- *Corresponding Author:
- V. Kumar
Regional Ayurveda Research Institute for Drug Development, Amkhoh, Gwalior-474 009, India
E-mail: vijaychem99@gmail.com
| Date of Submission | 01 April 2017 |
| Date of Revision | 10 March 2018 |
| Date of Acceptance | 15 September 2018 |
| Indian J Pharm Sci 2018;80(6):1151-1155 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Binding of pesticides to serum albumin significantly influence their absorption, metabolism, distribution and excretion. In the present study, interactions of acephate, glyphosate, monocrotophos and phorate with bovine serum albumin were explored employing the UV/Vis and Fourier-transform infrared spectroscopy methods. The observed values of binding constant for titled pesticides were. phorate (1.85×107 M-1)>acephate (3.93×104 M-1)>glyphosate (1.31×104 M-1)>monocrotophos (1.12×104 M-1). Results have shown that out of four pesticides, phorate could show strong interactions with bovine serum albumin.
Keywords
Pesticides, UV/Vis and FTIR, BSA, binding constant
Crop protection has become necessary in order to increase the food production and use of pesticides has become unavoidable act for that purpose [1-5]. Pesticides are considered as an integral part of modern agriculture [6-10]. At the same time, food safety issues have gained considerable attention due to its effects on environment and human health. Pesticides used to protect the crops or foods at various stages like sowing to storage, and these synthetic chemicals are allow to enter into human body through different modes of applications of pesticides [1-13]. Binding of chemicals to serum albumin significantly influence their absorption, distribution, metabolism and excretion mechanisms. Therefore, basic understandings of interactions of pesticides to serum albumins are also very important with respect to human health [2,14-17].
Acephate, glyphosate, monocrotophos and phorate are widely used organophosphate pesticides (OPs) for agricultural applications worldwide [1-13]. OPs inhibit the reversible hydrolysis of acetylcholine and therefore the persistence of OPs for longer time in the environment is a problem [2-10]. The physiochemical and biological behavior of OPs are well exposed with a number of available literatures, whereas relatively less information is available on their behavior and impact on serum proteins. Interactions of few pesticides with macromolecules and serum proteins, and their mode of binding are also available in recent literature [14-19]. Most importantly, our special interest in the pesticides of different classes allows us to describe the interaction of acephate, glyphosate, monocrotophos and phorate with bovine serum albumin (BSA).
All reagents and solvents used were of the commercial quality and were used without purification. Technical grade level acephate, glyphosate, monocrotophos and phorate were obtained as gift samples from Gautami Ltd., Andhra Pradesh, India and BSA was purchased from Loba Chemie Pvt. Ltd., Mumbai, India.
The absorption spectra were recorded on a Shimadzu 1800S double beam spectrophotometer with quartz cuvettes of 1 cm. The UV absorption of BSA, complexes of acephate-BSA, glyphosate-BSA, monocrotophos- BSA and phorate-BSA solutions were measured at pH 7.2 by keeping the concentration of BSA constant (0.05 mM), while varying the concentration of the pesticides (0.010, 0.0075, 0.0050, 0.0025, 0.00125 mM), in the range of 230-400 nm. The binding constants of the pesticides-BSA complexes were calculated by using previously employed method of Abdi et al. [19]. FTIR spectrophometric analyses qualitatively and quantitatively were performed as reported by Abdi et al. [19].
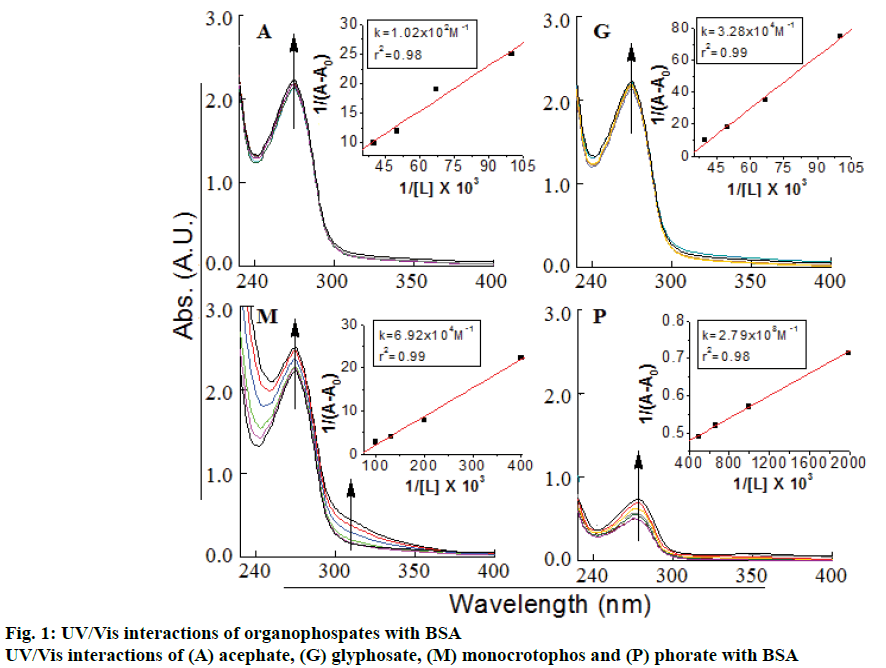
Interactions of acephate, glyphosate, monocrotophos and phorate were analyzed with BSA by employing the UV/Vis spectrometric method. The interactions were reported in terms of binding constant. The double reciprocal plot of 1/(A-A0) versus 1/(ligand concentration) is linear and the binding constant (K) can be estimated from the ratio of the intercept to the slope. A0 is the initial absorbance of the free BSA at 275 nm and A is the recorded absorbance at different product concentrations (Figure 1). An increase in the absorbance was resulted with an increase in OPs concentration level (0.010, 0.0075, 0.0050, 0.0025, 0.00125 mM) of OPs, and shifting of 5-10 nm of BSA band at 275 nm was observed due to OP(s)-BSA complex formation (Figure 1).
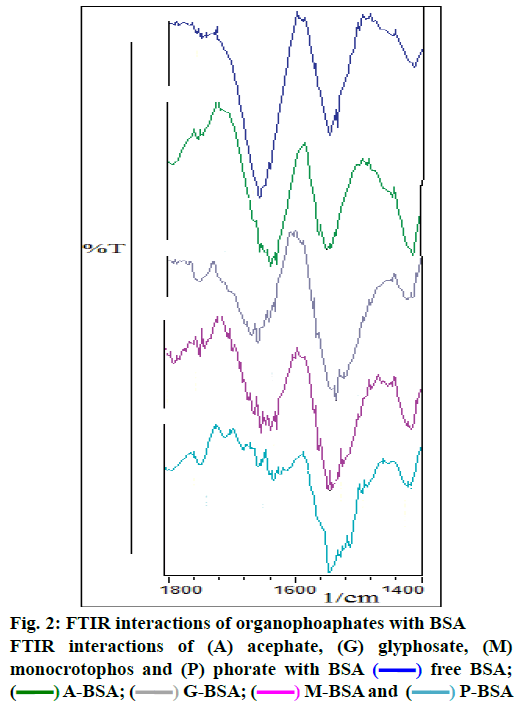
The observed binding constant for individual pesticide to BSA complex was as: 3.93(±0.04)×104 M-1 (acephate-BSA), 1.31(±0.03)×104 M-1 (glyphosate- BSA), 1.12(±0.05)×104 M-1 (monocrotophos-BSA) and 1.85(±0.04)×107 M-1 (phorate-BSA). In present study, it was observed that the binding constant for the acephate-BSA, glyphosate-BSA and monocrotophos– BSA complexes suggest a low affinity for complex formation with BSA protein, compared to strong complexation of phorate-BSA complex. As per literature, molecules with binding constants ranging from 10-6 to 10-8 M were considered as strong binding with BSA [20-22], hence phorate could show strong complexation or interactions with BSA. Here, interaction behavior (in terms of binding constant) of four pesticides was observed (Table 1 and Figure 1 to 3). The average values of protein binding constants are mentioned above and shown under Figure 1. All these results suggested average to very strong interaction of various pesticides with BSA. Acephate, glyphosate, monocrotophos and phorate and BSA interactions were characterized by infrared spectroscopy and its derivative methods. Since, there was major spectral shifting was observed for the protein amide I band at 1653 cm-1 (mainly C=O stretch) and amide II band at 1541 cm-1 (C–N stretching coupled with N–H bending modes) upon pesticide interactions with BSA [20-25]. The difference spectra [(protein solution+pesticide solutions)+(protein solution)] were obtained, in order to monitor the intensity variations of these vibrations and the results are shown in Figure 2. Similarly, the infrared self-deconvolution with second derivative resolution enhancement and curve-fitting procedures were used to determine the protein secondary structures in the presence of pesticides-BSA complexes (Figure 2).
| Quantitative % change in BSA by FTIR spectroscopy | |||||
|---|---|---|---|---|---|
| Complexes | a-Helix 1656 cm-1 | ß-Sheet 1626 cm-1 | Turn structure 1674 cm-1 |
ß-anti parallel 1695 cm-1 |
Random coil 1633 cm-1 |
| BSA-A | 7.4 | 14.6 | 7.4 | 5.4 | 4.4 |
| BSA-G | 14.7 | 11.7 | 3.1 | 2.7 | 2.4 |
| BSA-M | 3.7 | 4.3 | 1.4 | 2.3 | 13.4 |
| BSA-P | 4.7 | 3.9 | 1.2 | 17.6 | 5.1 |
| BSA-SA | 1.2 | 12.5 | 1.8 | 3.2 | 1.9 |
Table 1: Quantitative FTIR Interactions of Organophosphates with BSA
In literature, quantitative analysis of the protein secondary structure for the free BSA in hydrated films has been carried out [18-25]. The observed FTIR peaks for the free protein were as: α-helix (1656 cm-1), β-sheet (1618 and 1628 cm-1), turn structure (1670 cm-1), β-antiparallel (1693 and 1680 cm-1) and random coil (1638 cm-1). The β-sheet structure is composed of two components at 1618 (inter β-strand) and 1628 cm-1 (intra β-strand, hydrated) that are consistent with the spectroscopic current studies of BSA. In current study, with the addition of individual pesticide (0.01 mM) to BSA, a decrease in intensity as well as shifting (4 cm-1 to 20 cm-1) of the amide I band at 1658 cm-1 (BSA) was observed with features at 1654 cm-1. In the spectra of pesticide(s)-BSA complexes, negative features are due to the reduction of intensity due to loss of protein structure (Figure 2) of the amide I band and suggest a major reduction of protein α-helical structure. Similar infrared spectral changes were observed for the protein amide I band in several ligand-protein complexes, where major protein conformational changes occurred [22-25]. The present results are consistent with the decrease in intensity of the protein amide I band discussed above. The decrease in α-helix structure and increase in β-sheet and turn structures is indicative of protein destabilization upon pesticide(s)-BSA interactions and results are very consistent with literature [20-25].
A quantitative analysis of the protein secondary structure for the free BSA and its OPs analogues adducts (acephate-BSA, gl-637 cm-1; Figures 2 and 3; Table 1). The results are consistent with the spectroscopic studies of BSA previously reported [20-25]. Upon OPs analogues interactions (acephate-BSA, glyphosate- BSA, monocrotophos-BSA and phorate-BSA), a major decrease of α-helix from 69 % (free BSA) to 7.4 % (acephate-BSA,1 mM), 14 % (glyphosate-BSA, 1 mM), 3.7 % (monocrotophos-BSA, 1 mM) and 4.7 % (phorate-BSA, 1 mM) with changes in β-sheet from 12 % (free BSA) to 14.6 % (acephate-BSA,1 mM), 11.6 % (glyphosate-BSA, 1 mM), 4.3 % (monocrotophos-BSA, 1 mM) and 3.9 % (phorate- BSA) were observed (Figure 3). A similar change was observed for the turn structure from 14 % (free BSA) to 7.4 % (acephate-BSA, 1 mM), 3.1 % (glyphosate-BSA, 1 mM), 1.4 % (monocrotophos- BSA, 1 mM) and 1.2 % (phorate-BSA, 1 mM; Figure 3). A similar change was also observed for the β-antiparallel from 3 % (free BSA) to 5.4 % (acephate- BSA, 1 mM), 2.7 % (glyphosate-BSA, 1 mM), 2.3 % (monocrotophos-BSA, 1 mM), and 17.6 % (phorate- BSA, 1 mM) and random coil from 2 % (free BSA) to 4.4 % (acephate-BSA, 1 mM), 2.4 % (glyphosate- BSA, 1 mM), 13.4 % (monocrotophos-BSA, 1 mM), and 5.1 % (phorate-BSA, 1 mM; Figure 3). These results are consistent with the decrease in intensity of the protein amide I band discussed above. The decrease in α-helix structure, β-sheet (except acephate) and turn structure and increase in β-antiparallel and random coil is indicative of protein destabilization upon OPs and its derivatives interaction. It was found that binding constant of complexes of acephate-BSA, glyphosate- BSA, monocrotophos-BSA and phorate-BSA was almost vary linearly with β-antiparallel FTIR frequency at 1695 cm-1 (°1).
In pharmaceutical industry, protein binding studies are most common studies to check the influence of drugs with respect to absorption, metabolism, distribution and excretion. Strong interactions or biding of drugs may alter the effect and alter the efficacy of drug. Very strong interactions may leads to toxicity consequently such molecule is not used as drug [24-28]. Basically, drug delivery is a complex mechanism. It is assumed that drugs show their interactions to various proteins before reaching to target. Mode of interactions may define the toxicity, potency and efficacy of a drug. Generally, strong interactions of any medicine with proteins with binding constant (k) ranging from 106-1011 M-1 is not a good thing for the proper drug delivery [18,19,24-28].
The observed order of binding constants of the four pesticides with BSA was; phorate (1.85×107 M-1) >>>acephate (3.93×104 M-1)>glyphosate (1.31×104 M-1) >monocrotophos (1.12×104 M-1). Current study highlighted the fact that due to strong binding ability of phorate with BSA, it might severely affect the living organisms. The remaining three pesticides have not shown stronger binding constant so these could be considered as less toxic in terms of protein binding. Binding of pesticides or drugs to serum albumins significantly influence their absorption, metabolism, distribution and excretion. Strong interactions or biding of drugs may alter the effect and alter the efficacy of drug. In case of pesticides strong binding might lead to high toxicity. Future work will address the effect of mixed pesticides on BSA interactions in the presence of metal ions and most probable mechanism involved for these interactions.
Conflict of interest
There is no conflict of interest associated with this project.
Financial support and sponsorship
Nil.
References
- Duke SO, Powles SB. Glyphosate: a once-in-a-century herbicide. Pest Manag Sci 2008;64:319-25.
- Kaur A, Kumar V, Singh S, Singh J, Upadhyay N, Datta S, et al. Toll-like receptor-associated keratitis and strategies for its management. 3 Biotech 2015;5:611-9.
- Kumar V, Upadhyay N, Wani AB, Singh J, Singh S, Kaur P. Spectroscopic Methods for the Detection of Organophosphate Pesticides –A Preview. Curr World Environ 2013;8:313-8.
- Kumar V, Upadhyay N, Singh S, Singh J, Kaur P. Thin-Layer Chromatography: Comparative Estimation of Soil’s Atrazine. Curr World Environ 2013;8:469-73.
- Kumar V, Upadhyay N, Kumar V, Kaur S, Singh J, Singh S, et al. Environmental Exposure and Health Risks of the Insecticide Monocrotophos-A Review. J Bio Env Sci 2014;5:111-20.
- Kumar V, Kumar V, Upadhyay N, Sharma S. Chemical, biochemical and environmental aspects of atrazine. J Bio Env Sci 2014;5:149-65.
- Kumar V, Kumar V, Upadhyay N, Sharma S. Interactions of atrazine with transition metal ions in aqueous media: Experimental and computational approach. 3 Biotech 2015;5:791-8.
- Kumar V, Upadhyay N, Kumar V, Sharma S. A review on sample preparation and chromatographic determination of acephate and methamidophos in different samples. Arab J Chem 2015;8:624-31.
- Kumar V, Singh S, Singh J, Upadhyay N. Potential of Plant Growth Promoting Traits by Bacteria Isolated from Heavy Metal Contaminated Soils. Bull Environ Contam Toxicol 2015;94:807-15.
- Kumar V, Kaur S, Singh S, Upadhyay N. Unexpected formation of N-phenyl-thiophosphorohydrazidic acid O,S-dimethyl ester from acephate: chemical, biotechnical and computational study. 3 Biotech 2016;6:1-11.
- Kumar V, Upadhyay N, Manhas A. Designing, syntheses, characterization, computational study and biological activities of silver-phenothiazine metal complex. J Mol Struct 2015;1099:135-41.
- Prasad R, Upadhyay N, Kumar V. Simultaneous determination of seven carbamate pesticide residues in gram, wheat, lentil, soybean, fenugreek leaves and apple matrices. Microchem J 2013;111:91-7.
- Singh S, Singh N, Kumar V, Datta S, Wani AB, Singh D, et al. Toxicity, monitoring and biodegradation of the fungicide carbendazim. J Environ Chem Lett 2016;14:317-29.
- Yang B, Hao F, Li J, Wei K, Wang W, Liu R. Characterization of the binding of chrysoidine, an illegal food additive to bovine serum albumin. Food Chem Toxicol 2014;65:227-32.
- Lv Y, Li X, Wang Z, Zheng H, Zhang Q, Huo R, et al. Interaction of bovine milk protein with chlorpyrifos. J Dairy Sci 2014;97:2056-60.
- Saquib Q, Al-Khedhairy AA, Siddiqui MA, Roy AS, Dasgupta S, Musarrat J. Preferential binding of insecticide phorate with sub-domain IIA of human serum albumin induces protein damage and its toxicological significance. Food Chem Toxicol 2011;49:1787-95.
- Yue Y, Zhang Y, Zhou L, Qin J, Chen X. In vitro study on the binding of herbicide glyphosate to human serum albumin by optical spectroscopy and molecular modeling. J Photochem Photobiol B 2008;90:26-32.
- Han XL, Tian FF, Ge YS, Jiang FL, Lai L, Li DW, et al. Spectroscopic, structural and thermodynamic properties of chlorpyrifos bound to serum albumin: A comparative study between BSA and HAS. J Photochem Photobiol B 2012;109:1-11.
- Abdi K, Nafisi SH, Manouchehri F, Bonsaii M, Khalaj A. Interaction of 5-Fluorouracil and its derivatives with bovine serum albumin. J Photochem Photobiol B 2012;107:20-6.
- Byler DM, Susi H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 1986;25:469-87.
- Kragh-Hansen U. Structure and ligand binding properties of human serum albumin. Dan Med Bull 1990;37:57-84.
- Krimm S, Bandekar J. Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv Protein Chem 1986;38:181-364.
- Ahmed-Ouameur A, Diamantoglou S, Sedaghat-Herati MR, Nafisi Sh, Carpentier R, Tajmir-Riahi HA. The effects of drug complexation on the stability and conformation of human serum albumin: protein unfolding. Cell Biochem Biophys 2006;45:203-14.
- Bourassa P, Dubeau S, Maharvi GM, Fauq AH, Thomas TJ, Tajmir-Riahi HA. Locating the binding sites of anticancer tamoxifen and its metabolites 4-hydroxytamoxifen and endoxifen on bovine serum albumin. Eur J Med Chem 2011;46:4344-53.
- Kumar V, Chawla M, Cavallo L, Wani AB, Manhas A, Kaur S, et al. Complexation of trichlorosalicylic acid with alkaline and first row transition metals as a switch for their antibacterial activity. Inorganica Chim Acta 2018;469;379-86.
- Liu J, Tian J, Hu Z. Binding of isofraxidin to bovine serum albumin. Biopolymers 2004;73:443-50.
- Kumar V, Singh S, Singh R, Upadhyay N, Singh J. Design, synthesis, and characterization of 2,2-bis(2,4-dinitrophenyl)-2-(phosphonatomethylamino)acetate as a herbicidal and biological active agent. J Chem Biol 2017;10;179-90.
- Kaur S, Kumar V, Chawla M, Cavallo L, Poater A, Upadhyay N. Pesticides Curbing Soil Fertility: Effect of Complexation of Free Metal Ions. Front Chem 2017;5;1-9.
 ) free BSA; (
) free BSA; ( ) A-BSA; (
) A-BSA; ( ) G-BSA; (
) G-BSA; ( ) M-BSA and (
) M-BSA and ( ) P-BSA
) P-BSA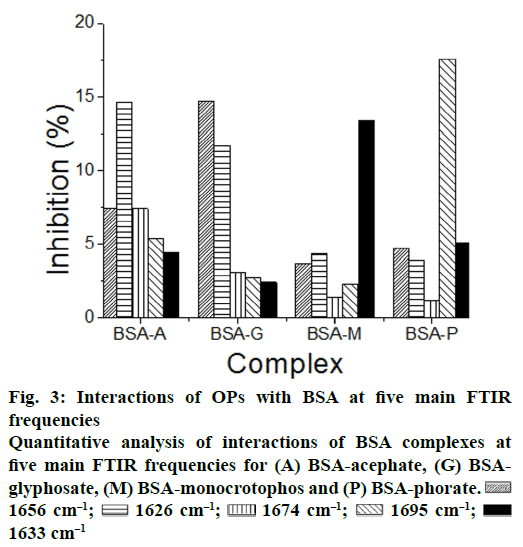
 1656 cm–1;
1656 cm–1;  1626 cm–1;
1626 cm–1;  1674 cm–1;
1674 cm–1;  1695 cm–1;
1695 cm–1;  1633 cm–1
1633 cm–1