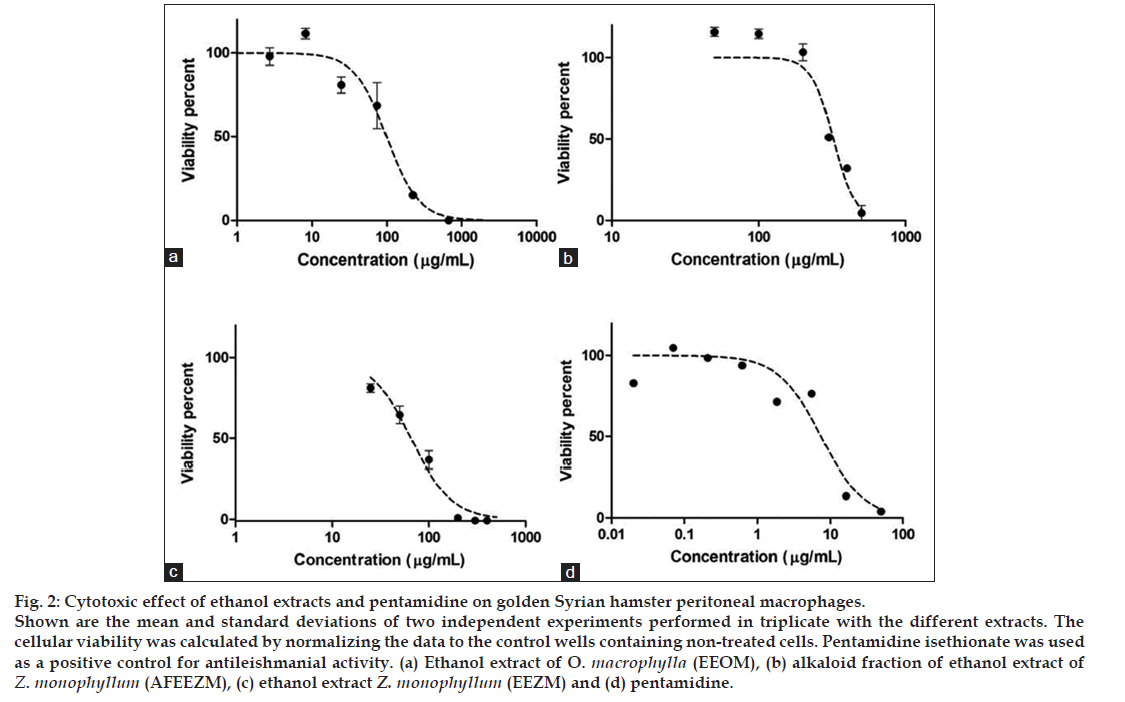
This assay was designed to identify the toxicity of extracts on peritoneal macrophages. The LC50 for each of the extracts was 100.46±13.24, 316.45±5.95 and 71.41±2.33 μg/ml for EEOM, AFEEZM, and EEZM, respectively (fig. 2).
Figure 2: Cytotoxic effect of ethanol extracts and pentamidine on golden Syrian hamster peritoneal macrophages.
Shown are the mean and standard deviations of two independent experiments performed in triplicate with the different extracts. The cellular viability was calculated by normalizing the data to the control wells containing non-treated cells. Pentamidine isethionate was used as a positive control for antileishmanial activity. (a) Ethanol extract of O. macrophylla (EEOM), (b) alkaloid fraction of ethanol extract of Z. monophyllum (AFEEZM), (c) ethanol extract Z. monophyllum (EEZM) and (d) pentamidine.
All of the evaluated extracts showed a cytotoxicity level lower than that of pentamidine, the positive control for these assays. Among the rutaceae extracts evaluated, the AFEEZM fraction displayed the lowest toxicity (LC50: 316.45±5.95 μg/ml) as compared with EEZM (LC50 71.41±2.33 μg/ml). As AFEEZM is a fraction obtained from EEZM, this result suggests that the likely toxic components of EEZM were removed during the AFEEZM isolation process.
Antileishmanial activity of EEOM, AFEEZM, and EEZM on Leishmania promastigotes
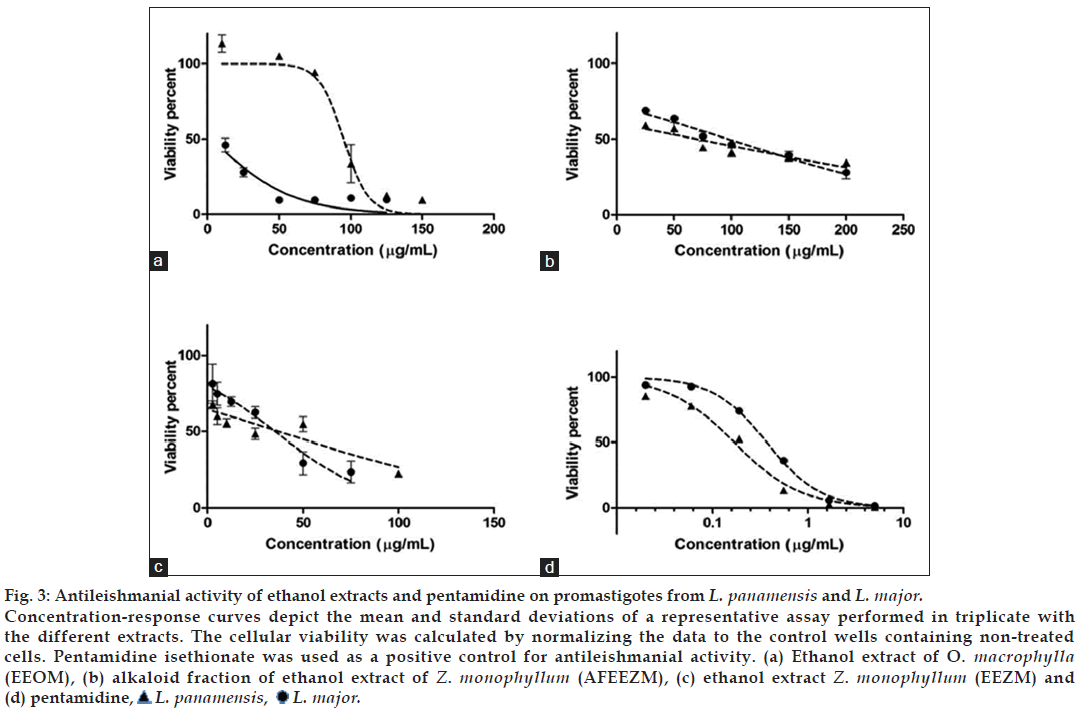
The concentration-response curves for EEOM, EEZM, and AFEEZM extracts on Leishmania promastigotes are shown in fig. 3. The biological activities of the extracts derived from the Rutaceae family (AFEEZM and EEZM) were similar. The EC50 for AFEEZM on L. panamensis and L. major promastigotes was 61.43±3.05 and 77.04±3.72 μg/ml, respectively, and the EC50 for EEZM on L. panamensis and L. major promastigotes was 17.06±1.49 and 25.82±3.15 μg/ml, respectively. The EC50 values of EEOM from the Lauraceae family were significantly different (P<0.001) between the two Leishmania species (L. panamensis: 100.64±2.96 and L. major: 9.66±1 μg/ml).
Figure 3: Antileishmanial activity of ethanol extracts and pentamidine on promastigotes from L. panamensis and L. major.
Concentration-response curves depict the mean and standard deviations of a representative assay performed in triplicate with the different extracts. The cellular viability was calculated by normalizing the data to the control wells containing non-treated cells. Pentamidine isethionate was used as a positive control for antileishmanial activity. (a) Ethanol extract of O. macrophylla (EEOM), (b) alkaloid fraction of ethanol extract of Z. monophyllum (AFEEZM), (c) ethanol extract Z. monophyllum (EEZM) and (d) pentamidine,  L. panamensis,
L. panamensis,  L. major.
L. major.
The antileishmanial activity of the two extracts derived from the lauraceae family was similar to what was observed in the macrophage assay: greater activity was found for EEZM, which is the extract from which the alkaloid fraction AFEEZM was derived.
The behavior of AFEEZM was similar against the promastigotes of both Leishmania spp; both species showed similar S/I values, and the EC50 values were 61.425±3.05 μg/ml and 77.04±3.72 against L. panamensis and L. major, respectively (Table 1). This result provided evidence that the activity of AFEEZM is lower against both macrophages and promastigotes in comparison to EEZM.
| Treatment | Peritoneal macrophages LC50 (μg/ml) | L. panamensis promastigotes EC50 (μg/ml) | I/S | L. major promastigotes EC50 (μg/ml) | I/S |
|---|---|---|---|---|---|
| EEOM | 100.46 ± 13.24 | 100.64 ± 2.96 | 1 | 9.7 ± 1 | 10 |
| AFEEZM | 316.45 ± 5.95 | 61.43 ± 3.05 | 5 | 77.04 ± 3.72 | 4 |
| EEZM | 71.41 ± 2.33 | 17.06 ± 1.495 | 4 | 28.99 ± 3.17 | 2 |
| PTM | 6.13 ± 1.343 | 0.17 ± 0.003 | 35 | 0.31 ± 0.061 | 20 |
Table 1: Cytotocixity and antileishmanial activity (promastigotes) of extracts derived from laureceae and rutaceae plants
Antileishmanial activity of EEOM, AFEEZM, and EEZM on internalized Leishmania amastigotes
The results of the antileishmanial activity of the ethanol extracts on promastigotes allowed us to propose a selective biological response against Leishmania when compared to other prokaryotic cells, particularly macrophages. Next, an experimental model of natural infection was chosen to provide a more reliable representation of the activity of these extracts. In this case, internalized amastigotes that differentiated from promastigotes upon infection were used. The hamster peritoneal macrophages were infected with Leishmania and treated with extracts to determine EC50 values. An EC50 of 6.155±0.115 μg/ml was found for L. panamensis amastigotes in response to EEZM extract, and an EC50 of 30.135±0.685 μg/ml was found for L. major amastigotes in response to AFEEZM extract. S/I values were 12 and 11, respectively (Table 2).
| Treatment | Peritoneal macrophages LC50 (μg/ml) | L. panamensis amastigotes EC50 (μg/ml) | I/S | L. major amastigotes EC50 (μg/ml) | I/S |
|---|---|---|---|---|---|
| EEOM | 100.46 ± 13.24 | 12.67 ± 0.11 | 8 | 29.16 ± 2.08 | 3 |
| AFEEZM | 316.45 ± 5.95 | 46.23 ± 1.05 | 7 | 30.135 ± 0.685 | 11 |
| EEZM | 71.41 ± 2.33 | 6.16 ± 0.115 | 12 | 24.95 ± 3.15 | 3 |
| STB | 2562 ± 202 | 704.7 ± 361.6 | 4 | 932.8 ± 289.63 | 3 |
Table 2: Cytotocixity and antileishmanial activity (amastigotes) of extracts derived from laureceae and rutaceae plants
In analyzing the S/I ≥10, we found that all of the analyzed extracts demonstrated preferential selectivity for the Leishmania genus. Interestingly, Z. monophyllum extracts showed greater activity, but the selectivity was dependent on the species; EEZM was more selective against L. panamensis, and AFEEZM was more selective against L. major.
Discussion
Many members of the Lauraceae family have been found to have ethno botanical uses in Colombia. For example, Ocotea quixos has shown anti-bacterial activity against Escherichia coli, Pseudomonas aureoginosa, Enterococcus foecali, and Staphylococcus aureus, as well as antifungal activity [24]. In terms of antiparasitic functions, a crude alkaloid extract of Ocotea lancifolia showed in vitro activity against promastigotes of three Leishmania species (L. braziliensis, L. amazonensis, and L. donovani) at a concentration of 100 μg/ml [25].
The Citrus genus is one of the most studied of the Rutaceae family. Members of this genus contain flavonoids, one of the most important compounds in plants [26]. A crude aqueous extract of the root bark of Zanthoxylum xanthoxyloides is widely used in Ghana and Nigeria for the treatment of various inflammatory conditions, as it has been shown to reduce the vascular response during inflammation. Additionally, a compound derived from this plant species was found to inhibit the migration of inflammatory cells to the wound site [27]. The Rutaceae family is characterized by high levels of flavonoids, which are compounds that can pharmacologically inhibit enzymes involved in cellular activation [26].
The cytotoxic activity of EEOM on hamster peritoneal macrophages was also lower than the control molecule (LC50: 100.46±13.24 μg/ml), confirming previous results on J774 macrophages by Sánchez et al. (2011) [10]. We propose that the activity of Lauraceae-derived extracts on macrophages is independent of the macrophage species of origin. The Ocotea genus is known as a source of furofuran-type metabolites, lignans, neolignans, and alkaloids [28]. The findings of EEOM activity on macrophages can now be added to the already-existing descriptions for species of this genus, including antiparasitic activity of O. lancifolia [25], antidiarrheal and disinfectant properties of O. quixos [29], and antileishmanial activity of O. macrophylla [10].
In an earlier report evaluating the activity of EEOM on L. panamensis and L. braziliensis promastigotes, there was no observable difference in EC50 values between the two Leishmania species (98.03±19.87 and 85.7±22.88 μg/ml for L. panamensis and L. braziliensis, respectively [10]. Interestingly, L. major was more susceptible to EEOM exposure in our assays, with an EC50 of 9.7±1 μg/ml (Table 1). Comparing these results with those observed in species of the Viannia subgenus, it appears that members of the Leishmania subgenus are more susceptible to EEOM exposure. When the hamster peritoneal macrophages were infected and treated with the extracts, we were able to establish the EC50 of EEOM, EEZM and AFEEZM over internalized amastigotes of L. panamensis y L. major, we calculated the S/I (this index is an arbitrary value that denotes the specificity of the effect expected, if exist higher value of difference, we expect a higher selectivity/antileishmanial activity, See Table 2). Analyzing the S/I (above 10, which is considered as promising) we find that all products tested showed preferential selectivity against Leishmania spp, however derivatives from Z. monophyllum exhibit increased activity, besides apparently this products show a selectivity species-dependent being EEZM more selective against L. panamensis, whereas AFEEZM resulted frankly more selective on L. major.
However, confirmation of this hypothesis would require further evaluation of other species belonging to the subgenus. L. major, the species responsible for cutaneous leishmaniasis in the old world [30], was nearly 10 times more susceptible to EEOM than L. panamensis. Therefore, its use as a leishmanicide could be useful in cases of cutaneous leishmaniasis, particularly in the old world. Related with the non-selective reduction of AFEEZM activity on macrophages and parasites, we suggests that the activity is dependent on compounds that are removed from EEZM during fractionation. Although the best way to calculate the S/I is with results obtained from amastigotes, Table 1 shows an approximation of the expected antileishmanial effect on the promastigotes and the accompanying S/I calculations.
The antileishmanial activity of EEOM on internalized L. panamensis amastigotes was higher than its activity on promastigotes, reaching an S/I of 8 (Table 2). However, its activity on L. major amastigotes was much lower than that shown for promastigotes. Treating to explain the different activity of extracts and the fraction over internalized amastigotes and promastigotes, we understand that both parasite forms can have different metabolism and physiologic conditions linked to factors like pH and temperature, factors that can induce different reactions on different parasite forms, even using the same molecule [31]. In the other hand, the activity of extracts can modify over amastigotes form by the metabolism of the host cell (macrophages in this case) where perhaps the extracts or fractions could metabolize inducing other active mediators with variable toxicity on the intracellular amastigotes.
This further justifies the need for evaluating the antileishmanial potential of compounds using the intracellular amastigote model to better approximate a natural infection, where the amastigote itself is responsible for pathology and is the primary target of Leishmaniasis therapies.
This study provides new results for antileishmanial activity on L. panamensis and L. major strains by plant extracts from the Lauraceae and Rutaceae families. An in vitro infection assay was performed for macrophages isolated from golden Syrian hamsters, which have been validated as an experimental model for cutaneous leishmaniasis by new world parasites [12].
We highlight the value of screening with internalized amastigotes in macrophages, for which results are much more relevant than extracellular promastigotes. Analyzing the results of the most promising products (the extracts and the fraction), we notice that the parasiticidal activity is generally higher against internalized parasites than promastigotes. This represents an advantage when evaluating drug treatments, as the amastigote is the parasite stage responsible for human disease. In our experiments, L. panamensis was more sensitive to EEOM and EEZM extracts (S/I=8 and 12, respectively), whereas L. major was more sensitive to the AFEEZM fraction (S/I=11). This result is not surprising given that not all parasite species are equally susceptible to the currently used antileishmanial drugs. This is the case for pentamidine isethionate, whose sensitivity is higher against L. guayanensis strains, making this medicine one of the main treatment options in the Guyanas [6]. The pharmacological activity of hexadecylphosphocholine (Miltefosine®) has also shown specificity for L. panamensis strains over other Leishmania species [6]. With this in mind, the study of these extracts for different models, strains, and Leishmania species should be continued, including in vivo evaluations in models such as the golden hamster.
The extracts from the Rutaceae family showed higher antileishmanial activity in our assay. This family is known for its high content of different alkaloids, particularly quaternary alkaloids, such as berberins and xanthophilin pyranoquinolines [32]. Many of the alkaloids present in Zanthoxyllum have shown antibiotic properties [33]. They have also shown antifungal properties against Aspergillus flavus, Penicillium digitatum and Candida albicans [33]. Other studies have described inhibitory effects on protozoans, including decreased reproduction of Entamoeba histolytica and Giardia lamblia trophozoites upon exposure to a crude ethanol extract from Z. liebmannianun [34].
The presence of berberine was shown to be effective in a clinical study of leishmaniasis [35]. Vennerstrom et al. (1990) used hamsters as a model for infection with L. donovani and L. panamensis. This study revealed a 56% reduction in infection following a 200 mg/kg/day treatment with this compound [36]. In the EEZM extract and the AFEEZM fraction was reported berberin as constituent [15,16]. Thus, we hypothesize that the activity reported here could be generated by berberin alone or through synergism between berberin and other molecules present in the extracts. Mishra et al. (2008) reported various alkaloids with antileishmanial activity against promastigotes and amastigotes in vitro and in vivo [37]. Berberine stood out in this study due to its promising activity on the development of promastigotes in in vivo assays. Berberine was reported as a metabolite identified in a Z. monophyllum extract [38]. Other alkaloids reported to have antileishmanial effects do not appear to be present in the AFEEZM and EEZM extracts, suggesting that the metabolite responsible for the antileishmanial activity in our assays is berberine. However, more studies of the different alkaloid and non-alkaloid compounds making up the Z. monophyllum ethanol extract are needed to confirm that berberine is the only compound with promising antileishmanial activity.
In 2008 Mishra et al. reported different type of alkaloids with antileishmanial activity over promastigotes and amastigotes in vitro and in vivo assays into which we find also the berberine with antileishmanial properties, however for the other compounds identified as part of AFEEZM we do not find reports related with antileishmanial activity [38]; these finding let us suggest that the metabolite responsible of antileishmanial activity in our assays is the berberine, or maybe other compounds no alkaloids into of EEZM (which require subsequent studies).
It has been proposed that Leishmania lipophosphoglycans can subvert macrophage function by activation of the extracellular signal related kinase (ERK 1/2), leading to enhanced levels of IL-10 along inhibition of IL-12. This decrease in IL-12 has been attributed to downregulation of p38 MAPK which favors parasite survival. The berberine has been associated with the induction of IL-12 production following activation of p38 MAPK, thereby establishing a new chemotherapeutic target against leishmaniasis [39].
According to the World Health Organization (WHO), 80% of the world population depends on traditional medicine for primary health care needs. The use of plant extracts, as well as other alternative medicinal treatments, has been highly popular since the 1990s [40]. Natural products from medicinal plants, as pure compounds or as standardized extracts, provide unlimited opportunities for new medicines due to an incomparable availability of chemical diversity. There is a rising demand for chemical diversity in screening programs and in the search for medicines derived from natural sources. There is particular interest in edible plants grown around the world [41]. With the emergence of adverse effects and microbial resistance to synthesized medicines, research in the area of ethnopharmacology has grown. To develop a natural extract or a pharmaceutical product that might be used in a modern clinical practice, pharmaceutical companies prefer to use alcoholic extracts as a starting material before moving on to aqueous extracts [42]. However, for any type of extract used, standardization is necessary to assure quality control for natural medicines [42]. Natural extracts have been used as ointments for topical antibacterial treatments. For example, 4% tea tree oil used for Staphylococcus aureus infections has shown positive results for the treatment of acne [43]. It has also been shown that topical treatment with Jasminum grandiflorum methanol extract accelerates the scarring process of wounds in rats [44]. The present study has produced novel results in which we describe antileishmanial activity by ethanol extracts derived from Z. monophyllum and O. macrophylla on a hamster (Mesocrisetus auratus) model, which most resembles human cutaneous leishmaniasis. In the present study we provide data that contributes to and advances research in the area of ethnopharmacology. We provide new results that corroborate the presence of compounds in the Rutaceae and Lauraceae families of plants, including berberin that display promising antileishmanial activity. This presents an opportunity for future research into each interesting compound found in the ethanol extracts. Hopefully studies will continue to characterize the promising compounds, preferably by using the golden Syrian hamster model.
References
- Bañuls AL, Hide M, Prugnolle F. Leishmania and the leishmaniases: A parasite genetic update and advances in taxonomy, epidemiology and pathogenicity in humans. AdvParasitol 2007;64:1-109.
- Davies CR, Reithinger R, Campbell-Lendrum D, Feliciangeli D, Borges R, Rodriguez N. The epidemiology and control of leishmaniasisin Andean countries. Cad SaudePublica 2000;16:925-50.
- Mitropoulos P, Konidas P, Durkin-Konidas M. New World cutaneous leishmaniasis: Updated review of current and future diagnosis and treatment. J Am AcadDermatol 2010;63:309-22.
- Minodier P, Parola P. Cutaneous leishmaniasis treatment. Travel Med Infect Dis 2007;5:150-8.
- INS. Epidemiological behavior of Leishmanisis in Cundinamarca, Colombia, Editor. Available from: http://www.ins.gov.co: Bogotá. 2012. p. 11. Last accessed Aug 2013.
- Chakravarty J, Sundar S. Drug resistance in leishmaniasis. J Glob Infect Dis 2010;2:167-76.
- Monte RL, Barbosa JM, Sousa LM, Athayde PF, Dias CS, Oliveira MR. Crude ethanolic extract, lignoid fraction and yangambin from Ocoteaduckei (Lauraceae) show antileishmanial activity. Z Naturforsch C 2007;62:348-52.
- Franca F, Lago EL, Marsden PD. Plants used in the treatment of leishmanial ulcers due to Leishmania (Viannia) braziliensis in an endemic area of Bahia, Brazil. Rev Soc Bras Med Trop 1996;29:229-32.
- Rocha LG, Almeida JR, Macedo RO, Barbosa-Filho JM. A review of natural products with antileishmanial activity. Phytomedicine 2005;12:514-35.
- Sanchez-Suarez J, Coy-Barrera E, Cuca LE, Delgado G. Leishmanicidal and cytotoxic activities of extracts and naturally-occurring compounds from two Lauraceae species. Nat Prod Commun 2011;6:231-4.
- Nylen S, Gautam S. Immunological perspectives of leishmaniasis. J Glob Infect Dis 2010;2:135-46.
- Osorio Y, Melby PC, Pirmez C, Chandrasekar B, Guarin N, Travi BL. The site of cutaneous infection influences the immunological response and clinical outcome of hamsters infected with Leishmaniapanamensis. Parasite Immunol 2003;25:139-48.
- Espitia CM, Zhao W, Saldarriaga O, Osorio Y, Harrison LM, Cappello M, et al. Duplex real-time reverse transcriptase PCR to determine cytokinemRNA expression in a hamster model of New World cutaneous leishmaniasis. BMC Immunol 2010;11:31.
- Mitra S, Ghosh L, Chakrabarty P, Biswas M, Bhattacharyya FK, Ghosh DK. Effect of bioamines on uptake of promastigotes of Leishmaniadonovani by hamster peritoneal macrophages. J Med Microbiol 1992;36:283-7.
- Patiño OJ, Cuca LE. Monophyllidin, a new alkaloid L-proline derivate from Zanthoxyllummonophyllum. PhytochemLett 2011;4:22-5.
- Cuca LE, Coy ED, Alarcon MA, Fernandez A, Aristizabal FA. Cytotoxic effect of some natural compounds isolated from Lauraceaeplants and synthetic derivatives. Biomedica 2011;31:335-43.
- Ponte-Sucre A, Vicik R, Schultheis M, Schirmeister T, Moll H. Aziridine-2,3-dicarboxylates, peptidomimetic cysteine protease inhibitors with antileishmanial activity. Antimicrob Agents Chemother2006;50:2439-47.
- Basselin M, Lawrence F, Robert-Gero M. Pentamidine uptake in Leishmaniadonovani and Leishmaniaamazonensispromastigotes and axenic amastigotes. Biochem J 1996;315:631-4.
- Frezard F, Demicheli C, Ribeiro RR. Pentavalentantimonials: New perspectives for old drugs. Molecules 2009;14:2317-36.
- Anoopkumar-Dukie S, Carey JB, Conere T, O’Sullivan E, van Pelt FN, Allshire A. Resazurin assay of radiation response in cultured cells. Br J Radiol 2005;78:945-7.
- Oliver M, gregory D, Interaction Between Leishmania and the Host Macrophage. In: Myler PJ, Fasel N, editors. Leishmania: After the genome. Norfolk, UK: Caister Academic Press; 2008.
- Rojas R, Valderrama L, Valderrama M, Varona MX, Ouellette M, Saravia NG. Resistance to antimony and treatment failure in human Leishmania (Viannia) infection. J Infect Dis 2006;193:1375-83.
- Perez-Cordero JJ, Sanchez-Suarez J, Delgado G. Use of a fluorescent stain for evaluating in vitro infection with Leishmaniapanamensis. ExpParasitol 2011;129:31-5.
- Villamizar VEM. Secondary metabolites with biological activity (pharmacology) applying ethnobotany and phytochemistry of some species of: Ocotea, Crytocarya, Litsea, Caryodaphnosis, Machilus and Actinodaphne (Lauraceae). Revista de la Facultad de Ciencias de laSalud 2009;7:19.
- Fournet A, Ferreira ME, Rojas de Arias A, Guy I, Guinaudeau H, Heinzen H. Phytochemical and antiprotozoal activity of Ocotealancifolia. Fitoterapia 2007;78:382-4.
- Benavente-Garcia O, Castillo J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem 2008;56:6185-205.
- Prempeh A, Mensah-Attipoe J. Crude aqueous extract of the root bark of zanthoxylumxanthoxyloides inhibits white blood cells migration in acute inflammation. Ghana Med J 2008;42:117-9.
- Coy Barrera ED, Cuca Suarez LE. In vitro inhibitory activities of Lauraceaeaporphine alkaloids. Nat Prod Commun 2010;5:383-6.
- Ballabeni V, Tognolini M, Bertoni S, Bruni R, Guerrini A, Rueda GM, et al. Antiplatelet and antithrombotic activities of essential oil from wild Ocoteaquixos (Lam.) Kosterm. (Lauraceae) calices from Amazonian Ecuador. Pharmacol Res 2007;55:23-30.
- Bates PA. Transmission of Leishmaniametacyclicpromastigotes by phlebotomine sand flies. Int J Parasitol 2007;37:1097-106.
- Coombs GH, Hart DT, Capaldo J. Proteinase inhibitors as antileishmanial agents. Trans R Soc Trop Med Hyg 1982;76:660-3.
- De Garcia L, Calle J, Reguero M, Joseph-Nathan P. Phytochemical study of Zanthoxylum-monophyllum. Fitoterapia 1989;60:447-50.
- Gomez Y, Gil K, Gonzalez E, Farias LM. Anti-fungi activity of organic extracts from the tree Fagaramonophylla (Rutaceae) in Venezuela. Rev Biol Trop 2007;55:767-75.
- Arrieta J, Reyes B, Calzada F, Cedillo-Rivera R, Navarrete A. Amoebicidal and giardicidal compounds from the leaves of Zanthoxylumliebmannianun. Fitoterapia 2001;72:295-7.
- Croft SL, Yardley V. Chemotherapy of leishmaniasis. Curr Pharm Des 2002;8:319-42.
- Serrano MX. Chemotherapy against leishmaniasis; the state of the art, challenges, and new proposals from Venezuela. EstudTransdisciplinarios 2010;2:69-75.
- Mishra B, Kalea R, Singh R, Tiwari V. Alkaloids: Future Prospective to combat Leishmaniasis. Fitoterapia 2009;80:81-90.
- Patiño O, Cuca L. Isolated metabolites of Zanthoxylum XIV. Colombian Congress of Chemistry. Colombian Chemistry Association 2006.
- Saha P, Bhattacharjee S, Sarkar A, Manna A, Majumder S, Chatterjee M. Berberine chloride mediates its anti-leishmanial activity via differential regulation of the mitogen activated protein kinase pathway in macrophages. PLoS One 2011;6:e18467.
- Cowan MM. Plant products as antimicrobial agents. ClinMicrobiol Rev 1999;12:564-82.
- Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga Latha L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr J Tradit Complement Altern Med 2011;8:1-10.
- Samarakoon SR, Thabrew I, Galhena PB, De Silva D, Tennekoon KH. A comparison of the cytotoxic potential of standardized aqueous and ethanolic extracts of a polyherbal mixture comprised of Nigella sativa (seeds), Hemidesmusindicus (roots) and Smilax glabra (rhizome). Pharmacognosy Res 2010;2:335-42.
- Martin KW, Ernst E. Herbal medicines for treatment of bacterial infections: A review of controlled clinical trials. J AntimicrobChemother 2003;51:241-6.
- Chaturvedi AP, Kumar M, Tripathi YB. Efficacy of Jasminumgrandiflorum L. leaf extract on dermal wound healing in rats. IntWound J 2013;10:675-682.