- Corresponding Author:
- S. Swain
Department of Pharmaceutics, Roland Institute of Pharmaceutical Sciences, Khodasingi, Berhampur-760 010
E-mail: swain_suryakant@yahoo.co.in
| Date of Submission | 25 July 2013 |
| Date of Revision | 30 June 2014 |
| Date of Acceptance | 01 July 2014 |
| Indian J Pharm Sci 2014;76(4):354-363 |
Abstract
The objective of present research work was to design and characterize the venlafaxine HCl-loaded sodium alginate-based mucoadhesive microcapsules by ionic gelation technique using HPMC K100M as mucoadhesive polymer. The Placket-Burman Design was applied for preliminary screening of the formulations and systematic optimization by using Box-Behnken Design. The prepared microcapsules were characterized for drug content, entrapment efficiency, micromeritic properties, particle size, swelling index, mucoadhesive strength, in vitro drug release and in vivo antidepressant activity. FTIR and differential scanning calorimetry studies showed no incompatibility. Surface morphology studies revealed spherical nature of the prepared microcapsules. In vitro drug release studies revealed sustained release by diffusion mechanism. Further, the microcapsules were effective in reducing the depression induced by forced swimming test in Sprague-Dawley rats compared to the pure drug. The microcapsules were found to be stable under accelerated stability conditions, which suggest them as better alternative delivery systems for enhanced therapeutic efficacy of antidepressant drug, venlafaxine HCl.
Keywords
Antidepressant activity, in vitro drug release, mucosal drug delivery, sustained release, optimization
Oral route is most sought-after for administration of drug molecules to the systemic circulation due to low cost therapy, ease of administration, patient compliance. Several attempts have been made to develop the effective drug delivery systems to combat the unmet patient needs. Despite the tremendous strides in oral drug delivery, the success of conventional dosage forms is limited due to their inability to increase the residence time of the drug in the stomach and proximal portion of the small intestine for prolonged periods of time [1,2]. On contrary, the sustained release dosage forms have merits of reduced dosing frequency, enhanced gastric residence time, and provide desired therapeutic clinical benefits [3]. Mucoadhesive microcapsules are the multiple unit particulate systems contain mucoadhesive polymers, which provide sustained release action upon intimate contact with the epithelial mucous membrane lining. Also such formulation protects the drug from gastric acidic environment, increases the oral bioavailability of drugs and reduces the variability [4].
Venlafaxine, (1- [2-(dimethylamino)-1-(4- methoxyphenyl) -ethyl]-cyclohexan-1-ol), is a serotonin-norepinephrine reuptake inhibitor, widely used as an antidepressant agent [5]. It blocks the action of transporter reuptake proteins affecting mood, thereby leaving more active neurotransmitter in the synapse [6]. Also, it inhibits the neuronal uptake of norepinephrine, serotonin neurotransmitter which helps in alleviating the mood [7,8]. Venlafaxine is freely soluble in water (0.58 g/ml) and exhibit short elimination half-life of less than 5 h. It is prescribed in the dose of 100 mg twice or thrice daily, thus leads to poor patient complianc [9]. Moreover, venlafaxine has narrow absorption window and is mainly absorbed from proximal areas of gastrointestinal tract [10]. Further, it undergoes high hepatic first-pass metabolism in liver, which leads to poor oral bioavailability (<45%) [11]. The conventional dosage forms available in the market fail to satiate the needs of enhancing the oral bioavailability and to improve the patient compliance. This calls for the development of venlafaxine-loaded novel mucoadhesive multiparticulate drug delivery systems to enhance its oral bioavailability with improved pharmacodynamic potential [12]. Optimization of pharmaceutical experiments involving both product and process are being practiced widely for systematic development of dosage forms. Design of Experiment (DoE) methodology, in this regard, helps in designing the experiments by complete understanding of the product and process variables and optimization of them with the help appropriate experimental design [13,14]. Examples of various experimental designs used for screening and optimization of novel drug delivery systems include factorial design, Placket- Burman design, Taguchi design, Box-Behnken design, Central-Composite design and mixture design [15]. As discussed, the mucoadhesive drug delivery systems contain polymers of diverse chemical nature, thus requires optimization of them to obtain the robust formulations with desired therapeutic performance [16].
The present studies, therefore, aims to prepare the mucoadhesive microcapsules by ionic gelation method employing various polymer viz. sodium alginate, HPMC K4M, HPMC K15M, HPMC K100M, carbopol 974P and chitosan. Initially different batches of microcapsules were prepared by ionic gelation method to evaluate the suitability of a particular mucoadhesive polymer. Further, the preliminary prototype formulations were prepared as per suitable screening design, i.e., Placket-Burman design and evaluated for selecting the influential variables. Further, the mucoadhesive microcapsules were optimized using Box-Behnken design for the influential factors to obtain the robust formulations. The optimized microcapsules were evaluated for physiochemical characterizations, in vitro drug release, and antidepressant activity. Also, the microcapsules were evaluated by SEM, FT-IR and DSC to investigate the shape and distribution of drug and for solid-state characterization.
Materials and Methods
Venlafaxine HCl was generously gifted by Ranbaxy Labs. Ltd., Gurgaon, India. Various excipients like sodium alginate, HPMC (K4M, K15M, K100M) were provided by Colorcon, Goa, India, whereas CaCl2, carbopol 974P and chitosan provided by Evonik, Mumbai, India. While all other chemicals and reagents like sodium hydroxide, potassium dihydrogen phosphate used was of analytical reagent grade. Deionized distilled water was used throughout the experimental procedure.
Preparation of mucoadhesive microcapsules
The microcapsules were prepared as per the procedure reported by Nayak et al., with little modifications [2]. Required quantities of sodium alginate and the selected mucoadhesive polymers were dissolved in purified water to form a homogeneous solution. The drug was added to the polymer solution and mixed thoroughly with the help of a mechanical stirrer at 1000 rpm for 1 h to form a viscous dispersion. The resulting dispersion was then added manually drop wise into 10% w/v calcium chloride solution through a syringe with a needle of size no. 18. The added droplets were allowed to retain in the calcium chloride solution for 3 h to complete the curing reaction and to produce spherically rigid microcapsules. The microcapsules were collected by decantation, and washed with water and dried at 45° for 12 h. The composition of preliminary batches of mucoadhesive microcapsule formulations prepared is shown in Table 1.
| Ingredients | Batch code | ||||
|---|---|---|---|---|---|
| B1 | B2 | B3 | B4 | B5 | |
| Venlafaxine HCl | 500 | 500 | 500 | 500 | 500 |
| Sodium alginate | 400 | 400 | 400 | 400 | 400 |
| Carbopol 974P | 100 | - | - | - | - |
| HPMC K4M | - | 100 | - | - | - |
| HPMC K15M | - | - | 100 | - | - |
| HPMC K100M | - | - | - | 100 | - |
| Chitosan | - | - | - | - | 100 |
| CaCl2 (%w/v) | 10 | 10 | 10 | 10 | 10 |
| Distilled water | QS | QS | QS | QS | QS |
Formulations designed in the proportions of drug:sodium alginate: mucoadhesive polymer:5:4:1. All the quantities depicted in the table are in mg, QS: Sufficient quantity
Table 1: Formulation Design of Mucoadhesive Microcapsules
Optimization of microcapsule formulations
In order to optimize the mucoadhesive microcapsule formulations, initially the influential factors were selected by employing the Plackett-Burman design [17]. In total seven different factors were selected at their respective ranges for microcapsule formulations to evaluate their effect on the chosen responses, as shown in Table 2. Further, the Box-Behnken design was used for optimization of the microcapsule formulations. The mucoadhesive microcapsules were prepared as per the suggested experimental runs and evaluated for selected responses. Response surface analysis was carried out to observe the effect of various factors on the response variables and interactions among them.
| Trials | Sod. | HPMC | Cross | Calcium | Span | Stirring | Stirring |
|---|---|---|---|---|---|---|---|
| Alginate | K100M | linking | Chloride | 80 | speed | time | |
| time | |||||||
| 1 | -1 | -1 | 1 | -1 | -1 | 1 | -1 |
| 2 | -1 | 1 | -1 | 1 | 1 | -1 | 1 |
| 3 | -1 | 1 | 1 | -1 | 1 | 1 | 1 |
| 4 | 1 | 1 | 1 | -1 | -1 | -1 | 1 |
| 5 | 1 | -1 | -1 | -1 | 1 | -1 | 1 |
| 6 | 1 | -1 | -1 | -1 | -1 | 1 | -1 |
| 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 8 | 1 | -1 | -1 | 1 | 1 | 1 | -1 |
Low level (-1); high level (+1): Sodium alginate (mg) 250 (-1) and 750 (+1); HPMC K100M (mg) 50(-1) and 150(+1); cross linking time (h) 3 (-1) and 6 (+1); calcium chloride (%w/v) 5 (-1) and 15 (+1); Span 80 (%w/w) 3 (-1) and 5 (+1); stirring speed (rpm) 1000 (-1) and 2000 (+1) and stirring time (min), 30 (-1) and 60 (+1), HPMC: Hydroxypropyl methylcellulose
Table 2: Formulation And Influence of High and Low Level Process Variables Per Plackett-Burman Design
Percent yield and drug content
The weight of all batches of dried mucoadhesive microcapsule formulations prepared as per the experimental design was noted to calculate the percent yield. For calculation of the percent drug content in the prepared microcapsules, an amount of microcapsules, equivalent to 25 mg of venlafaxine HCl was weighed, powdered and suspended in 100 ml of phosphate buffer pH 7.4. The resultant dispersion was kept for 24 h for complete solubilization of the drug and filtered though a Whattman filter paper and spectrophotometrically at 225 nm (Shimadzu 1800 UV-ENG240V, Japan) [18]. The total drug content was determined by employing absorbance obtained in the linear regression equation (Y=0.017x+0.006) of the calibration curve.
Entrapment efficiency
The drug entrapment efficiency was determined by dissolving microcapsules equivalent to 25 mg of venlafaxine HCl in 100 ml of pH 7.4 phosphate buffer. The resultant dispersion was kept for 24 h for complete mixing and filtered though a Whattman filter paper [18]. The venlafaxine content was measured analyzed spectrophotometrically at 225 nm using UV spectrophotometer (Shimadzu-1800, Tokyo, Japan). The drug entrapment efficiency was calculated using Eqn.1 [19], which is, entrapment efficiency=(actual drug content/theoretical drug content)×100.
Loose surface crystal study
The drug-loaded microcapsules were evaluated for loose surface crystal study to calculate the excess amount of drug present on the surface of microcapsules. For this 100 mg of microcapsule were shaken in 20 ml of pH 7.4 phosphate buffer for 5 min and filtered through 0.45 µm membrane filter. The amount of drug present in filtrate was determined spectrophotometrically, which yield the total drug content [2,20].
In vitro wash off test
The mucoadhesive properties of prepared microcapsules were evaluated by the in vitro wash-off method. Freshly excised piece of goat intestinal mucosa collected from the local slaughter house were cut into appropriate pieces and the required pieces were mounted on a glass slide using thread. About 25 microcapsules were spread out on each piece of mucosa and then hung from the arm of the tablet disintegration test apparatus [20]. The disintegrating test apparatus was operated in a way that the tissue specimen was exposed to the media (both 0.1 N HCl and Phosphate buffer, pH 7.4 maintained at 37±0.5°), with regular 10-12 dips per minute. The test was performed up to 6 h and at a regular interval of 1 h, the total number of microcapsules adhere remain to the intestinal mucosa were counted and percentage mucoadhesion was calculated.
Percent moisture loss
The microcapsules were evaluated for percentage moisture loss, which gives an idea about the hydrophilic nature. The weighed amount of microcapsules (W1) was initially kept in a desicator containing calcium chloride at 37° for 24 h. The final weight (W2) was noted when no further change in weight of sample was observed. Finally the percent moisture content was determined by Eqn. 2 [2], Percent moisture loss=(W1-W2/W1)×100.
Micromeritic properties of microcapsules
The prepared microcapsules were evaluated for various micromeritic properties like angle of repose, bulk density, Carr?s index and Hausner?s ratio. All the tests were performed as per the procedure given in USPXXXI. The angle of repose was determined by fixed funnel method using Eqn. 3, Tan θ=h/r, where, h=height in cm of the powder pile, r=radius in cm of the powder pile.
For determination of tapped density, microcapsules were tapped using USP tapped density tester (Electrolab, model ETD?1020, Mumbai) for 100 taps and the change in volume was measured [21]. Carr?s index and Hausner?s ratio were calculated by the Eqn. 4 and 5, respectively, which are, Carr?s index=(bulk density-tapped density/bulk density)×100 and Hausner?s ratio=(bulk density/tapped density)×100.
Particle size
The particle of the prepared microcapsules was measured by optical microscopy. Accurately weighed 50 mg of the prepared microcapsules were mounted on a micrometer slide and observed under microscope to observe the particle size [2]. The particles size was obtained in terms of circulatory factor (S) which can be calculated by using Eqn. 6, S=P×P/12.56×A, where, A is area (cm2) and P is the perimeter of the circular tracing.
Swelling index
The swelling index was determined by keeping the prepared microcapsules in pH 7.4 phosphate buffer solution for 12 h. After overnight wetting, the swollen microcapsules were collected and their weight was noted. From the weight of the microcapsules before and after wetting, the swelling index was calculated by following Eqn. 7, Sw=(Wt-Wo/Wo)×100, where, Sw=percent swelling of microcapsules, Wt=weight of the microcapsules at time t, Wo=initial weight of the microcapsules.
In vitro drug release study
In vitro drug release studies of the prepared microcapsules were carried out employing USP Type-I dissolution apparatus (Electrolab TDT-06P Mumbai, India) in 900 ml of phosphate buffer pH 7.4 maintained at 37±0.5° and 50 rpm. At specified time intervals (0, 1, 2, 3, 4, 6, 8, 10 and 12 h), an aliquot of 5 ml samples were withdrawn with replacement of fresh medium to maintain the sink condition. The samples were filtered through a membrane filter of 0.45 µm and analyzed spectrophotometrically at λmax225 nm. From the drug content, the cummulative percentage drug release vs. time was plotted to compare the in vitro drug release from the prepared microsphere formulations.
In vivo pharmacodynamic study
The approval of the Institutional Animal Ethics Committee was obtained before starting the study. The registration number, approval number and date is 926/PO/ac/06/CPCSEA, 73 and 07/06/2012 respectively. Animal experiments are conducted in full compliance with local, national, ethical, and regulatory principles and local licensing regulations, per the spirit of Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International expectations for animal care and use/ ethics committees. In vivo antidepressant activity of the venlafaxine loaded microcapsules was carried out in Sprague-Dawley rats by forced swim test. To carry out the study the animals were divided into four different groups (n=6), as control, naïve, pure drug treated and microcapsules treated. The animals were provided with housing in polypropylene cages having artificial lighting to simulate the day-night cycles, and access to food (Lipton pellet diet, Mumbai, India) and water ad libitum. As per the test protocol, the forced swimming test was performed two days prior to the administration of the treatment to induce the depression in animals. It is a common behavioral test where rodents were subjected to force swimming for assessing the depression and testing the efficiency of antidepressant drugs. The animals were allowed for swimming in a plexiglass cylinder (18 cm width and 40 cm height) containing water for 15-20 min till the animals get immobilized, which is an indication of depression like state. The swimming practice in the animals were carried out for 2 days, and then on the third day clinically effective doses of antidepressant drugs were administered prior to 5 min of test swimming session. The animals receiving the test formulations containing drug loaded mucoadhesive microcapsules were observed for total taken to become immobilize compared with other groups. The attentions were made for false positive results do occur due to the generalized increase in motor activity in the animals. The test was performed in replicates to get confirmatory results.
Fourier-transformed infrared (FTIR) spectroscopy
FTIR spectroscopy was carried out to characterize the possible interaction between the drug and excipients, if any. The FTIR spectra of pure drug, physical mixture of drug with selected polymers and prepared microcapsules were recorded using KBr disc using an FTIR spectrophotometer (Shimadzu, Tokyo, Japan).
Differential scanning calorimetry (DSC)
DSC was performed for pure drug, physical mixture of drug with polymers and optimized microcapsules in DSC apparatus (Shimadzu DSC 60, Tokyo, Japan). Samples were heated from 30-300° at the heating rate of 10°/min in nitrogen atmosphere (flow rate, 20 ml/min).
Scanning electron microscopy (SEM)
The shape and surface morphology of prepared microcapsules were observed by SEM. The dried microcapsules were mounted on brass stub using carbon paste and subjected to gold coating to make the surface electrostatic and kept in a dessicator. The stub was mounted on the sample holder tray in the electron microscope (Joel Scanning Microscope JSM-5800, Denton, USA), and the acceleration voltage of 20 kv was used with secondary electron image as the detector to observe the surface picture of the microcapsules.
Stability study
The stability studies of the optimized microcapsules were carried out as per the ICH guidelines. The microcapsule were suitably packed in the high density plastic bottles and stored at 40°/75% RH for 3 months in the stability chamber. The microcapsules were evaluated for physiochemical properties, drug content and in vitro drug release at specified time periods (0, 1, 2 and 3 months) to assess the stability.
Results and Discussion
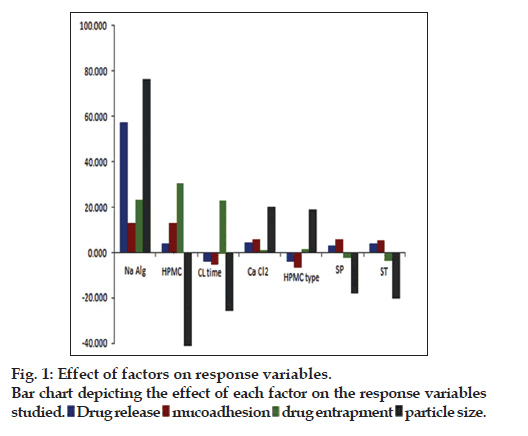
The factor screening studies were carried out employing Placket-Burman design yielded the solutions with most influential factors affecting the response variables. The coefficients of linear polynomial equations generated for each of the response variable were used to screen out the factors. The influence of each of the factors studied on the response variables is depicted pictographically in fig. 1. It has been observed that among the factors studied, the concentration of sodium alginate, HPMC type (i.e., HPMC K100M), amount of HPMC K100M and crosslinking time had higher influence on the chosen response variables. The selected factors were subjected for optimization with the help of Box- Behnken design. Total 13 formulations were prepared as per the experimental design and characterized for the selected response variables, which are represented in Table 3. The model was analyzed for fitting into an appropriate mathematical model, i.e., quadratic model to obtain the curvature unveiling interaction effects among the variables. Further, the model was evaluated statistically for ANOVA and lack of fit, which showed that all the model terms were significant (P<0.05) with insignificant lack of fit indicated that the model was best fitted. The response surface analysis was carried out employing the 3D response surfaces. It was observed that concentration of all three factors have positive impact on the %drug release. Further, it was observed that among the three factors, the concentration of HPMC K100M had significantly higher influence on the mucoadhesion, whereas concentration of sodium alginate had influence on drug entrapment efficiency, respectively. However, increasing the concentration of cross-linking agent and HPMC K100M has higher influence on particle size of the microcapsules. Finally, the optimized formulation was selected by numerical optimization procedure and desirability function. The optimized formulation was found to contain 283 mg of sodium alginate and 106 mg of HPMC K100M, while the optimum crosslinking time was found to be 14 h.
| Formulation code |
Na alginate (mg) |
HPMC K100M (mg) |
Cross linking time (h) |
Drug release (%) |
Mucoadhesion (%) |
Drug entrapment (%) |
Particle size (µM) |
|---|---|---|---|---|---|---|---|
| F1 | -1 | -1 | 0 | 71.35 | 65 | 12.51 | 1005.43 |
| F2 | 1 | 1 | 0 | 45.56 | 88 | 12.22 | 1014.22 |
| F3 | -1 | 0 | -1 | 66.54 | 74 | 22.56 | 1099.15 |
| F4 | 1 | -1 | 0 | 48.43 | 79 | 31.44 | 1173.59 |
| F5 | 0 | 0 | 0 | 67.31 | 64 | 21.67 | 1059.87 |
| F6 | -1 | 1 | 0 | 69.34 | 81 | 11.58 | 1012.42 |
| F7 | 0 | 1 | 1 | 71.05 | 71 | 33.24 | 1022.13 |
| F8 | 1 | 0 | 1 | 45.26 | 72 | 13.59 | 1067.28 |
| F9 | 1 | 0 | -1 | 44.61 | 68 | 23.14 | 1176.43 |
| F10 | 0 | -1 | 1 | 46.11 | 61 | 32.52 | 1189.45 |
| F11 | -1 | 0 | 1 | 68.23 | 73 | 31.23 | 1162.36 |
| F12 | 0 | 1 | -1 | 45.84 | 72 | 30.48 | 1054.65 |
| F13 | 0 | -1 | -1 | 55.88 | 56 | 28.45 | 1028.12 |
Low level (-1), intermediate level (0) and high level (+1): Sodium alginate (mg) 250, 500 and 750; HPMC K100 (mg) 50, 100 and 150; cross linking time (h) 3, 4.5 and 6, HPMC: hydroxypropyl methylcellulose
Table 3: Responses of Experimental Trials Using Box-Behken Design
The percent yield of different batches of microcapsules was found to be ranging between 63.24 to 86.79%, respectively. The yield was good, indicating that all the polymers undergo gelation by crosslinking agent to form the microcapsules. Percent drug content of the prepared microcapsules was found to be between 12.83 to 33.31%. Similarly, entrapment efficiency was found to be between 11.83 to 32.29%, respectively. The entrapment efficiency was dependent on the concentration of sodium alginate. Loose surface crystal studies showed that maximum fraction of free drug was found on the surface of microcapsule formulations containing lower amount of the matrix forming polymer, HPMC K15M. The results of loose surface crystal studies are enlisted in Table 4. It was revealed that formulation F5 (8.22±0.01) had maximum and formulation F1 (2.14±0.02) had minimum amount of loose surface crystals.
The minimum percentage of moisture loss was observed in formulation F4 and maximum percentage of moisture loss was observed in formulation F2. This ensured that presence of simulative water content may be due to the utilization of water as a manufacturing vehicle during preparation of microcapsules and may also be attributed to the hygroscopic nature of the drug or mucoadhesive polymers [2]. The low water content indicated proper drying of the microcapsules. The percentage moisture loss values in different batches of microcapsules are depicted in Table 4.
| Formulation code |
(%±SD) | ||||
|---|---|---|---|---|---|
| Production yield |
Drug content |
Entrapment efficiency |
Loose surface crystal study |
Total moisture loss |
|
| F1 | 63.24±0.01 | 12.83±0.01 | 11.83±0.01 | 2.14±0.02 | 5.31±0.02 |
| F2 | 69.58±0.02 | 20.42±0.01 | 19.42±0.01 | 6.99±0.01 | 6.49±0.01 |
| F3 | 82.25±0.01 | 24.97±0.01 | 23.66±0.01 | 4.23±0.02 | 4.67±0.01 |
| F4 | 86.79±0.03 | 33.31±0.02 | 32.29±0.02 | 3.88±0.01 | 2.36±0.02 |
| F5 | 55.61±0.02 | 25.77±0.01 | 24.45±0.01 | 8.22±0.01 | 3.22±0.02 |
| F6 | 65.68±0.02 | 17.81±0.01 | 12.58±0.01 | 5.44±0.02 | 4.33±0.02 |
| F7 | 75.41±0.01 | 24.52±0.01 | 31.44±0.02 | 7.89±0.01 | 5.69±0.01 |
| F8 | 55.61±0.02 | 26.27±0.01 | 14.09±0.01 | 5.33±0.02 | 5.27±0.01 |
| F9 | 58.86±0.01 | 34.31±0.02 | 24.11±0.01 | 4.08±0.01 | 2.56±0.02 |
| F10 | 66.61±0.02 | 26.57±0.01 | 32.52±0.01 | 6.02±0.01 | 4.02±0.02 |
| F11 | 75.78±0.02 | 18.33±0.01 | 30.33±0.01 | 3.04±0.02 | 6.30±0.02 |
| F12 | 54.31±0.03 | 26.42±0.01 | 31.68±0.02 | 7.89±0.01 | 5.69±0.01 |
| F13 | 55.61±0.02 | 23.44±0.01 | 27.45±0.01 | 4.03±0.02 | 5.58±0.01 |
Production yield (%), drug content (%), entrapment efficiency (%) loose surface crystal study and circulatory factor and percent moisture loss of selected runs of mucoadhesive capsules
Table 4: Evaluation of Mucoadhesive Microcapsules
The tapped density was found in the range between 0.312±0.02 to 0.510± 0.03 g/cm3 and bulk density was found to be ranging between 0.234±0.1 to 0.471±0.07 g/cm3. The prepared microcapsules showed good flow property as Carr?s index was found to be between 6.25±0.04 to 16.21±0.12 and angle of repose between 11.13° to 29.43°, which also indicated good flow behaviour of the microcapsules [22].
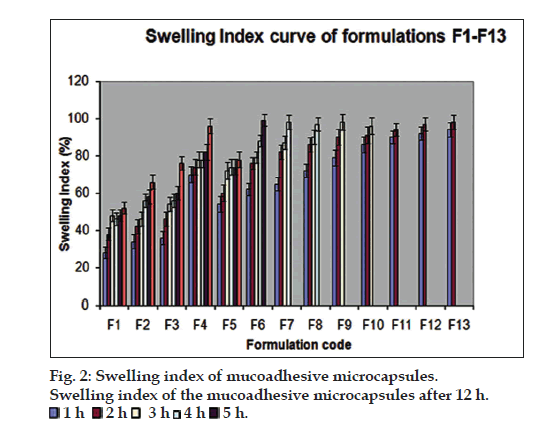
The particle sizes of the microcapsules were found between 1042.34 and 1285.61 µm. The variability of particle size between different batches was depends on the factors such as polymer concentration, amount of cross linking agent and stirring time. fig. 2 pictorially depicts the swelling index of the mucoadhesive microcapsules prepared as per the experimental design. The formulation F4 showed highest swelling index may be due to the higher water uptake and concentration of the mucoadhesive polymer, HPMCK 100M.
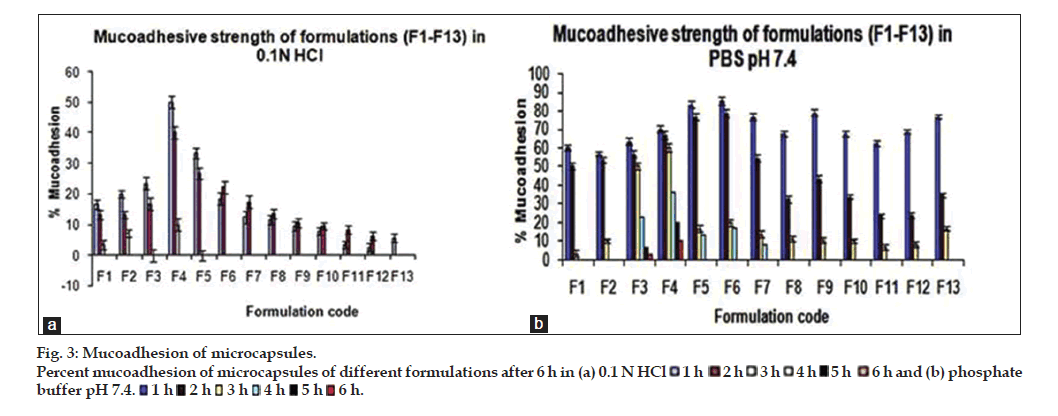
The results of in vitro wash off test showed good mucoadhesion properties of the prepared microcapsule formulations in both 0.1 N HCl and pH 7.4 phosphate buffer. It was observed that microcapsules subjected to 0.1 N HCl was completely washed off in 3 h, however microcapsules subjected to pH 7.4 phosphate buffer were adhered to the tissue up to 6 h as shown in fig. 3a and b. The formulation F4 exhibited higher bioadhesion strength in pH 7.4 phosphate buffer as compared to other formulations. The formulations containing higher concentration of mucoadhesive polymer showed higher mucoadhesion strength and longer wash-off periods. This may be due to the electrostatic attraction between the polymer chains and mucin. The swelling index of the polymer also has influence on the mucoadhesion strength. Higher swelling index provides attachment of the microcapsules with mucosal surface for longer period of time [23].
In vitro drug release studies showed that all the prepared batches of mucoadhesive microcapsules followed a sustained release profile of drug release, as shown in fig. 4a and b. The cumulative percentage drug release from the prepared microcapsule formulations were found to be between 44.61 to 71.351%, respectively. It has been observed that the sustained release action was higher in formulations containing higher concentration of the mucoadhesive polymer and vice-versa. The in vitro release profile of venlafaxine HCl from microcapsules showed that with increasing the concentration of sodium alginate the release of the venlafaxine HCl from the polymer matrix was retarded. In case of formulation (F4), containing 750 mg of sodium alginate and 50 mg of HPMC K100M, the drug release was retarded up to 12 h among the formulations between (F1-F6). However, in case of microcapsule formulations (F7?F13), the percent release of venlafaxine HCl was quite faster and sustained release action was found up to 6 h.
The in vitro drug release data were fitted into various mathematical models corroborated the diffusion mechanism of drug release form the microcapsules, as the regression coefficient (r2) value of the Higuchi model was found to be higher. Further, the Korsmeyer-Peppa?s release exponent (n), suggested that the release mechanism was found to be supercase-II transport (n>1.0). Table 5 depicts the r2 values of various mathematical models and release exponent (n). The diffusion mechanism of drug release was attributed to the swelling behaviour and polymer chain relaxation phenomena of the hydrophilic polymers, i.e., sodium alginate and HPMC employed in the formulation of microcapsules [24,25].
| Formulation code |
Zero order (r2) |
First order (r2) |
Higuchi (r2) |
Baker and Lansdale (r2) |
Korsmeyer-Peppa’s model release exponent (n) |
|---|---|---|---|---|---|
| F1 | 0.917 | 0.950 | 0.955 | 0.917 | 1.355 |
| F2 | 0.992 | 0.839 | 0.933 | 0.992 | 1.558 |
| F3 | 0.933 | 0.869 | 0.952 | 0.932 | 1.470 |
| F4 | 0.931 | 0.873 | 0.968 | 0.931 | 1.290 |
| F5 | 0.937 | 0.979 | 0.953 | 0.937 | 1.560 |
| F6 | 0.927 | 0.956 | 0.955 | 0.907 | 1.445 |
| F7 | 0.982 | 0.829 | 0.933 | 0.952 | 1.358 |
| F9 | 0.973 | 0.866 | 0.952 | 0.942 | 1.451 |
| F10 | 0.942 | 0.853 | 0.968 | 0.962 | 1.260 |
| F11 | 0.948 | 0.959 | 0.953 | 0.947 | 1.350 |
| F12 | 0.967 | 0.960 | 0.955 | 0.977 | 1.245 |
| F13 | 0.942 | 0.889 | 0.933 | 0.997 | 1.488 |
Table 5: In Vitro Dissolution Kinetics Data of Selected Formulations
In vivo pharmacodynamic evaluation of antidepressant activity showed that the duration of immobility (s) after specified time intervals of 1, 2 and 6 h was significantly higher for animals treated with microcapsule in comparison with the naive, control and pure drug treated groups, as depicted in fig. 5. Further, the microcapsules were found to be better inhibiting the forced swimming induced depression in experimental animals compared to the aqueous solution of the pure drug. This confirmed the enhanced antidepressant activity of the drug, when administered in microcapsule formulations. This may be possibly due to the mucoadhesive nature and sustained release profile of drug release from the microcapsules for prolonged periods of time.
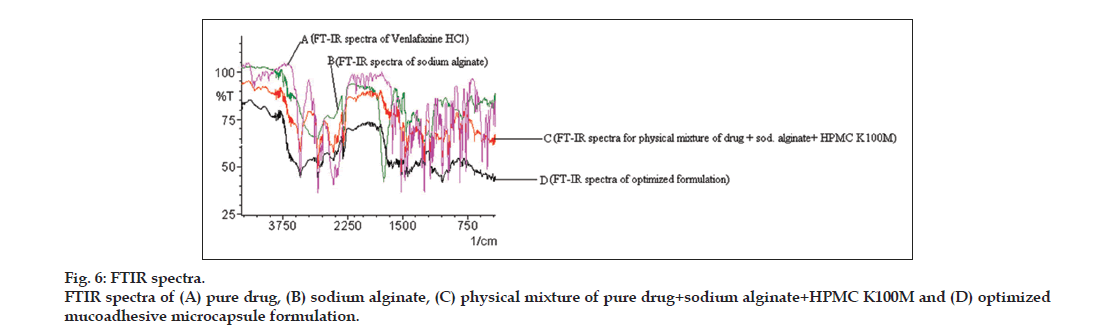
FTIR spectra analysis revealed no significant interaction between various rational combinations containing physical mixture of drug with polymers (i.e., sodium alginate and HPMC K100M) and in the optimized microcapsules, as shown in fig. 6. FTIR spectra of pure drug showed the major peaks for C-H stretching (alkane) at 2935.66 cm-1, C-O stretching (phenol) at 1247.94 cm-1, O-H bending (phenol) at 1180.44 cm-1, C-H bending vibrations at 1471.69 cm-1, C=N vibrations at 1035.77 cm-1, C-H (aromatic) bending vibrations at 837.11cm-1 and C=C stretching (aromatic) at 1514.12 cm-1, which were compared with the peaks of physical mixture of drug with polymers. The FT-IR spectra of drug-polymer mixture confirmed neither any shift in the wave numbers of the peaks nor in the intensity, construed lack of interaction.
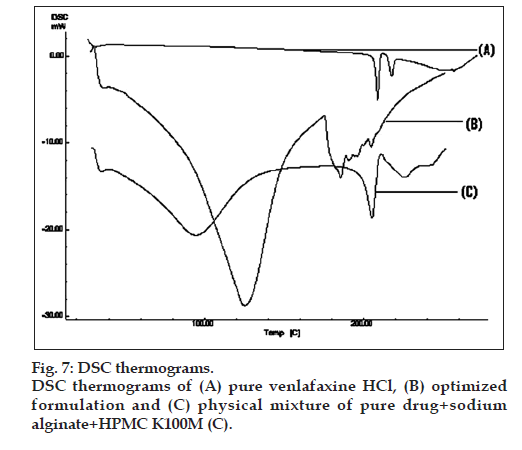
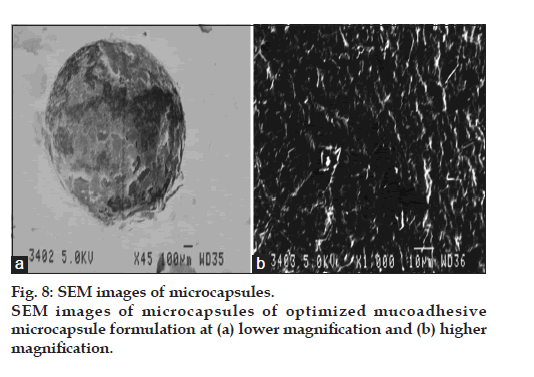
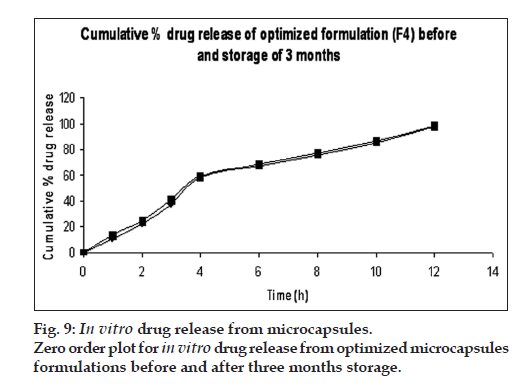
DSC thermograms of pure drug, physical mixture of drug with polymers and microcapsules are dipected in fig. 7. In pure drug and physical mixture a sharp peak at 208° was observed due to melting, whereas in drug-loaded microcapsules, no characteristic peak was observed at 208°, suggesting that drug is molecularly dispersed in the matrix [26]. The SEM images of the drug loaded optimized mucoadhesive microcapsules are shown in fig. 8. The microcapsules were found to be spherical in shape with rough outer surface due to the entanglement of the polymer chains, which in turn responsible for sustained release property. As per the three month stability studies the optimized mucoadhesive microcapsules were found to be stable. There was no significant change in the in vitro drug release and drug content. fig. 9 depicts the in vitro drug release profile from the microcapsules at initial time point and after three months of stability study under accelerated conditions.
The present studies, therefore, shows the successful formulation of mucoadhesive microcapsules of venlafaxine HCl by ionic gelation method using sodium alginate and HPMC K100M as suitable polymers. The application of experimental design methodology helped to prepare the optimized formulation, which showed good sustained release profile of drug release with enhanced mucoadhesion property and antidepressant activity. The drug release was found to be diffusion controlled and followed Higuchi kinetics. FTIR and DSC studies used for solid-state characterization confirmed absence of any physiochemical interaction between drug and polymers. SEM analysis showed a smooth surface with spherical appearance of microcapsules. Further, in vivo pharmacodynamic studies by forced swimming test observed superior antidepressant activity of mucoadhesive microcapsules compared to the aqueous solution of the pure drug in male Sprague-Dawley rats. The prepared formulations were found to be stable under accelerated stability studies for three months. This confirmed that mucoadhesive microcapsules can be an alternative dosage form for effective delivery of venlafaxine HCl.
Acknowledgements
The authors are thankful to Mr. Dhananjay Panigrahi, Scientist, Ranbaxy Labs Ltd., Gurgoan, India for providing gift sample of venlafaxine HCl. The authors are also thankful to Dr. Piyush Ranjan Mishra, Department of Pharmacology, Principal, Management and Staff of Roland Institute of Pharmaceutical Sciences for their kind co-operation and providing necessary facilities to carry out the present research work.
References
- Malakara J, Nayak AK. Formulation and statistical optimization of multiple-unit ibuprofen-loaded buoyant system using 23-factorial design. Chem Eng Res Des 2012;90:1834-46.
- Nayak AK, Hasnain MS, Beg S, Alam MI. Mucoadhesive beads of gliclazide: Design, development and evaluation. Sci Asia 2010;36:319-25.
- Vasir JK, Tambwekar K, Garg S. Bioadhesive microspheres as a controlled drug delivery system. Int J Pharm 2003;255:13-32.
- Mathiowitz E, Chickering DE, Jacob JS. Mucoadhesive drug delivery system. U.S. 6197346, 2001.
- Keith S. Advances in psychotropic formulations. Prog Neuropsychopharmacol Biol Psychiatry 2006;30:996-1008.
- Holliday SM, Benfield P. Venlafaxine: A review of its pharmacology and therapeutic potential in depression. Drugs 1995;49:280-94.
- Makhija SM, Vavia PR. Once dailys ustained release tablets of venlafaxine, a novel antidepressant. Eur J Pharm Biopharm 2002;54:9-15.
- Punitha S, Girish Y. Polymers in mucoadhesivebuccal drug delivery system-A review. Int J Res Pharm Sci 2010;1:170-86.
- Chowdary KP, Srinivasa RY. Mucoadhesive microspheres and microcapsules: Current status. Indian J Pharm Sci 2005;67:141-50.
- Bindu MB, Nath AR, Banji D, Ramalingam R, Madhu NM, Arjun G, et al.: Formulation and evaluation of venlafaxine HCl enclosed in alginate microbeads prepared by iontophoretic gelation method. Int J Pharm Res Dev 2009;8:1-11.
- Steven TM. Pharmacokinetics of once-daily venlafaxine extended release in healthy volunteers. Cur Ther Res 1997;58:504-14.
- Simona JS, Aguiar LM. Extended-release venlafaxine in relapse prevention for patients with major depressive disorders. J Psych Res 2004;38:249-57.
- Singh B, Dahiya M, Saharan V, Ahuja N. Optimizing drug delivery systems using systematic ?design of experiments.? Part II: retrospect and prospects. Crit Rev Ther Drug Carrier Syst 2005;22:215-94.
- Singh B, Kumar R, Ahuja N. Optimizing drug delivery systems using systematic ?design of experiments.? Part I: fundamental aspects. Crit Rev Ther Drug Carrier Syst 2005;22:215-94.
- Singh B, Ahuja N. Development of controlled release buccoadhesive hydrophilic matrices of diltiazem hydrochloride: Optimization of bioadhession, dissolution and diffusion parameters. Drug Dev Ind Pharm 2002;28:431-42.
- Gohel MC, Amin AF. Formulation design and optimization of modified-release microspheres of diclofenac sodium. Drug Dev Ind Pharm 1999;25:247-51.
- Kapil R, Dhawan S, Beg S, Singh B. Buccoadhesive films for once-a-day administration of rivastigmine: Systematic formulation development and pharmacokinetic evaluation. Drug Dev Ind Pharm 2012;39:466-80.
- Sharma HK, Pradhan SP, Sarangi B. Preparation and in vitro evaluation of enteric controlled release pantoprazole loaded microbeads using natural mucoadhesive substance from Dillenia indica. Int J Pharm Tech Res 2010;2:542-51.
- Rahman Z, Kohli K, Zhang SQ, Khar RK, Ali M, Charoo NA, et al. In vivo evaluation in rats of colon-specific microspheres containing 5-fluorouracil. J Pharm Pharmacol 2008;60:615-23.
- Swain S, Behera U, Beg S, Sruti J, Patro CN, Dinda SC, et al. Design and characterization of enteric-coated controlled release mucoadhesive microcapsules of rabeprazole sodium. Drug Dev Ind Pharm 2012;39:548-60.
- Swain S, Patro CN, Sruti J, Rao ME. Design and evaluation of sustained release solid dispersions of verapamil hydrochloride. Int J Pharm Sci Nanotech 2011;3:1252-62.
- Loyd A, Nicholas GP. Pharmaceutical dosages forms and drug delivery systems, powders and granules. 9th ed. New Delhi: Wolters Kluwer/ Lippincott Williams and Wilkins; 2011. p. 184-202.
- Prajapati SK, Tripathi P, Ubaidulla U, Anand V. Design and development of gliclazide mucoadhesive microcapsules: In vitro and in vivo evaluation. AAPS PharmSci Tech 2008;9:224-30.
- Emmerichs N, Wingender J, Flemming HC, Mayer C. Interaction between alginates and manganese cations: Identification of preferred cation binding sites. Int J Biol Macromol 2004;34:73-9.
- Tao Y, Lu Y, Yinjing S, Gu B, Lu W, Pan J. Development of mucoadhesive microspheres of acyclovir with enhanced bioavailability. Int J Pharm 2009;378:30-6.
- Albertini B, Passerini N, Sabatino MD, Vitali B, Brigidi P, Rodrigueza L. Polymer?lipid based mucoadhesive microspheres prepared by spray-congealing for the vaginal delivery of econazole nitrate. Eur J Pharm Sci 2009;36:591-601.
 1 h
1 h  2 h
2 h  3 h
3 h  4 h
4 h  5 h
5 h  6 h and (b) phosphate
buffer pH 7.4.
6 h and (b) phosphate
buffer pH 7.4.  1 h
1 h  2 h
2 h  3 h
3 h  4 h
4 h  5 h
5 h  6 h.
6 h.
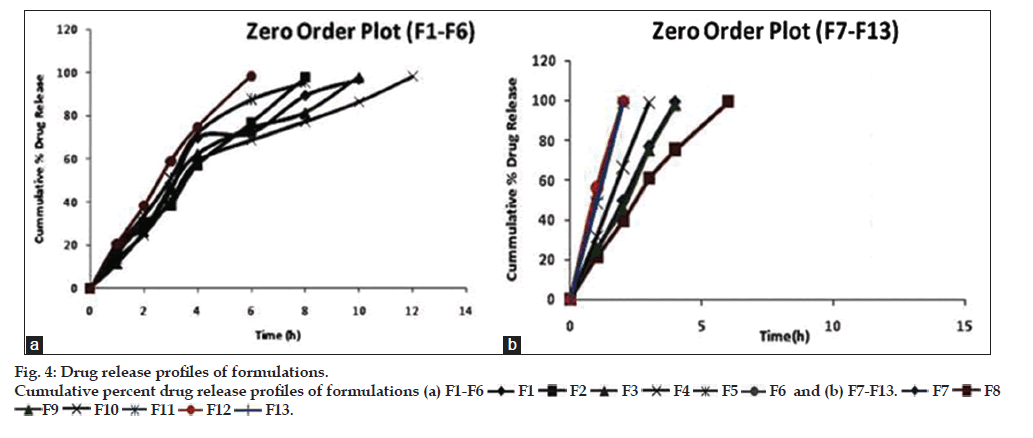
 F1
F1  F2
F2  F3
F3  F4
F4  F5
F5  F6 and (b) F7-F13.
F6 and (b) F7-F13.  F12
F12  F13.
F13.
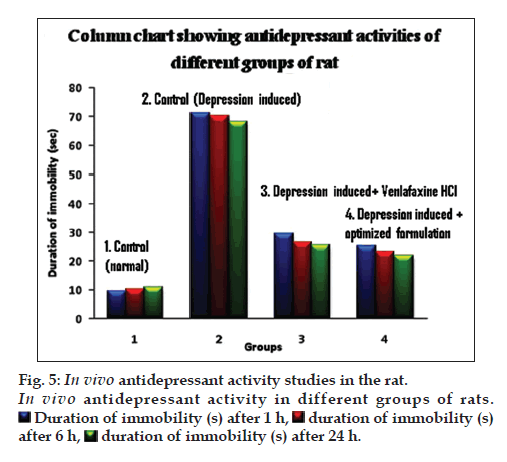
 Duration of immobility (s) after 1 h,
Duration of immobility (s) after 1 h,  duration of immobility (s)
after 6 h,
duration of immobility (s)
after 6 h,  duration of immobility (s) after 24 h.
duration of immobility (s) after 24 h.