- *Corresponding Author:
- Seema Thakral
G. V. M. College of Pharmacy, Murthal Road, Sonepat-131 001, India
E-mail: seemathakral@rediffmail.com
| Date of Submission | 28 February 2005 |
| Date of Revision | 23 August 2005 |
| Date of Acceptance | 2 February 2006 |
| Indian J Pharm Sci, 2006, 68 (1): 13-19 |
Abstract
Ever since their experimental discovery in 1985, fullerenes have attracted considerable attention in different fields of sciences. Investigations of chemical, physical and biological properties of fullerenes have yielded promising information. Their unique carbon cage structure coupled with immense scope for derivatization makes fullerenes a potential therapeutic agent. Henceforth various potential therapeutic applications of fullerenes have been reviewed in the present paper. These include antiHIV- protease activity, photodynamic DNA cleavage, free radical scavenger, antimicrobial action and use of fullerenes as diagnostic agents.
Carbon, the common element in organic compounds, is known to exist in two allotropic forms, viz, diamond and graphite. In 1985, a third form of carbon called fullerenes was discovered [1]. The group of scientists led by Smalley, Kroto and Curl attempted to simulate the conditions under which carbon nucleates in the atmosphere of red giant stars. In the experiment, a surface of solid graphite was vaporized by irradiation with the laser into plasma containing atoms and free ions. The free atoms and ions were chilled down due to the collision with the helium atoms. Through the collision, clusters containing various numbers of carbon atoms were formed. The clusters were examined in a mass spectrometer, and it was found that clusters that had 60 and 70 carbon atoms dominated, and that most clusters had 60 carbon atoms. In the beginning, the scientists had problem in producing sufficient amount of fullerenes. They had succeeded to prepare only less than 10-15 g. But after 5 years, Kratschmer and Huffmann [2], and then Kroto et al [3] developed new high yielding preparative methods for fullerenes.
These scientists named the newly found molecule after the architect Richard Buckminster Fuller, who created the dome in 1967 with the same shape as the carbon cluster. Fullerenes generated so much interest and excitement among research scientists that the three scientists who discovered fullerenes [1] received Nobel Prize in Chemistry in 1996. Fullerenes were later found to exist naturally in interstellar dust as well as in geological formations on Earth, though only in the ppm-range. Some of these places are Shunga/Russia [4], New Zealand [5] and Sudbury/ Canada [6].
Structure and Properties
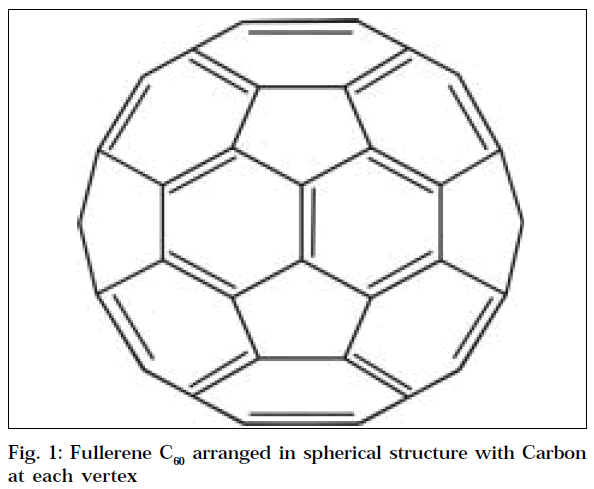
Fullerenes are large carbon cage molecules (Fig.1) considered to be three-dimensional analogues of benzene. The most abundant form of fullerenes is Buckminster fullerene (C60) with 60 carbon atoms arranged in a spherical structure. The shape of the molecule, known as truncated icosahedron [2], resembles that of a soccer ball, which contains 12 pentagons and 20 hexagons. Fullerenes fulfill the EULER’s theorem, i.e., if a polyeder is to build a closed structure from pentagons and hexagons; it has to contain exact 12 pentagons. Following this rule, the smallest stable fullerene is C60, which has no two pentagons side by side, making it the most stable structure.
An important property of C60 molecule is its high symmetry. There are 120 symmetrical operations, like rotation around the axis and reflection in a plane, which map the molecule onto itself. This makes C60 the most symmetrical molecule [7]. There are two types of bond lengths in the fullerene: C5-C5 single bonds in the pentagons and C5-C6 double bonds in the hexagons [8]; the first is 1.45±0.015 Å and the other one is 1.40±0.015 Å. Each carbon atom forms bond to three other adjacent atoms with sp [2] hybridization. The set of orbitals is arranged at 120-degrees angles and is centred in the xy-plane. Hence these delocalized pi electrons stabilize the spheroid structure by resonance [9].
A C60 molecule, also known as Buckyball or Buckminsterfullerene, is about 7 Å in diameter. C60 molecules condense to form a solid of weakly bound molecules [10]. This crystalline state is called fullerites. This solid is cubic, weakly bound [11] with a lattice constant a=14.71 Å and electrically insulating. It occurs as yellow powder, which turns pink when dissolved in toluene. On exposure to strong UV light, the Buckyballs polymerize, forming bonds between adjacent balls. In the polymerized state, C60 no longer dissolves in toluene [12]. NMR studies of C60 benzene solvates show free rotation at room temperature [13]. At about -13°, the balls spin freely in their crystalline positions [14]. At lower temperature, their movements begin to limit to certain orientations. Eventually, below -183°, the balls become completely struck [15]. Chemically the molecule is quite stable; breaking the balls requires temperature of over 1000°. By heating fullerenes up to 1500° in absence of air, they transform to graphite.
Other than C60, fullerenes can contain between 30 to 980 carbon atoms forming different structures with different properties and field of applications. As hexagons are added or removed from the basic soccer ball structure, the molecule begins to lose its roundness. C70, which has 25 hexagons, is shaped more like a rugby ball. Giant fullerenes take on a pentagonal shape. Smaller fullerenes look like asteroids. With the loss of roundness comes a loss of stability, C60 being the most stable of all. Other than C60 and C70, C76, C78, C84, C86 have been isolated and studied in detail. Fullerenes have low density relative to diamond (1.65 g/cc compared to 3.51 g/cc). Fullerenes are unstable in water. Good solvents for fullerenes are carbon disulphide, o-dichlorobenzene, toluene and xylene [16-18]. Thin layers of fullerenes are coloured from yellow to yellowish-green. The colour of fullerene solutions is attributed to the pi-pi electron transition (Table 1).
| Fullerenes | Molar Mass (g/mol) | Colour of the solution |
|---|---|---|
| C60 | 720.6 | Purple/violet |
| C70 | 840.7 | Brick red |
| C76 | 912.76 | Light yellow green |
| C84 | 1008.84 | Brown |
| C86 | 1032.86 | Olive-green |
Table 1: Fullerenes and Colour Of Their Solutions
Species Of Fullerenes
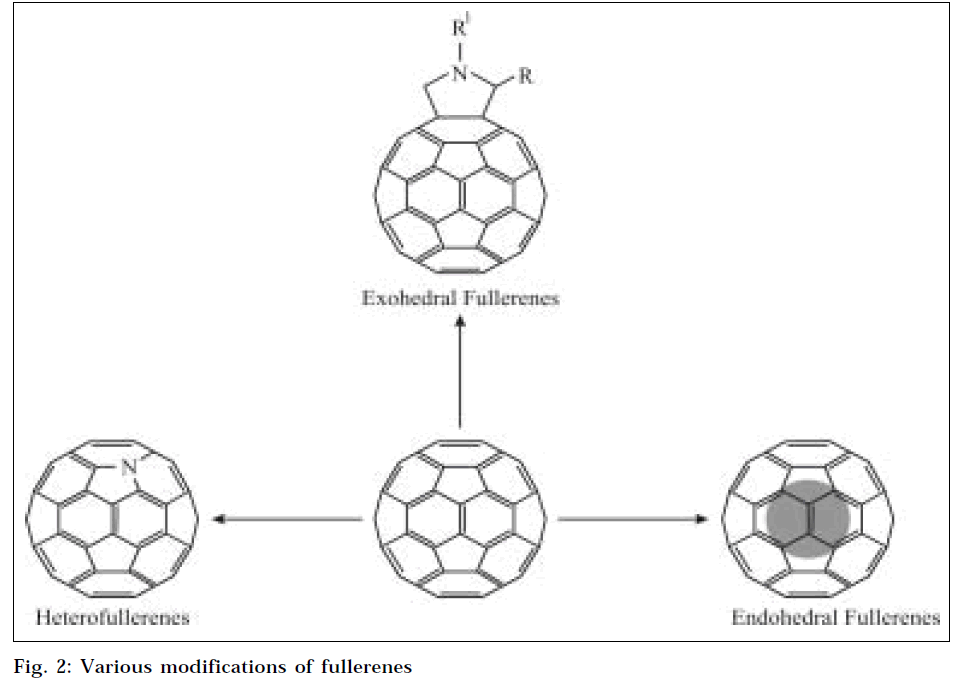
Some time after discovery of fullerenes, many chemical modifications of fullerenes were discovered (Fig. 2). Some of the important fullerene species are as follows.
Alkali-doped fullerenes
As fullerene molecule is highly electronegative, it readily forms compounds with electron donating atoms, the most common examples being alkali metals [19]. This reaction leads to production of an interesting class of compounds known as alkali-doped fullerides, wherein alkali metal atoms fill in the space between Buckyballs and donate valence electron to the neighbouring C60 molecule. If alkali atoms are potassium or rubidium, the compounds are superconductors, and they conduct electric current without any resistance at temperatures below 20-40 K [20], e.g., K3C60, Rb3C60.
Endohedral fullerenes
Since fullerenes are hollow with a closed shell of carbon atoms, it is possible to enclose another atom inside. This class of fullerene derivatives is known as Endohedral fullerenes. When the atom trapped inside is a metal, they are known as metallofullerenes [21]. Even though C60 is the most common fullerene, few endohedral materials have been synthesized using C60 as cage molecule, the reason being small size of the molecule. Most of endohedral materials are made out of C82, C84 or even higher fullerenes. The atoms that form stable endohedral compounds include lanthanum, yttrium, scandium, and some of the noble gases [22]. As it is very difficult to open up carbon cage molecules to enclose a foreign atom inside, endohedral material must be synthesized while formation of the cage itself [23]. (The accepted notation for endohedral material is to use the @ symbol to show that the first material is inside the second, e.g., La@C82 and Sc2@C84.)
Exohedral fullerenes
The most important and most versatile of all species of fullerenes is Exohedral fullerenes or fullerene derivatives, which are molecules formed by a chemical reaction between fullerenes and other chemical groups. Fullerene derivatives are also known as functionalized fullerenes. As fullerenes possess the conjugated π-system of electron, two main types of primary chemical transformations are possible on fullerene surface: addition reactions and redox reactions, which lead to covalent exohedral adducts and salts respectively [24]. As fullerenes are insoluble in water, numerous derivatives of fullerenes have been synthesized with improved solubility profile.
Heterofullerenes
Heterofullerenes represent another fundamental group of modified fullerenes. They represent heteroanalogues of C60 and higher fullerenes; one or more carbon atoms of the cage are substituted by hetero-atoms, e.g., trivalent nitrogen or boron atom [25]. The simplest derivative of nitrogen fullerene is aza (60) fullerene C59N. and its dimer (C59N)2.
Another significant spin-off product of fullerene research is carbon nanotubes based on carbon or other elements [26,27]. These systems consist of graphitic sheets seamlessly wrapped into cylinders. The diameter of nanotubes range from 0.4 to >3 nm for Single-Walled NanoTubes (SWNT) to 1.4 to at least 100 nm for Multi- Walled NanoTubes (MWNT) [28,29]. Also nanotubes are quite stiff and exceptionally strong, having high tensile strength. The calculated Young’s Modulus for an individual nanotube is ≈ 0.64TPa [30]. This unique combination of diameter and strength is potentially useful in everything from tennis rackets to space travel.
Biological Applications of Fullerenes
Fullerenes are inert, hollow and indefinitely modifiable. When administered orally in the water-soluble form, they are not absorbed; while on i.v. injection, they get rapidly distributed to various body tissues. They are excreted unchanged by kidney [31]. Acute toxicity of water-soluble fullerenes was found to be quite low [32]. All these interesting properties offer possibilities of utilizing fullerenes in biology and medicinal chemistry and promise a bright future for fullerenes as medicinal agents. However, this possibility faces a significant problem, i.e., natural repulsion of fullerenes to water. To overcome this limitation, a number of methodologies are being developed. These include synthesis of fullerene derivatives having modified solubility profile, encapsulation of C60 in cyclodextrins [33] or in calixarenes [34] or water suspension preparations [35].
Many derivatives of fullerenes have been synthesized. There have been many patents written on fullerenes, and fullerene patent database is growing rapidly. Fullerenes and their derivatives are being intensively investigated as they exhibited promising preliminary activities in the following medical streams.
Diagnostic applications
Endohedral metallofullerenes are the fullerenes with metal ion trapped inside fullerene cage. These have shown potential applications in diagnostics. As for example, water solublised forms like M@C82(OH)30 are being used as Magnetic Resonance Imaging contrast agents (M=Gd3+)36, X-ray contrast agents (M=Ho3+)37 and radiopharmaceuticals (M=166Ho3+and 170Tm2+) [38,39]. One of these derivatives, i.e., 166Ho3+@C82(OH)30 has been extensively studied as radioactive tracer for imaging of diseased organs and for killing cancerous tumours. The radioactive metal is trapped inside the carbon shell, which is very stable and resistant to metabolism by body. The metallofullerenes have been found to be non-toxic, and they stay in the body for approximately one hour, allowing imaging of circulatory system.
AntiHIV activity
The enzyme protease specific to HIV-1 has been shown to be a viable target for antiviral therapy. The active site of this enzyme can be roughly described as an open-ended cylinder lined almost exclusively by hydrophobic amino acids except for two catalytic aspartic acids. Wudl et al hypothesized that since C60 molecule has approximately same radius as the cylinder that describes the active site of HIV-P, an opportunity exists for a strong hydrophobic interaction between C60 derivatives and the active site surfaces. Inhibition of HIV-P in presence of C60 was demonstrated through molecular modelling studies and experimental observations [40]. Virus inactivation assays confirmed the activity of fullerene derivatives against HIV-1 and HIV-2 [41,42]. A water-soluble derivative was found to be active against HIV-1 and HIV- 2 (EC50 approximately 6 μM) in acute and chronic infected human lymphocytes and also noncytotoxic up to 100 μM in peripheral mononuclear cells and H9, Vero and CEM cells [43].
As the binding constant found experimentally was not significant in terms of affinity [40], structural optimization of C60 derivatives for HIV-P interaction was investigated. On this basis, some authors proposed ideal inhibitors, in which two ammonium groups at distance of 5.5 Å or 5.1 Å are directly linked to C60. Molecular modelling studies on these derivatives showed that they could fit well inside the HIV-P cavity and can interact with carboxylic residues of aspartatic acid [44].
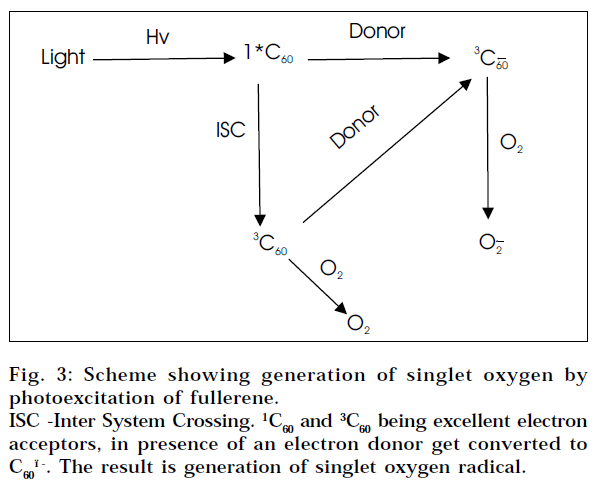
DNA photocleavage
A water-soluble fullerene carboxylic derivative was found to be cytotoxic when exposed to visible light. In an initial experiment, C60 carboxylic acid was incubated with cultured cells for three days. No biological activity was observed. However on exposure to low power visible light, the compound killed the cells. It was later shown that cytotoxicity of C60 derivatives was mediated by its ability to cleave DNA [45]. Hence other potential application of fullerenes is related to the easy photoexcitation of fullerenes [46] (Fig. 3) on the basis of which fullerene-based photodynamic compounds are being developed for the treatment of cancer.
In this field, many fullerene conjugates with different units possessing biological affinity to nucleic acids or proteins are being investigated for anticancer activity. In particular, conjugate between C60 and specific agents that interact with nucleic acids, such as acridine [47] or complementary oligonucleotide [46,48], have been synthesized with the objective of increasing both cytotoxicity and sequence selectivity. However, DNA cleavage was found to occur at guanine residue without any significant sequence selectivity [45]. A C60-PEG conjugate irradiated by light strongly induced tumour necrosis without any damage to the overlying normal tissue, with complete cure achieved by a C60-PEG dose of 0.424 mg/kg and irradiation power of 1011 J/m2, making it an excellent candidate for photodynamic tumour therapy [49]. Cytotoxicity of dendritic C60 monoadduct and malonic acid C60 trisadduct was recently investigated on Jurkat cells, and on exposure to UV light, the cell number was found to reduce to approximately 19% within two weeks [50].
Free radical scavenger
Free radicals have been linked to onset and progression of foetal neurogenerative diseases like amytrophic lateral sclerosis, commonly known as Lou Gehrig’s disease. Fullerene compounds with their unique cage structure, combined with high number of conjugated double bonds (30) in the core, interact with biomolecules and have avid reactivity with free radicals. Buckminster fullerenes, for example, are capable of adding multiple radicals per molecule; the addition of as many as 34 methyl radicals to a single C60 sphere has been reported. Hence they are regarded as ‘‘free radical sponge’’[51]. However to improve native solubility profile of fullerenes, several fullerene derivatives have been prepared, and these are reported to possess protective effect in various systems. A water-soluble C60 derivative, fullerenol, has been shown to scavenge free radicals in vitro [52] and in vivo [53]. Fullerenol has been shown to block hydrogen peroxide-induced inhibition of population spikes in rat hippocampus [53]. Carboxy-fullerene, another water-soluble derivative of fullerene, has been shown to prevent apoptotic injury evoked by N-methyl D-aspartate (NMDA) without interfering with NMDA-induced Ca2+ influx in cortical cell cultures [54] and has been found to have effective neuroprotective antioxidant activity in vitro and in vivo (hundreds of times more powerful than Vitamin E). As a free radical scavenger, the protective effect of fullerene derivatives has been demonstrated in various systems, including reduced injury on ischemia reperfusion intestine [55], protecting cell types from undergoing apoptosis [56,57], reduced free radical level in organ perfusate [58], and having neuroprotective effect [59,60].
Additional factors that fullerene derivatives have shown promising results are in pretoxicity studies and the fact that water as wall as lipid soluble derivatives are possible greatly increases their usefulness as antioxidant in health and personal care products, e.g., skin creams, burn creams and nutritional supplements.
Antimicrobial activity
Fullerenes were anticipated to possess antibacterial activity based on the hypothesis that C60 could produce membrane disruption by insertion into phospholipids bilayers [61]. The resulting membrane disorder, causing altered permeability, leads to the release of metabolites and cell death. As for example, monomethoxy triethylene glycol (mTEG) substituted fulleropyrollidines showed complete inhibition of Mycobacterium avium at dose 260 μg/ml and M. tuberculosis at dose ≅ 50 μg/ ml [62]. The plausible explanation for the antitubercular activity has been proposed that presence of carbon cage destabilizes the tubercular cell wall by intercalation in the hydrophobic part. The affinity of carbon cage for the hydrophobic constituents of Mycobacterium cell wall, i.e., ceramide has been further proved by administration of intraperitoneal injection of mTEG fullerene derivative to mice. The derivative was found to be deposited on Tyson’s gland, which is again a tissue rich in ceramide [63].
Antimicrobial activity of another fullerene derivative, i.e., carboxyfullerenes has been intensively investigated. Carboxyfullerenes were found to inhibit E. coli-induced meningitis by reducing the damage caused by infiltrating neutrophils on the blood brain barrier but not by direct inhibition of E. coli [64]. Moreover, it was found that the carboxyfullerenes inhibited the growth of Streptococcus pyrogens strain A-20 (MIC 5-50 mg/l) [61]. Since these derivatives exhibited varying effects on Gram+ve and Gram-ve bacteria, further investigations led to the observations that carboxyfullerenes can insert into cell wall of Gram+ve cocci, disrupt the cell wall structure and cause bacterial death. The cell wall of Gram-ve organisms, having an outer membrane consisting of lipoproteins, lipopolysachharides and phospholipids, is not susceptible to access by fullerenes [65]. This suggests that carboxyfullerenes could be considered as new antimicrobial agents against Gram+ve cocci.
Osteoporosis
To date, biphosphonate compounds and fluoride anions are the drugs used in the treatment of osteoporosis and other bone disorders. However, the former are not absorbed orally and latter are fairly toxic. Advantage is being taken of the preferential localization of fullerene derivatives in bones. Hence polyfluoro biphosphonated fullerene derivatives are being developed as bimodal drug for osteoporosis therapy [66].
However, the use of fullerenes as biological or pharmacological agents requires the measurement of dosage and serum levels by sensitive and simple immunological procedures. This in turn requires that specific antibodies to fullerenes should be available. The requirement led to the question of the ability of these carbon-made spherical structures to induce the production of specific antibodies. However, it was determined that immunization of mice with a water-soluble C60 fullerene derivative conjugated to bovine thyroglobulin yielded a population of fullerene-specific antibodies of the IgG isotype, showing that the immune system was diverse enough to recognize and process fullerenes as protein conjugates [67]. A monoclonal fullerene-specific antibody has been isolated and the sequences of its light and heavy chains have been reported [68]. These findings further substantiate the practical utility of fullerenes as therapeutic agents, diagnostic agents and as imaging vectors.
Conclusions
The exponential increase in patent filing and publications indicate growing industrial interest that parallels academic interest. The discovery of fullerenes has been compared to the discovery of benzene by many researches. The hydrophobic spheroid structure and radical sponge character of the fullerene molecule is responsible for activities in many fields. In addition to some of the important medical applications as mentioned above, fullerenes are being intensively investigated for practical utilization in a number of other fields, e.g., as room temperature superconductors, as component of solar batteries, for production of synthetic diamonds and as lubricants.
However, there are two important facts that are considered to be barriers to fullerene applications. The first one is relative insolubility and instability of fullerenes to water. This however is being successfully overcome with the development of numerous water-soluble fullerene derivatives. The second and more important limitation is the high cost of fullerenes. Compared to gold that is available at the cost of $10/g, the cost of fullerenes, as listed on web, varies from $45 to $1000/g, depending on degree of purification. This high cost, which is mainly attributed to very high temperature involved in the production process, is restricting the numerous potential applications of fullerenes. One possible development that may take place in near future is creation of modest demand of fullerenes in medicine, as medicine is considered to be the cost-insensitive application. This in turn will encourage larger production leading to lower prices and still more demand. The preliminary biological investigations of fullerene derivatives as discussed above do promise encouraging results. Hence research efforts in both chemistry and biology should be to further investigate the potential applications of these interesting spheroidal molecules.
References
- Kroto, H.W., Heath, J.R., O’Brein, S.C., Curl, R.F. and Smalley, R.E., Nature, 1985, 318, 162.
- Kratschmer, W., Lamb, L.D. and Hoffman, D.R., Nature, 1990, 347, 354.
- Kroto, H.W., Allaf, A.W. and Balm,. S.P., Chem. Rev., 1991, 91, 1231.
- Busek, P.R., Tsipursky, S.J. and Hettich, R., Science, 1992, 257, 215.
- Heymann, D., Chibante, L.P.F., Brooks, R.R., Wolbach, W.S. and Smalley R.E., Science, 1994, 265, 645.
- Becker, L., Bada, J.L., Winans, R.E., Hunt, J.E., Bunch, T.E. and French, B.M., Science, 1994, 265, 642.
- Taylor, R., Hare, J.P., Abdul-Sada, A.K. and Kroto, H.W., J. Chem.Soc. Chem. Commun., 1990, 1423.
- Hawkins, J.M., Meyor, A., Lewis, L.A., Loren, S. and Hollander, P.J., Science, 1991, 252, 312.
- Haddon, R.C., Brus, L.E. and Raghavachari, K., Chem. Phys. Lett., 1986, 131, 165.
- Balch, A.L., Catalano, V.J., Leen, J.W., Olmstead, M.M and Parkin, S.R., J. Amer. Chem. Soc., 1991, 113, 8953.
- Heiney, P.A., Fischer, J.E., Mcghie, A.R., Romanow, W.J., Denestein, A.M., McCauley, J.P. and Smith, A.B., Phys. Rev. Lett., 1991, 66, 2911.
- Kolodzeijski, W. and Kilnowski, J., Chem. Phys. Lett., 1995, 247, 507.
- He, H.Y., Barras, J., Foulker, J. and Klinowski, J., J. Chem. Phys., 1997, B101, 117.
- He, H.Y., Teixeira-Dias, J., Foulkes, J. and Klinowski, J., Phys.Chem. Chem. Phys., 2000, 2, 2651.
- Meidine, M.F., Hitchcock, P.B., Kroto, H.W., Taylor, R. and Walton, D.R.M., J. Chem. Soc. Chem. Commun., 1992, 1534.
- Sivaraman, N., Dhamodaran, R., Kalippan, I., Srinivasan ,T.G., Vasudeva Rao, P.R. and Mathews, C.K., Fullerene Sci. andTech., 1994, 2, 233.
- Sivaraman, N., Dhamodaran, R., Kalippan, I., Srinivasan, T.G., Vasudeva Rao, P.R. and Mathews, C.K., J. Org. Chem., 1992, 57, 6007.
- Rouff, R.S., Tse, D.S., Malhotra, R. and Lorents, D.C., J. Phys.Chem., 1993, 97, 3379.
- Haddon, R.C., Hebard, A.F., Rosseinsky, M.J., Murphy, D.W., Ducloss, J. and Lyons, K.B., Nature, 1991, 350, 320.
- Hebard, A.F., Rosseinsky, M.J., Haddon, R.C., Murphy, D.W., Glarum, S.H., Palstra, T.T.M., Ramirez, A.P. and Kortan, A. R., Nature, 1991, 350, 600.
- Saunders, M., Jimenz-Vazquez, H.A., Cross, R.J., Mroczkowski, S., Gilbin, D.E., and Poreda, R.J., J. Amer. Chem. Soc., 1994, 116, 2193.
- Saunders, M., Cross, R.J., Jimenz-Vazquez, H.A., Shimshi, R. and Khong, A., Science,1996,271,1693.
- Lee, H.M., Olmstead, M.M., Suetsuna, T., Shimotani, H., Dragoe, N. and Cross, R.J., Chem. Commun., 2002, 2002, 1352.
- Schick, G., Hirsch, A., Mauser, H. and Clark, T., Chem. Eur. J., 1996, 2, 935.
- Hirsch, A. and Nuber, B., Acc. Chem. Res., 1999, 32, 795.
- Iijima, S., Nature, 1991, 354, 56.
- Ebbesen, T.W. and Ajayan, P.M., Nature, 1992, 358, 220.
- Wang, N., Tang, Z.K., Li, G.D. and Li, J.S., Nature, 2000, 408, 50.
- Ding, R.G., Lu,G.Q.,Yan, Z.F. and Wilson, M.A., J. Nanosci.Nanotech.,2001, 1, 7.
- Gao, G., Cagin, T. and Goddard, W.A., Nanotech.,1998, 9, 184.
- Wilson, L.J., Science News, 1999, 155, 292.
- Yamago, S., Tokuyama, H., Nakamura, E., Kikuchi, K., Kananishi, S., Sueki, K., Nakahara, H., Enomoto, S. and Ambe, F., Chem. Biol., 1995, 2, 385.
- Fillipone, S., Hiemarn, F. and Ransat, A., Chem. Commun., 2002, 14, 1508.
- Shinkai, S. and Ikeda, A., Pure Appl. Chem., 1999, 71, 275.
- Scrivens, W.A., Tour, J.M., Creek, K.E. and Pirisi, L., J. Amer.Chem. Soc., 1994, 116, 4517.
- Wharton, T., Alford, J.M., Husebo, L.O. and Wilson, L.J., In; Kadish, K.M. and Rouff, R.S., Eds., Recent Advances in Chemistry and Physics of Fullerenes and Related Materials, 9th Edn., The Electrochemical Society, Pennington, NJ, 2000, 258.
- Wilson, L.J., Interface, 1999, 8, 24.
- Cagle, D.W., Kennel, S.J., Mirzadeh, S., Alford, J.M. and Wilson, L.J., Proc. Natl. Acad. Sci. USA, 1993, 96, 5182.
- Ehrhardt, G.J. and Wilson, L.J., In; Braun, T., Eds., Nuclear and Radiation Chemical Approaches to Fullerene Science, Kluwer Academic Publishers, Dordrecht, Netherlands, 2000, 174.
- Friedman, S.H., DeCamp, D.L., Sijbesma, R.P., Srdanov, G., Wudl, F. and Kenyon, G.L., J. Amer. Chem. Soc., 1993, 115, 6503.
- Sijbesma, R., Srdanov, G., Wudl, F., Castoro, J.A., Wilkins, C., Friedman, S.H., DeCamp, D.L. and Kenyon, G.L., J. Amer. Chem.Soc., 1993, 115, 6510.
- Friedman, S.H., Ganapati, P.S., Rubin, Y. and Kenyon, G.L., J. Med.Chem., 1998, 41, 2424.
- Schinazi, R.F., Sijbesma, R., Srdanov, G., Hill, C.L., and Wudl, F., Antimicrob Agents Chemother.,1993, 37, 1707.
- Marcorin, G., Ros, T. Da, Castellano, S., Stefancich, G., Borin, I., Miertus, S. and Prato, M., Org. Lett., 2000, 2, 3955.
- Tokuyama, H., Yamago, S., Nakamura, E., Shiraki, T. and Sugiura, Y., J. Amer. Chem. Soc., 1993, 115, 7918.
- An, Y.-Z., Chen, C.-HB, Anderson, J.L., Sigman, D.S., Foote, C.S., and Rubin, Y., Tetrahedron, 1996, 52, 5179.
- Yamakoshi, Y., Yagami, T., Sueyoshi, S., and Miyata, N., J. Org.Chem., 1996, 61, 7236.
- Boutorine, A.S., Tokuyama, H., Takasugi, M., Isobe, H., Nakamura, E., and Helene, C., Angew. Chem. Int. Ed. Engl., 1994, 33, 2462.
- Tabata, Y., Murakami, Y., and Ikada, Y., Jpn. J. Cancer Res., 1997, 88, 1108.
- Rancan, F., Rosan, S., Boehm, F., Cantrell, A., Brellreich, M., Schoenberger, H., Hirsch, A. and Moussa, F., J. Photochem.Photobiol.B, 2002, 67, 157.
- Kusic, P.J., Wasserman, E., Keizer, P.N., Morton, J.R. and Preston, K.F., Science, 1991, 254, 1183.
- Chiang, L.Y., Lu, F.J., and Lin, J.T., J. Chem. Soc. Chem.Commun., 1995, 1283.
- Tsai, M.C., Chen, Y.H. and Chiang, L.Y., J. Pharm. Pharmacol., 1997, 49, 438.
- Dugan, L.L., Gabrielesen, J.K., Yu, S.P., Lin, T.S., and Choi, D.W., Neurobiol.Dis., 1996, 3, 129.
- Lai, H.S., Chen, W.J. and Chiang, L.Y., World J. Surgery, 2000, 24, 450.
- Hsu, S.C., Wu, C.C., Lun, T.Y., Chou, C.K. and Hans, S.H., Blood, 1998, 91, 2658.
- Bisaglia, M., Natalini, B., Pellicciari, R., Straface, E., Manlorni, W. and Monti, D., J. Neurochem., 2000, 74, 1197.
- Cheuh, S.C., Lai, M.K., Lee, M.S., Chiang, L.Y., Ho, Ti. and Chen, S.C., Transplantation Proceedings, 1999, 31, 1976.
- Dugan, L.L., Turetsky, D.M., Du, C., Lobner, D., Wheeler, M., Almli, C.R., Shen, C. K. F., Luh, T.Y., Choi, D.W. and Lin, T.S., Proc. Natl.Acad. Sci. USA, 1997, 94, 9434.
- Lin, A.M.Y., Chyi, B.Y., Wang, S.D., Yu, H.H., Kanakamma, P.P. and Luh, T.Y.,et al,J. Neurochem., 1999, 72, 1634.
- Tsao, N., Luh, T.Y., Chou, C.K., Wu, J.J., Lin, Y.S., and Lei, H.Y., Antimicrob. Agents Chemother.,2001, 45, 1788.
- Ros, T. Da, Spalluto, G., and Prato, M., Croatia ChemicaActa, 2001, 74, 743.
- Maggini, M., Martin, N. and Guldi, D.M., Fullerenes 2000: Funtionalized Fullerenes. 1st Ed. The Electrochemical Soc. Inc., Pennington NJ. 2000, 9, 240.
- Tsao, N., Kanakama, P.P., Luh, T.Y., Chou, C.K. and Lei, H.Y., Antimicrob. Agents Chemother.,1999, 43, 2273.
- Tsao, N., Luh, T.Y., Chou, C.K., Chang, T.Y., Wu, J.J., Liu, C.C., and Hie, H.Y.J., Antimicrob. Agents Chemother., 2002, 49, 641.
- Gonzalez, K.A., Wilson, L.J., Wenju, W.U. and Nancollas, C.H., Biorg.Med. Chem., 2002, 16, 1991.
- Chen, B.X., Wilson, S.R., Das, M., Coughlin, D.J. and Erlange, B.F., Proc. Natl. Acad. Sci., 1998,95, 10809.
- Braden, B.C., Goldbaum, F.A., Chen, B.-X., Kirschner, A.N., Wilson, S.R., and Erlanger, B.F., Proc. Natl. Acad. Sci., 2000, 97, 12193.