- Corresponding Author:
- Dawn W Foster
Psychiatry Department at Yale Schol of Medicine Yale University, New Haven, CT 06519, USA
Tel203 974 7892
E-mailvmhiremath2004@yahoo.com
| Date of Submission | 5 November 2009 |
| Date of Revision | 19 April 2011 |
| Date of Acceptance | 24 April 2011 |
| Indian J Pharm Sci, 2011, 73 (2): 193-198 |
Abstract
The present study was designed to compare the curative role of proton pump inhibitors, omeprazole, rabeprazole and lansoprazole against dexamethasone-induced ulcer model. Dexamethasone (5 mg/kg/day) was used as an ulcerogen. Dexamethasone suspended in 1% CMC in water was given orally to all rats. Omeprazole (20 mg/ kg), rabeprazole (20 mg/kg), and lansoprazole (20 mg/kg) were administered by oral route 30 minutes prior to dexamethasone for ulcer protective studies, gastric secretion and mucosal studies. Effects of proton pump inhibitors were determined by the evaluation of various biochemical parameters such as estimation of myeloperoxidase, cortisol, alkaline phosphatase, malondialdehyde, endogenous anti-oxidants like superoxide dismutase, catalase and reduced glutathione. In dexamethasone induced ulcer model, omeprazole showed significant decrease in malondialdehyde, myeloperoxidase, alkaline phosphatase level and increase in superoxide dismutase, catalase and reduced glutathione level as compared to rabeprazole and lansoprazole. Omeprazole showed significant reduction in cortisol content where as rabeprazole and lansoprazole did not show significant changes as compared to control. The result indicates that omeprazole is the most effective and selective proton pump inhibitor in dexamethasone induced ulcer model as compared to rabeprazole and lansoprazole.
Keywords
Catalase, cortisol, dexamethasone, myeloperoxidase, proton pump inhibitors, superoxide dismutase
Peptic ulcer is the most common gastrointestinal disorder in clinical practice. Acute gastric ulcers result from erosion of the mucosal membrane generally in the gastric and duodenal regions and are also thought of an outcome of alterations in the balance between mucosal damaging agents and mucosal defense mechanisms. Peptic ulcer is affecting about 5-10% of the world’s population living in different geographical regions. There are several etiological factors, which lead to ulceration of gastric and duodenal mucosa. It has been reported earlier that heavy smoking, alcohol and steroids intake may delay healing of ulcers. This could be due to increased gastric acid secretion, reduction in gastric mucosal blood flow, inhibition of duodenal bicarbonate production, prostaglandin synthesis, Helicobacter pylori infection, reduced generation of nitric oxide and increased generation of free radicals [1-4].
Ulcerogenic potential of corticosteroids is well known and believed to a result of increased gastric acid and pepsin secretion, which aggravate peptic ulcer [5]. Frequent usage of corticosteroids in the treatment of bronchial asthma, brain metastasis, cerebral edema, shock, autoimmune diseases, allergy, and inflammatory conditions like rheumatoid arthritis, osteoarthritis has increased the risk of peptic ulcer disease [6].
Corticosteroids cause gastric erosions by damaging surface epithelial cells and makes gastric mucosa susceptible to ulceration by inhibiting prostaglandin synthetase to block the gastroprotective action of prostaglandin and also by inhibiting the peroxidase, thereby elevating the endogenous H2O2 level to generate more reactive hydroxyl radical [7] and reduction in the levels of nitric oxide [8] responsible for further increase in gastric mucosal damage. Dexamethasone, which is a potent corticosteroid delays rat gastric ulcer healing by inhibition of angiogenesis in rat stomachs [9]. Dexamethasone significantly suppresses EGF-stimulated gastric epithelial cell proliferation and one of the pathways involved is via inhibiting activation of ERK1/ ERK2, followed by inhibition of COX-2, Cyclin D1 expression and DNA synthesis [10]. Corticosteroids reduce regenerative repair of epithelium in experimental gastric ulcers [11].
Omeprazole, rabeprazole, lansoprazole inhibit gastric acid secretion by blocking H+/K+ ATPase pump. Although these drugs share a common structure (all are substituted benzimidazoles) and pharmacological actions but each differs somewhat in its clinical pharmacology [12]. Therefore, the present work has been undertaken with an aim to compare different proton pump inhibitors for the treatment of dexamethasone induced gastric mucosal damage in albino rats.
Materials and Methods
Healthy Wistar adult rats of either sex weighing between 150-200 g were used. Animals were housed individually in polypropylene cages, maintained under standard conditions (25±3° and 35-60% humidity; the animals were feed with standard rat pellet diet, Hindustan Lever Ltd., Mumbai, India) and water ad libitum. The study was conducted after obtaining institutional animal ethical committee clearance (KLESCOPH/IAEC.Clear/2006-2007/06).
The chemicals and solvents used were nitroblue tetrazolium, NADH, phenazine methosulphate, sodium pyrophosphate, trichloro acetic acid, thiobarbituric acid, Hexadecyl trimethyl ammonium bromide, o-dianisidine, hydrochloric acid, concentrated sulfuric acid, absolute alcohol, hydrogen peroxide, glacial acetic acid, dichloromethane. All chemicals and solvents used were of analytical grade. Omeprazole was obtained from Cipla Ltd, Goa, India. Rabeprazole, lansoprazole and dexamethasone were obtained from Cadila health care, Ahmedabad, India.
Treatment protocol
Albino Wistar rats of either sex weighing between 150-200 g were divided into 5 groups of 6 animals each. In this method dexamethasone (5 mg/kg/day) was used to induce the gastric damage [13]. Group I normal saline (5 ml/kg). Group II served as control in which only dexamethasone (5 mg/kg/day) for 10 days was administered. Remaining groups were treated with dexamethasone (5 mg/kg/day) for 10 days followed by omeprazole (20 mg/kg), rabeprazole (20 mg/kg) and lansoprazole (20 mg/kg), respectively by oral route once daily for 10 days. At the end of the treatment animals were fasted overnight, sacrificed by cervical dislocation and ulcer index was calculated. Stomachs were collected and were subjected to estimation of anti-oxidant enzymes like superoxide dismutase, catalase, reduced glutathione and lipid peroxides. Stomachs were fixed in 10% buffered formalin for histopathology.
Estimation of reduced glutathione [14]
Scrapped glandular tissue of the stomach weighing 1 g was homogenized with 10 ml of 10% TCA in ice cold condition and centrifuged at 3000 rpm for 10 min. The supernatant was separated and 2 ml of 0.2M phosphate buffer of pH 8 was added to 0.5 ml of supernatant. Then 0.2 ml of DTNB solution was added and absorbance was taken immediately at 412 nm. The amount of glutathione was calculated using a molar extinction coefficient of 13600 M-1 cm-1.
Estimation of malondialdehyde [15]
Malondialdehyde was estimated in terms of thiobarbituric acid reactive species (TBARS), using malondialdehyde (MDA) as standard. To 1 ml of the sample extract was added with 2 ml of the TCA-TBA-HCL reagent (15% w/v TCA, 0.375% w/v TBA in 0.25N HCl). The contents were boiled for 15 min, cooled and centrifuged at 10000 rpm to remove the precipitate. The absorbance was read at 535 nm and malondialdehyde concentration of sample was calculated using extinction coefficient of 1.56×105 M-1 cm-1.
Estimation of superoxide dismutase [16]
The gastric fundic mucosal scrap was homogenized by using a Potter-Ellvehjem glass homogenizer for 30 s in ice cold 0.9% saline (5%). To 0.4 ml of the homogenate was added 1.2 ml of sodium pyrophosphate buffer (pH 8.3, 0.052 M), 0.1 ml of 186 μM of phenazine metha sulphate (PMS), 0.3 ml of 300 μM nitro blue tetrazolium (NBT) and 0.8 ml of distilled water was added to make up the volume up to 2.8 ml. The reaction was started by addition of 0.2 ml of NADH (780 μM). It was incubated at 30° for 60 s. The reaction was stopped by addition of 0.1 ml of glacial acetic acid. The reaction mixture was stirred vigorously and shaken with 4 ml of n-butanol. The mixture was allowed to stand for 10 min, centrifuged and butanol layer was taken out. Colour intensity of the chromogen was measured against butanol at 560 nm using a spectrophotometer. A system devoid of enzyme activity was defined as enzyme concentration required to decrease the rate of reaction by 50% in one min under the assay conditions. The results are expressed as units (U) of SOD activity/g of wet tissue.
Estimation of catalase
Gastric mucosal scrap was weighed and 10% homogenate was prepared with 0.2M phosphate buffer pH 8.0. After centrifugation, the clear supernatant was used for assay of enzyme activity. The reaction mixture (1.5 ml, vol.) contained 1.0 ml of 0.01M pH 7.0 phosphate buffer, 0.1 ml of tissue homogenate (supernatant) and 0.4 ml of 2M H2O2. The reaction was stopped by the addition of 2.0 ml of dichromateacetic acid reagent (5% potassium dichromate and glacial acetic acid were mixed in 1:3 ratio). Colour intensity was measured colorimetrically at 620 nm and expressed as μmoles of H2O2 consumed/min/mg protein as described by Sinha et al. [17].
Estimation of myleoperoxidase [18]
To measure MPO activity, scrapped tissue was minced on ice and homogenized in 10 ml of ice-cold 50mM potassium phosphate buffer (pH 6.0), containing 0.5% HETAB and 10 mM EDTA. The homogenates were then sonicated and centrifuged for 20 min at 12 000 g. MPO activity was measured spectrophotometrically as follows: 0.1 ml of supernatant was combined with 2.9 ml of 50 mM phosphate buffer containing 0.167 mg/ml O-dianisidine hydrochloride and 0.0005% H2O2. The change in absorbance was measured spectrophotometrically at 460 nm. One unit of MPO activity was defined as the change in absorbance per minute at room temperature, in the final reaction. MPO activity (U/g) = X/weight of piece of tissue taken, where X= 10 x change in absorbance per minute/volume of supernatant taken in the final reaction.
Estimation of cortisol [19]
Blood samples were centrifuged at 1000 g for 10 min at 4° and plasma was removed. To 200 μl of plasma, 1.5 ml of dichloromethane was added and centrifuged at 5000 g for 5 min. After discarding the top aqueous layer, 500 μl of fluorescent reagent (absolute alcohol+concentrated sulphuric acid) was added and shaken. After 20 min the fluorescence of the bottom layer was measured at 470 nm (excitation) and 530 nm (emission) using spectroflourimeter and fluorescence was translated to the corticosterone values (μg/mg) by using the standard curve. Alkaline phosphatase was estimated using alkaline phosphatase kit (Erba Pvt Ltd).
Histopathological observation
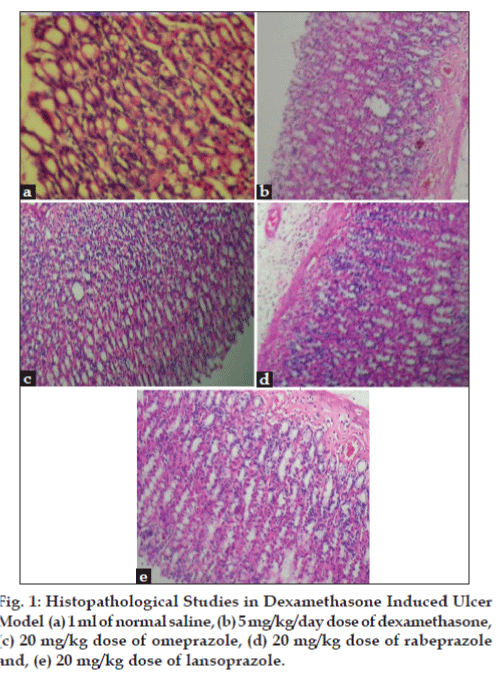
The stomach tissue sections were fixed in 10% formalin. The specimens were processed by standard procedure and embedded in paraffin wax. The blocks were sectioned at 5 micron and stained by hematoxylin and eosin and examined using light microscopy.
Statistical analysis
The results were expressed as the mean±SEM. The results obtained from the present study were analyzed using one-way ANOVA followed by Dunnett’s multiple comparison test. Data was computed for statistical analysis by using Graph Pad Prism Software. P<0.05 was considered as statistically significant.
Results and Discussion
In dexamethasone-induced ulcer model, Omeprazole showed significant increase in superoxide dismutase (SOD), reduced glutathione (GSH) and catalaes (CAT) level (P<0.05) as compared to rabeprazole and lansoprazole treated group (Table 1). Omeprazole also showed decrease in MPO, MDA and ALP level (P<0.05) as compared to rabeprazole and lansoprazole (Table 2). Control (induced ulcer) showed increase in cortisol content. Omeprazole showed significant reduction in cortisol content (P<0.05) as compared to rabeprazole and lansoprazole, which did not show significant changes as compared to control (Table 3). It is evident from Table 4 that the ulcer index in the control, omeprazole, rabeprazole and lansoprazole was 10.16±0.235, 4.330±0.5498, 4.750±0.3976 and 4.916±0.1743, respectively. The percentage protection of omeprazole, rabeprazole and lansoprazole was 57.67, 52.50, and 50.84, respectively. Omeprazole was found to be more significant. The histology of the stomach tissue from normal and PPI’s treated animals showed normal morphological appearances, whereas in dexamethasone-treated group showed significant disruptions in architecture of the stomach tissue (edema, congestion, hemorrhage and necrosis). The histology of stomach tissues from omeprazole treated group showed less disruptions (edema, congestion, hemorrhage and necrosis) and thus showed significant protection from dexamethasone treatment as compared to rabeprazole and lansoprazole (Table 5, fig. 1).
| Group | Oral treatment | GSH (n mol/mg wet gland) | SOD (UnitsA/mg protein) | CAT (UnitsB/mg protein) |
| I | Normal | 3.132±0.223 | 38.74±2.351 | 43.23±1.626 |
| II | Control | 0.751±0.053 | 18.74±3.326 | 21.23±1.539 |
| III | Omeprazole | 1.863±0.105a | 29.26±1.602a | 28.72±0.814a |
| IV | Rabeprazole | 1.796±0.150a | 25.69±1.565a | 26.43±1.415 |
| V | Lansoprazole | 1.703±0.135a | 23.29±1.609 | 25.07±1.327 |
Table 1 Effect of proton pump inhibitors on GSH, SOD and CAT levels
| Group | Oral treatment | MDA (n mol MDA/g wet gland) | ALP (IU/L) | MPO (U/g) |
|---|---|---|---|---|
| I | Normal | 21.17±2.267 | 28.54±1.942 | 0.185±0.033 |
| II | Control | 45.16±1.720b | 46.13±1.720b | 6.477±0.252b |
| III | Omeprazole | 28.57±1.571a | 31.80±2.138a | 2.131±0.425a |
| IV | Rabeprazole | 31.95±2.433a | 33.30±1.525a | 4.051±0.526a |
| V | Lansoprazole | 33.92±1.235a | 35.36±3.148a | 4.688±0.703a |
Table 2 Comparison of MDA, ALP and MPO levels in PPIs treated rats.
| Group | Oral treatment | Cortisol content |
|---|---|---|
| (Curative model) | ||
| I | Normal | 10.32±0.2040a |
| II | Control | 16.32±0.5924b |
| III | Omeprazole | 10.82±0.3420a |
| IV | Rabeprazole | 14.50±0.3706a |
| V | Lansoprazole | 15.27±0.4208 |
Table 3Comparison of cortisol content in proton pump inhibitors treated rats.
| Group | Oral treatment | Ulcer index | Protection (%) |
|---|---|---|---|
| I | Control | 10.16±0.235 | --- |
| II | Omeprazole | 4.330±0.5498a | 57.67 |
| III | Rabeprazole | 4.750±0.3976a | 52.50 |
| IV | Lansoprazole | 4.916±0.1743a | 50.84 |
Table 4 Effect of proton pump inhibitors on ulcer index
Dexamethasone-induced development of gastric ulceration by many mechanisms viz, dexamethasone increases lipid peroxidation and increases the generation of free radicals, stimulates both basal and drug induced gastric acid secretion and inhibits arachidonic acid metabolism [2,4,5]. Dexamethasone makes the gastric mucosa susceptible to ulceration by inhibiting prostaglandin synthetase and peroxidasetwo important gastroprotective enzymes [7] and by decreasing the level of nitric oxide [8]. Dexamethasone significantly suppresses epidermal growth factorstimulated gastric epithelial cell proliferation, and one of the pathways involved is via inhibiting activation of ERK1/ERK2, followed by inhibition of COX-2, cyclin D1 expression and DNA synthesis [10]. Apart from its mechanism of induction of ulcers, dexamethasone also delays ulcer healing by inhibition of angiogenesis in rat stomach and reduce regenerative repair of epithelium in experimental gastric ulcers [9].
In induced ulcer model omeprazole, rabeprazole and lansoprazole showed significant inhibition of ulcer index and increase in ulcer protection. Among these omeprazole showed more protective effect as compared to rabeprazole and lansoprazole. Omeprazole protects experimentally induced gastric ulcers by increased COX-2 expression, prostaglandin E2 synthesis, rate of regeneration of ulcerated mucosa and decreased inflammatory infiltrate [20], other than the inhibition of H+/K+ ATPase pump [21].
| Group | Oral treatment | Histopathological parameters | |||
|---|---|---|---|---|---|
| Oedema | Congestion | Haemorrhage | Necrosis | ||
| I | Normal | - | - | - | - |
| II | Control | +++ | +++ | ++ | +++ |
| III | Omeprazole | + | + | - | + |
| IV | Rabeprazole | ++ | + | + | + |
| V | Lansoprazole | ++ | + | ++ | + |
Table 5 Histopathological investigation in dexamethasone-induced ulcer model
Lipid peroxidation involves the formation and propagation of lipid radicals, the uptake of oxygen and rearrangement of double bonds in unsaturated lipids which eventually results in destruction of membrane lipids. The study also revealed a significant decrease in lipid peroxidation and increase in glutathione level by omeprazole suggesting its protective effect. Depletion of glutathione in ulcerated rats is known to result in enhanced lipid peroxidation [22] and excessive lipid peroxidation can cause increased glutathione consumption, as observed in present study. Reduced glutathione (GSH) is a major low molecular weight scavenger of free radicals in the cytoplasm and an important inhibitor of free radical mediated lipid peroxidation. GSH protects thiol protein groups required for maintaining the integrity of cell against oxidant. The administration of omeprazole protects the gastric cells against dexamethasone injury by decreasing generation of free radicals and restores the level of glutathione [23]. Rabeprazole also showed decrease in lipid peroxidation and increase in glutathione level, which indicates its antioxidant property. But there is a paucity of information regarding rabeprazole as an antioxidant.
Lansoprazole exerts its antioxidant effect by preservation of mucosal GSH levels, by direct scavenging activity against reactive oxygen species and by reverting the increase in plasma levels of peroxidated lipids in gastric ulcers, which is further supported by the direct inhibitory influence on cellular oxidative injury and inhibition of the oxidative metabolism of the activated inflammatory cells [24].
It is well known that reactive oxygen species are involved in the pathogenesis of gastric mucosal injury. Results of the present study also indicate similar alterations in the antioxidant status in induced ulcers. In induced ulcer model MDA was significantly increased while CAT and SOD levels were found to be decreased significantly. Due to decreased activity of SOD there is decrease in the scavenging of the superoxide radical O2 -, leading to lipid peroxidation and due to decreased CAT level which scavenge H2O2 - along with gastric peroximes there is increase in the generation of H2O2 - and its accumulation which lead to further mucosal damage [7]. Omeprazole effectively alleviated induced ulcers by reversing the increased level of MDA and decreased level of SOD and CAT back near to the control values suggesting decrease in oxidative damage. Omeprazole directly interfere with the oxidant producing system that is commonly regulated by receptor associated and protein-kinase dependent process. Omeprazole inhibits the activation of NADPH oxidase, the key enzyme required to produce the superoxide anion. Omeprazole inhibited superoxide production. The protonated, active form of omeprazole might preserve gastric β-carotene (which is decreased in the gastric juice with inflammatory gastric diseases) in vivo through a direct HOCl hypochlorous antagonism and shows significant HOCl scavenging effects. Thus, the protective effect of Omeprazole may also be due to its antioxidant properties and preservation of the endogenous anti-oxidants apart from its effects on other defensive factors [23,25].
Myeloperoxidase is an enzyme found in neutrophils and its activity is linearly related to infiltration of neutrophils. Omeprazole, rabeprazole and lansoprazole showed decreased myeloperoxidase activity. Omeprazole significantly reduced myeloperoxidase level as compared to rabeprazole and lansoprazole. Our biochemical analysis showed that omeprazole has significantly decreased the inflammatory infiltrate. Omeprazole inhibits the activation of neutrophils and neutrophil’s system for generating oxidants [23]. Omeprazole protects against the gastric mucosal damage associated with activated neutrophils/inflammatory reaction. Lansoprazole blocked oxygen-derived free radical output from neutrophils activated [24].
Increased alkaline phosphatase activity results from damage to gastric tissues and the release of this enzyme has been suggested to have a role in tissue necrosis. The present results showed that omeprazole reduces the level of alkaline phosphatase as compared to rabeprazole and lansoprazole which implicates its anti-ulcerogenic property.
In dexamethasone-induced ulcer model, we found that there was an increased level of cortisol. Rabeprazole and lansoprazole doesn’t show any significant changes, where as omeprazole has showed decreased cortisol level. This effect of omeprazole may be due to its inhibitory effect on cortisol synthesis by inhibition of both basal and adrenocorticotropic hormone stimulated levels of cortisol [26].
In dexamethasone treated group histopathological observation showed oedema, congestion, heamorrhage and necrosis. Mucosal epithelium of the omeprazole treated rats showed less hemorrhage, oedema, congestion and no necrosis is observed when compared against the control group. This may be due to the cytoprotective effect of omeprazole, where as in rabeprazole and lansoprazole treated rats showed mild to moderate hemorrhage, oedema, congestion and necrosis. The ulcer index studies also showed that omeprazole showed significant protection as compared to other PPIs.
To conclude, omeprazole is the most effective and selective proton pump inhibitor as compared with rabeprazole and lansoprazole in the treatment of dexamethasone induced gastric mucosal damage in Wistar rats.
Acknowledgements
The authors thank Cadila Healthcare, Ahmedabad and Cipla Laboratories, Goa for providing the drug samples. The authors are grateful to Dr. B. M. Patil, Principal, K. L. E. S College of Pharmacy, Hubli for providing the necessary facilities to carry out the work.
References
- Friedman JD, Haile PM, Siegelaaub AB, Seltzer CE. Cigarette, alcohol, coffee and peptic ulcer. N Engl J Med 1974;290:469-73.
- Manjari V, Das UN. Oxidant stress, anti-oxidants, nitric acid and essential fatty acids in peptic ulcer disease. Prostaglandins LeukotEssent Fatty Acids 1998;59:401-6.
- Megraud F, Lamouliatte H. Helicobacter pylori and duodenal ulcer evidence suggesting causation. Dig Dis Sci 1992;37:769-72.
- Dujovne CA, Azarnoff DL. Clinical complications of corticosteroid therapy. Med Clin North Am 1973;57:1331-42.
- Tripathi KD. Essentials of medical pharmacology. 5thed. New Delhi, India: Jaypee brothers; 2004. p. 255-65.
- Pezner RD, Lipsett JA. Peptic ulcer disease and other complications in patients receiving dexamethasone palliation for brain metastasis. West J Med 1982;137:375-8.
- Bandyopadhyay U, Biswas K, Bandyopadhyay D, Ganguly CK, Banerjee RK. Dexamethasone makes the gastric mucosa susceptible to ulceration by inhibiting prostaglandin synthetase and peroxidase - Two important gastroprotective enzymes. Mol Cell Biochem 1999;202:31-6.
- McCall TB, Palmer RM, Moncada S. Induction of nitric oxide synthase in rat peritoneal neutrophils and its inhibition by dexamethasone . EurJImmunol 1991;21:2523-7.
- Luo JC, Shin VY, Liu ES, Ye YN, Wu WK, So WH, et al.Dexamethasone delays ulcer healing by inhibition of angiogenesis in rat stomachs. Eur J Pharmacol 2004;485:275-81.
- Luo JC, Chi CW, Lin HY, Chang FY, Lu CL, Lee SD, et al. Dexamethasone inhibits epidermal growth factorstimulated gastric epithelial cell proliferation. J PharmacolExpTher 2007;320:687-94.
- Kaski CD, Rentsch R, Levis R, Hodgson HJ. Corticosteroids reduce regenerative repair in experimental gastric ulcers. Gut 1995;37:613-6.
- John H. The proton pump inhibitors: Similarities and differences. ClinTher 2000;22:266-80.
- Manjari V, Das UN. Effect of polyunsaturated fatty acids on dexamethasone induced gastric mucosal damage. ProstagalnLeukotEssent Fatty Acids 2000;62:85-96.
- Ellman GL. Tissue sulfhydryl groups. Arch BiochemBiophys 1959;82:70-7.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351-8.
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometricassay of superoxide dismutase. Indian J BiochemBiophys 1984;21: 130-2.
- Sinha KA. Colorimetric assay of catalase. Anal Biochem 1972;47: 389-94.
- Krawisz JE, Sharon P, Stenson WF. Qualitative assay for acute intestinal inflammation based on myeloperoxidase activity. Gastroenterology 1984;87:1344-50.
- Mattingly D. A simple flourimetric method for the estimation of free 11-hydroxycorticoids in human plasma. J Clin Path 1962;15:374-9.
- Dharmani P, Chauhan Singh V, Palit G. Cyclo oxygenase-2-expression and prostaglandin E2 production in experimental chronic gastric ulcer healing. Eur J Pharmacol 2005;519:277-84.
- Puscas I, Coltau M, Baican M, Domuta G. Omeprazole has dual mechanism of action. It inhibits both H+K+-ATPase and gastric mucosa carbonic anhydrase enzyme in humans (in vitro and in vivo experiments). J PharmacolExptTher 1999;290:530-4.
- Mutoh H, Ota S, Hiraishi H, Ivey KJ, Terano A, Sugimoto T. Reduced glutathione protects cultured gastric mucosal cells from suckling rats against acid. Am J Physiol 1991;261:65-70.
- Suzuki M, Mori M, Miura S, Suematsu M, Fukumura D, Kimura H, et al. Omeprazole attenuates oxygen derived free radical productionfrom human neutrophils. Free Rad Biol Med 1996;21:727-31.
- Natale G, Lazzeri G, Lubrano V, Colucci R, Vassalle C, Fornai M, et al. Mechanisms of gastroprotection by lansoprazole pretreatmentagainst experimentally induced injury in rats: Role of mucosal oxidative damage and sulfhydryl compounds. ToxicolApplPharmacol 2004;195:62-72.
- Lapenna D, Gioia SD, Ciofani G, Festi D, Cuccurullo F. Antioxidant properties of Omeprazole. FEBS Lett 1996;382:189-92.
- Katagiri F, Inoue S, Sato Y, Itoh H, Takeyama M. Comparison of the effects of proton pump inhibitors on human adrenocorticotropic hormone and cortisol levels under the starved condition. Biomed Pharmacother 2006;60:109-12.