- *Corresponding Author:
- A. Semalty
Department of Pharmaceutical Sciences, H. N. B. Garhwal University, Srinagar (Garhwal)-246 174, India
E-mail: semaltyajay@gmail.com
| Date of Submission | 10 August 2009 |
| Date of Revision | 18 August 2010 |
| Date of Acceptance | 10 September 2010 |
| Indian J Pharm Sci, 2010, 72 (5): 571-575 |
Abstract
The present work was carried out to investigate the hepatoprotective effect of ginger, chicory and their mixture against carbon tetrachloride intoxication in rats. Carbon tetrachloride treatment significantly elevated the alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and gamma glutamyltransferase activities and the serum triglycerides and cholesterol concentration as compared to control group. It also increased RBCs counts and Hb concentration, total or differential leucocytes counts. However it decreased platelet counts, platelet distribution width, mean platelet volume, platelet larger cell ratio. Methanol extract of ginger (250 and 500 mg/kg) and chicory (250 and 500 mg/kg) given alone or mixed (1:1 wt/wt) significantly restored the carbon tetrachloride-induced alterations in the biochemical and cellular constituents of blood. No toxic symptoms were reported in doses up to 5 g/kg. Alkaloids and/or nitrogenous bases, carbohydrates and/or glycosides, tannins, flavonoids, saponins and unsaturated sterols and/or triterpenes are the main active constituents of their methanol extract. The hepatoprotective effect of ginger and chicory was also confirmed by the histopathological examination of liver tissue.
Keywords
Buccal film, enalapril, ex vivo studies, in vitro studies, mucoadhesive
Among the various routes of administration oral route is the most convenient, easy and preferred one. However, orally administered drugs are either prone to hepatic first-pass metabolism or metabolism in gastrointestinal (GI) tract or both. These are the main reasons for which some classes of drugs like peptides and proteins cannot be administered orally. Delivery of drugs through various mucosal surfaces (nasal, rectal, vaginal, ocular and oral mucosa) may form the potential alternative solution for delivery of such classes of drugs. These mucoadhesive drug delivery systems improve the bioavailability of the drugs by bypassing the first pass effects and avoiding the presystemic elimination of the drug within the GI tract [1]. Out of the various sites available for mucoadhesive drug delivery, buccal mucosa is the most suited one for local as well as systemic delivery of drugs. It’s anatomical and physiological features like presence of smooth muscles with high vascular perfusion, avoidance of hepatic first pass metabolism and hence can potentially improve bioavailability are the unique features which make it as an ideal route for mucoadhesive drug delivery. Moreover, these dosage forms are economic and patient-friendly.
The drug dissolution (release) and permeation through the mucosa are governed by microenvironment of the mucosa. The microenvironment of the mucosa can be adjusted or modified with the help of well-designed mucoadhesive drug delivery systems [2]. These systems are designed and formulated with the help of mucoadhesive polymers which are generally of high molecular weight and of high viscosity grades with greater flexibility and optimum chain length. Various mucoadhesive polymers have also been investigated for buccal drug delivery [3-5]. Among the various mucoadhesive drug delivery systems, buccal films are better than oral gels and buccal tablets due to relatively longer residence time, more flexibility to cover the buccal mucosa and better comfort.
Enalapril maleate is an angiotensin converting enzyme (ACE) inhibitor, used mainly in the treatment of hypertension and angina pectoris. It has low bioavailability (40-60%) due to hepatic first pass metabolism [6]. Hence to improve its therapeutic efficacy and bioavailability the drug may be administered by buccal route through buccal films. Buccal delivery of enalapril maleate may circumvent hepatic first pass metabolism and improve bioavailability. Hence the present work deals with the formulation and characterization of mucoadhesive buccal film of enalapril maleate using mucoadhesive polymer sodium carboxymethylcellulose (SCMC), hydroxylpropylmethylcellulose (HPMC), hydroxyethylcellulose (HEC) and polyvinyl pyrrolidone K-90 (PVP K-90).
Materials and Methods
Enalapril Maleate was a gift sample (Intas Pharmaceutical Ltd. Dehradun), and PVP K-90, HPMC (47 centipoise), SCMC (high viscosity grade) were obtained from Central Drug House, Mumbai. Other chemicals used were of analytical grade. The films were prepared by solvent casting method.
Preparation of mucoadhesive buccal films
Buccal films of enalapril maleate were prepared by solvent casting technique using film forming mucoadhesive polymers (Table 1). HPMC was weighed (200 mg) accurately and dissolved in 2 ml of ethanol. The beaker containing polymer and ethanol was kept aside for 5 min for swelling of the polymer. Further 3 ml of ethanol was added to the above polymer solution and the dispersion was stirred. Then one drop of (0.029 g) propylene glycol was added to the polymer solution. Simultaneously enalapril maleate was accurately weighed in quantity such that 1 cm2 film contained 20 mg and then dissolved in 1 ml of ethanol in another beaker. The drug solution was added to the polymer solution and was mixed thoroughly with the help of a magnetic stirrer. The whole solution was poured into the glass Petri dish placed over a flat surface. Inverted funnel was placed over the dish to avoid sudden evaporation. The mould containing polymeric solution of drug was kept 12 h at room temperature for drying. After drying, the films were observed and checked for possible imperfections upon their removal from the moulds. They were covered with wax paper and preserved in desiccators till the evaluation tests were performed. These new films were examined in order to select the film having the best characteristics. Similarly, films of F1 were prepared.
| Formulation Polymer | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 |
|---|---|---|---|---|---|---|---|---|---|---|
| PVP K-90 | 5% | ---- | ---- | ---- | ---- | 5% | 2% | 2% | ---- | ---- |
| HEC | ---- | 5% | ---- | ---- | 2% | ---- | ---- | 2% | 5% | ---- |
| HPMC | ---- | ---- | 5% | --- | ---- | ---- | 2% | ---- | 2% | 5% |
| SCMC | ---- | ---- | ---- | 2% | 2% | 2% | ---- | ---- | ---- | 2% |
| Ethanol/Water ml | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
PVP-Poly vinylpyrrolidone, HEC-Hydroxyethylcellusose, HPMC-Hydroxypropylmethylcellulose and SCMC- Sodiumcarboxymethylcellulose
Table 1: Composition Of Mucoadhesive Buccal Films
For preparing films F2, F4 polymer was placed in 20 ml of water and stirred for 1 h and drug was dissolved in another beaker in ethanol. Both the solutions were mixed and poured in to Petri dish. For F5, F6, F7, F8, F9, F10 the two polymeric solutions were mixed and then drug solution in ethanol was added in this. The Petri dishes containing polymeric solutions of drug were kept aside for 12 h at room temperature for drying. The dried films were cut into size of 2 cm diameter, packed in aluminum foil and stored in a desiccator until further use.
Characterization of mucoadhesive buccal films
Three films of every formulation were weighed individually in a digital balance (Fisher Brand PS- 200) and the mean weights were calculated. The mean value of film thickness was calculated by measuring thickness of three films of each formulation at three different places using Micrometer Screw Gauge (Mitutoyo MMO-25DS).
Folding endurance was determined by repeatedly folding a small strip of size (2×2 cm) of film at the same place till it broke. The mean value (three readings and standard deviation) of folding endurance (the number of times, the film could be folded at the same place without breaking) were shown in Table 2.
| Formulation code | Swelling index (2 h) | In vitro residence | Thickness (mm) | Content | Folding endurance | Surface pH |
|---|---|---|---|---|---|---|
| time (h) | uniformity | |||||
| F1 | 17.12±1.14 | 2.23±0.56 | 0.17±0.03 | 19.0±0.01 | 234.4±6.47 | 6.23±0.02 |
| F2 | 20.24±1.15 | 3.01±0.72 | 0.15±0.01 | 17.7±0.01 | 222.5±10.31 | 6.51±0.02 |
| F3 | 21.33±1.11 | 1.87±0.09 | 0.15±0.02 | 17.8±0.01 | 279.0±8.0 | 6.72±0.05 |
| F4 | 25.34±1.21 | 4.00±0.09 | 0.22±0.01 | 18.3±0.01 | 178.5±4.62 | 6.64±0.08 |
| F5 | 31.01±0.22 | 2.33±0.55 | 0.16±0.02 | 18.2±0.01 | 257.0±5.11 | 6.35±0.09 |
| F6 | 46.11±0.30 | 2.99±0.70 | 0.18±0.03 | 21.0±0.01 | 187.5±8.12 | 6.22±0.10 |
| F7 | 28.03±0.33 | 1.88±0.92 | 0.21±0.01 | 19.5±0.02 | 165.5±12.22 | 6.31±0.01 |
| F8 | 31.81±0.23 | 3.87±0.08 | 0.17±0.03 | 18.8±0.01 | 277.0±3.39 | 6.65±0.02 |
| F9 | 46.28±0.32 | 4.11±0.09 | 0.17±0.01 | 20.0±0.01 | 287.0±5.0 | 6.67±0.02 |
| F10 | 46.83±1.22 | 1.75±0.07 | 0.16±0.02 | 19.4±0.01 | 245.4±9.65 | 6.45±0.02 |
Data are expressed as mean values and standard deviations (±SD); n=3
Table 2: Physical Evaluation Of Mucoadhesive Buccal Films Of Enalapril Maleate
To determine the drug content uniformity, three film units of each formulation were taken in separate 100 ml volumetric flasks, 100 ml of pH 6.6 phosphate buffer was added and continuously stirred for 24 h. The solutions were filtered, diluted suitably and analyzed at 213 nm in a UV spectrophotometer (Lambda 25, Perkin Elmer, US). The average of drug contents of three films was taken as final reading.
To determine surface pH of films, buccal films were left to swell for 1 h on the surface of the agar plate, prepared by dissolving 2% (w/v) agar in warmed isotonic phosphate buffer of pH 6.6 under stirring and then poured the solution into the Petri dish allowed to stand till gelling at room temperature. The surface pH was measured by means of pH paper placed on the surface of the swollen film.
For studying swelling properties, a drug-loaded film of 10×10 mm2 was weighed on a pre-weighed cover slip. It was kept in a Petri dish and 50 ml of pH 6.6 phosphate buffer was added. After every five min, the cover slip was removed and weighed up to 30 min. The difference in the weights gives the weight increase due to absorption of water and swelling of film. The percent swelling, % S, was calculated using the following equation: percent swelling (% S)=(Xt–Xo/Xo)×100, where Xt is the weight of the swollen film after time t, Xo is the initial film weight at zero time [7].
The in vitro residence time was determined using IP disintegration apparatus using pH 6.6 phosphate buffer (PB) as the disintegration medium (800 ml, maintained at 37±2°). On the surface of a glass slab the segments of rat intestinal mucosa (each of 3 cm length) were glued and then the slab was vertically attached to the apparatus. Three films of each formulation were hydrated (on one surface using pH 6.6 PB) and the hydrated surface was brought into contact with the mucosal membrane. The glass slab was vertically fixed to the apparatus and allowed to move up and down. The film was completely immersed in the buffer solution at the lowest point and was out at the highest point. The time required for complete erosion or detachment of the film from the mucosal surface was recorded (n=3) as given in Table 2.
For the in vitro release study the USP XXIV six station dissolution apparatus type 1 (DA-6DR, Veego Ltd., India) with 900 ml pH 6.6 PB (dissolution medium) was used. One film of each formulation was fixed to the central shaft using a cyanoacrylate adhesive. During the release study the temperature and rotation speed of the apparatus was maintained at 37±0.50 and 50 rpm, respectively. The release study was carried out for 2 h. After every hour, samples were withdrawn from each station, filtered, diluted suitably and then analyzed spectrophotometrically at 213 nm.
The ex vivo permeation studies of mucoadhesive buccal films of enalapril through an excised layer of porcine buccal mucosa (washed in isotonic phosphate buffer (pH 6.6) after excising and trimming from the sides) were carried out using the modified Franz diffusion cell [7,8]. A 2.0 cm diameter film of each formulation under study was placed in intimate contact with the excised porcine buccal mucosa and the topside was covered with aluminum foil as a backing membrane. The contents of receptor compartment filled with 100 ml of pH 7.4 phosphate buffer (with a Teflon bead placed inside) were stirred with a magnetic stirrer and temperature of 37±10 was maintained throughout the experiment. The samples were withdrawn at every hour, filtered, diluted suitably and then analyzed using UV spectrophotometer at 213 nm.
Results and Discussion
Buccal films of enalapril maleate were prepared by solvent casting technique with the use of mucoadhesive polymers such as PVP K-90, HPMC,HEC and SCMC. The prepared films were evaluated for different physicochemical tests such as weight variation, thickness, content uniformity, swelling index, surface pH, in vitro residence time, and in vitro drug release studies.
All the films showed uniform thickness throughout. The film thickness was observed to be in the range of 0.216±0.001 to 0.145±0.014 mm and average thickness found was about 0.173 mm. The weights of different formulation were found to be in the range of 58±1.97 mg to 86±0.77 mg. The acidic or alkaline pH may cause irritation to buccal mucosa and may affect the drug release and degree of hydration of polymers. Therefore the surface pH of buccal film was determined to optimize both drug release and mucoadhesion. The surface pH of all formulations was within ±0.5 units of the neutral pH and hence no mucosal irritation were expected and ultimately achieve patient compliance.
Folding endurance was measured manually by folding the film repeatedly at a point till they broke. Films did not show any cracks even after folding for more than 289 times. Hence it was taken as the end point. The folding endurance was found to be in the range of 287±5.0 to 165±12.22. The values were found to be optimum to reveal good film properties. The results of content uniformity indicated that the drug was uniformly dispersed; the content was in range of 17.79 to 21.0 mg/cm2.
The swelling of the films were observed in pH 6.6 phosphate buffer solution. The comparative swelling in different formulations were in order of F10>F9>F6>F8>F5>F7>F4>F3>F2>F1. Swelling was more pronounced in films F10 which containing HPMC and SCMC due to presence of more hydroxyl group in SCMC molecules. The percentage swelling of F7 and F8 was reduced considerably by the addition of PVP K-90.
It was observed that incorporation of drug induced significant reduction of the residence time of various formulations. As also reported by some previous studies, the enhanced erosion rate was observed with the non ionic polymers (HPMC and SCMC). It might be explained by the particle swelling leading to the development of internal swelling force by matrix and this in turn promotes disintegration and leaching of drug leaving behind a highly porous matrix [7,9,10]. The in vitro residence time of various formulations was in order of F10>F3>F7>F1>F5>F6>F2>F8>F9. The in vitro residence time of the films were found to be optimum and therefore films exhibited good swelling and drug release properties.
As the drug was uniformly dispersed in the matrix of the polymer, a significantly good amount of drug was loaded in all the formulations. The drug content was found to be in the range of 21.00±0.009 (F6) to 17.66±0.008 (F2). The order of drug content was found to be F6>F9>F7>F10>F1>F8>F4>F5>F3>F2.
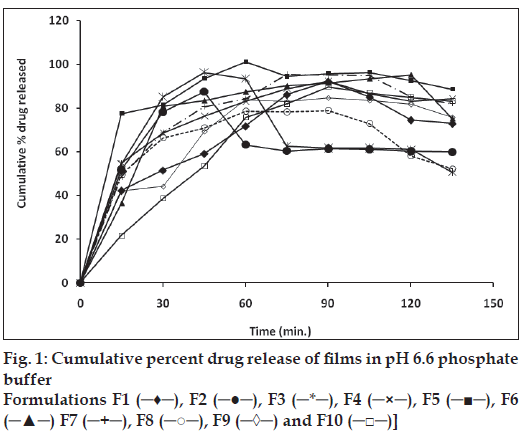
In vitro release studies of various formulations were performed using pH 6.8 phosphate buffer as dissolution medium. The drug concentration was determined spectrophotometrically at 213 nm. Significant difference was observed in the release pattern of enalapril maleate films containing PVP, HEC, HPMC and SCMC (fig. 1). It was observed that during dissolution, SCMC containing films swelled forming a gel layer on the exposed film surfaces [11,12]. The loosely bound polymer molecules in these films were readily eroded, allowing the easy release of enalapril maleate as compared to PVP. After two hours the release was found to be in the range of 78.96 to 96.35%. The rank order of drug release after 2 h was found to be 96.35>96.3 0>95.20>95.06>92.17>89.78>87.53>84.76>82.90>7 8.96 % for formulations F5>F3>F6>F7> F1>F10> F2>F9>F4>F8, respectively.
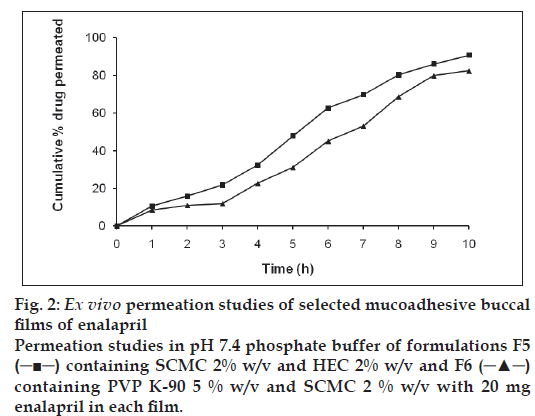
It was also concluded that formulation F5 (containing SCMC and HEC) and F6 (containing SCMC and PVP K-90) showed good swelling, a convenient residence time as well as promising drug release. On the basis of release pattern, swelling and residence time, F5 and F6 formulations were chosen for ex vivo study. In ex vivo study, drug permeation through the porcine buccal mucosa was determined for formulation F5 and F6 (fig. 2). The drug permeation was found to be 82.48 % and 90.86 % in F5 and F6 after 10 h. The drug permeation decreased in ex vivo study in comparison of in vitro release. This decrease in drug diffusion observed from ex vivo study compared to in vitro, may be due to the lesser permeability of porcine mucosa and also the presence of a backing membrane in the ex vivo study, which make the release unidirectional. The backing membrane restricting the contact of the film with the receptor fluid to one side alone slows down the water uptake, swelling and disruption of the matrix in turn releasing lesser amount of drug in specified time, compared to the one without the backing membrane. The correlation coefficient values were found to be 0.9852 and 0.9667 for F5 and F6, respectively showing good correlation. It may be concluded that the release kinetics followed zero order. The Higuchi Plots of F5 and F6 were found to be almost linear with correlation coefficient values of 0.9310 and 0.9748. This proves that the drug permeation followed the matrix diffusion process.
The results of all the physical characterization of all formulations (F1–F10) were found to be satisfactory. The results of the study show that therapeutic levels of enalapril can be delivered buccally. The present study concludes that these erodible mucoadhesive buccal films containing enalapril can be very promising for effective doses to systemic circulation. These may also provide an added advantage of circumventing the hepatic first pass metabolism. Films exhibited controlled release over more than 2 h. It was concluded that the films containing 20 mg of enalapril maleate in Sodium carboxymethylcellulose 2% w/v and hydroxyethyl cellulose 2% w/v (formulation F5), showed good swelling, a convenient residence time and promising controlled drug release, thus can be selected for the development of buccal film for effective therapeutic uses. Further, the study may be extended for assessing the in vivo release and in vitro-in vivo correlation.
Acknowledgements
Authors thankfully acknowledge Intas Pharmaceutical Ltd. Dehradun for providing the gift sample of enalapril maleate. Authors also acknowledge the DST project (SRSO/ HS/72/2006) laboratory, Department of Chemistry, H. N. B. Garhwal University Chauras campus, for providing the instrumental facilities.
References
- Shojaei AH, Chang RK, Guo X, Burnside BA, Couch RA. Systemic drug delivery via the buccal mucosa route. Pharm Tech 2001;6:70-81.
- Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery--a promising option for orally less efficient drugs. J Control Release 2006;114:15-40.
- Jasti B, Li X, Cleary G. Recent advances in mucoadhesive drug delivery systems. Pharma Tech 2003;194-6.
- Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev 2005;57:1666-91.
- Semalty A, Semalty M. Mucoadhesive polymers: a review, www. pharmainfo.net, [accessed on 2006 Sep 24].
- Houghton AR. Angiotensin II receptor antagonists in chronic heart failure: where do they fit? Drugs 2002;62:1433-40.
- Semalty M, Semalty A, Kumar G. Formulation and characterization of mucoadhesive buccal films of glipizide. Indian J Pharm Sci 2008;70:43-8.
- Junginger HE, Hoogstraate JA, Verhoef JC. Recent advances in buccal drug delivery and absorption: in vitro and in vivo studies. J Control Release 1999;62:149-59.
- El-Khodairy KA. Effect of physicochemical properties of the hydrophilic Gantrez matrix. Alex J Pharm Sci 2001;15:35-40.
- Samuelov Y, Donbrow M, Friedman M. Sustained release of drugs from ethylcellulose--polyethylene glycol films and kinetics of drug release. J Pharm Sci 1979;68:325-9.
- Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 1983;15:25.
- Nafee NA, Ismail FA, Boraie NA, Mortada LM. Mucoadhesive buccal patches of miconazole nitrate: in vitro/in vivo performance and effect of ageing. Int J Pharm 2003;264:1-14.