- Corresponding Author:
- Surti
Parul Institute of Pharmacy and Research, P.O. Limda, Waghodia-391 760, India
E-mail: naazsurti@gmail.com
| Date of Submission | 03 May 2014 |
| Date of Revision | 07 January 2015 |
| Date of Acceptance | 26 May 2015 |
| Indian J Pharm Sci 2015;77(3):290-298 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
This investigation was aimed to improve the dissolution rate of the poorly soluble drug lovastatin, by formulating it as a liquisolid compact. Different liquisolid compacts were prepared using mathematical formulae to calculate the required quantities of powder and liquid ingredients to produce acceptably flowable and compressible admixture. Avicel PH 200, Cab-O-Sil, sodium starch glycolate and PEG 400 were employed as carrier, coating material, disintegrant and non-volatile liquid vehicle, respectively. The various drug to liquid and carrier to coating ratio were used to prepare liquisolid compacts. The formulated liquisolid tablets were evaluated for weight variation, hardness, drug content, friability and disintegration time. The in vitro release characteristics of the drug from tablets formulated by direct compression and liquisolid technique were compared in two different dissolution media. The tableting properties of the liquisolid compacts were within the acceptable limits and drug release rates were distinctly higher as compared to directly compressed tablets. The FTIR spectra showed no interaction between drug-excipient and disappearance of the characteristic absorption band of lovastatin in liquisolid formulations could be attributed to the formation of hydrogen bonding between the drug and liquid vehicle, which resulted in dissolution enhancement. Thus, the liquisolid technique was found to be a promising approach for improving the dissolution of a poorly soluble drug like lovastatin.
Keywords
Liquisolid compacts, lovastatin, dissolution, carrier and coating material
The most convenient and commonly employed route of drug delivery is by oral ingestion. The oral route remains the preferred route of drug administration due to its convenience, better patient compliance and low production costs. After oral administration, the dissolution of a drug in the gastric fluids is a prerequisite for the absorption of drug into the systemic circulation. The absorption rate of poorly water soluble drug formulated as an orally administered solid dosage form is controlled by its dissolution rate in the fluid present at the absorption site i.e. the dissolution rate is often the rate limiting step in drug absorption [1,2].
As per biopharmaceutical classification system BCS, Class II drugs are defined as those with high permeability but whose solubility in aqueous media is not sufficient for the whole dose to be dissolved in the gastrointestinal tract. For these substances dissolution is therefore the rate?determining step for drug absorption [3].
Thus one of the major challenges in drug development today is poor solubility, as estimated 40% of all newly developed drugs are poorly soluble or insoluble in water. In addition, up to 50% of orally administered drug compounds suffer from formulation problems related to their low solubility and lipophilicity [4,5].
Different methods are employed to improve the dissolution characteristics of poorly water soluble drugs, which include, (a) solubilization in surfactants (b) pH adjustment (c) co?solvents (d) micro emulsion (e) self emulsification (f) polymeric modification (g) drug complexation (h) particle size reduction (i) the pro?drug approach and (j) solid solutions. Liquisolid system is the most promising method for promoting dissolution [6-10].
Rapid release rates are obtained in liquisolid formulations and these can be efficiently used for water insoluble solid drugs or liquid lipophilic drugs or water insoluble solid drugs dissolved in nonvolatile solvent and this liquid medication can be converted into free flowing, non adherent, dry looking, and readily compressible powders with use of carrier and coating materials. As the drug is in the form of liquid medication, it is either in solubilized or in molecularly dispersed state. Due to increased wetting and increased surface area for dissolution, liquisolid tablets of water insoluble drugs show improved dissolution profile and increase in bioavailability [11-13].
Hypertriglyceridemia is typically treated with a various class of medications called statins, fibrates, niacin (nicotinic acid) and bile acid sequestrants. Medications most commonly used to treat high LDL cholesterol levels are statins, such as atorvastatin or simvastatin. These drugs work by reducing the production of cholesterol within the body. But statins are poorly soluble in water and result in low bioavilability. Hence in this study, lovastatin was formulated into liquisolid tablets, which is expected to enhance dissolution of this poorly soluble drug in the stomach and hence improve its oral bioavailability.
Materials and Methods
The following materials were used, lovastatin (Gift sample, Emcure Pharmaceuticals, Pune, India), Avicel PH102, Avicel PH 200, lactose and dicalcium phosphate (Ozone international, Mumbai, India), Aerosil 200 and Cab?O?Sil M5 (Cabot Sanmar Limited, Mumbai, India), sodium starch gylcolate (Arihant Trading Co., Mumbai, India), talc and magnesium stearate (Loba Chemie Pvt. Ltd., Mumbai), propylene glycol, polyethylene glycol?200, polyethylene glycol?400 (Chemdyes corporation, Baroda, India). All reagents used were of analytical grade.
Solubility Studies
Solubility of the drug was determined in different solvents (propylene glycol, PEG?200, PEG?400). Accurately weighed drug was transferred in 5 ml solvent and shaken until saturation was achieved. Solution was allowed to stand for 12 h and then centrifuged at 3000 rpm. Drug dissolved in the supernatant was analyzed spectrophotometrically [14].
Theoretical aspects for designing the liquisolid systems
The amount of excipients (carrier and coating materials) used to prepare liquisolid compacts depend on the flowable liquid retention potential values (??value) and the liquid loading factors (Lf), Equation 1. The ??value of a powder is the maximum amount of a given nonvolatile liquid that can be retained inside powder bulk (w/w) while maintaining acceptable flowability. Whereas, Lf is the mass ratio (w/w) of the liquid medication to the carrier powder in the liquisolid formulation. Knowing the carrier: Coating ratio (R), liquid loading factor (Lf) can be calculated by the following Eqn. 1, Lf=?CA+?CO. 1/R.
The optimum weight of the carrier (Q), required for the respective vehicle could be calculated by rearranging the Eqn. 2, Lf=W/Q, where, W and Q are weight of the liquid medication (the drug+nonvolatile liquid vehicle) and weight of the carrier, respectively. The optimum weight of the coating material (q) could also be obtained from Eqn. 3, R=Q/q, where, Q and q are the weight of the carrier and coating material, respectively [15?17].
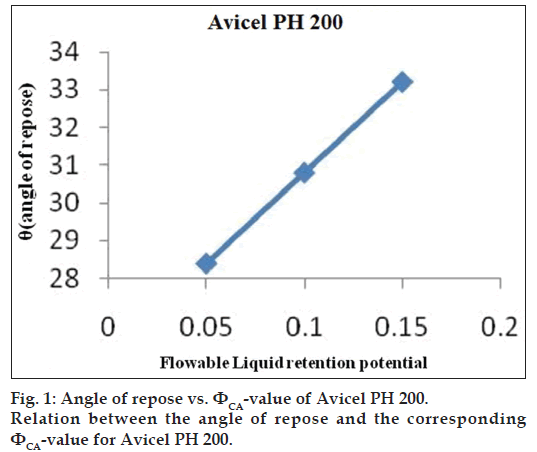
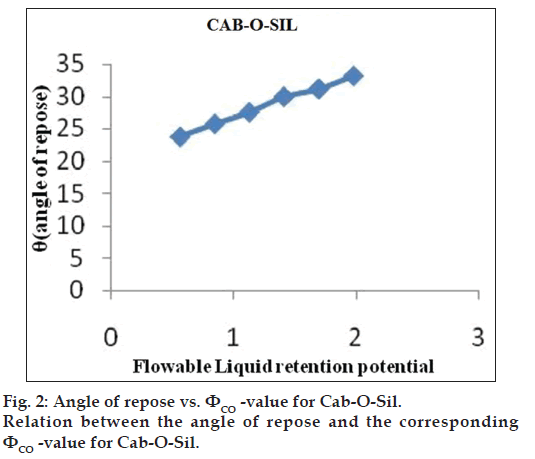
Flowable liquid retention potential for Avicel® PH 200 (ΦCA-value) and Cab-O?Sil (ΦCO-value)
An increasing amount of the nonvolatile liquid vehicles was added to 10 g of Avicel® PH 200 or silica powder and mixed using a mortar and pestle to give powder admixtures. The carrier and coating materials adsorbed the liquid vehicle resulting in a change in material flow properties compared to pure powder of Avicel® PH 200 or silica powder previously measured. At each concentration of the nonvolatile liquid vehicle, the angle of repose was determined as stated previously. The corresponding flowable liquid retention potentials were calculated using the Eqn. 4, ΦCA-value=weight of liquid/weight of solid.
Then, the obtained ??values were plotted against the corresponding angle of repose. The Φ-value, which corresponded to an angle of repose of 33°, represented the flowable liquid retention potentials of powder admixture. In cases where the Φ-value did not correspond to 33°, the highest Φ-value reached was chosen as the flowable liquid retention potential [18].
Preparation of liquisolid tablets
Calculated quantities of drug and nonvolatile solvent (Table 1) were accurately weighed in glass beaker and then heated till the drug dissolved. The resulting hot medication was incorporated into calculated quantities of carrier and coating materials. Mixing process was carried out in three steps as described by Spireas et al.[14].
| Formulation code | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| Drug: Liquid | 1:2 | 1:2 | 1:2 | 1:4 | 1:4 | 1:4 | 1:6 | 1:6 | 1:6 |
| Ca: Co (R) | 10 | 15 | 20 | 10 | 15 | 20 | 10 | 15 | 20 |
| Lf | 0.3475 | 0.2816 | 0.2487 | 0.3475 | 0.2816 | 0.2487 | 0.3475 | 0.2816 | 0.2487 |
| Drug (mg) | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| PEG400 (mg) | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| Avicel PH 200 (mg) Q | 172.6 | 213.0 | 241.2 | 287.7 | 355.1 | 402.1 | 402.8 | 497.1 | 562.9 |
| Cab? O? Sil (mg) q | 17.2 | 14.2 | 12 | 28.7 | 23.6 | 20.1 | 40.2 | 33.1 | 28.1 |
| SSG (mg) 5% | 12.5 | 14.3 | 15.6 | 20.8 | 23.9 | 26.1 | 29.1 | 33.5 | 36.5 |
| Magnesium stearate (mg) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Talc (mg) | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Unit weight (mg) | 277.3 | 316.5 | 343.8 | 452.2 | 517.6 | 563.3 | 627.1 | 718.7 | 782.4 |
Ca:Co (R) is carrier to coating ratio, SSG is sodium starch glycolate
Table 1: Composition Of Different Batches Of Liquisolid Tablets Of Lovastatin.
During the first stage, the system was blended at an approximate mixing rate of one rotation per second for approximately one minute in order to evenly distribute liquid medication in the powder. In the second stage, the liquid/powder admixture was evenly spread as a uniform layer on the surfaces of a mortar and left standing for approximately 5 min to allow drug solution to be absorbed in the interior of powder particles. In the third stage, the powder was scraped off the mortar surfaces by means of aluminum spatula and then blended with sodium starch glycolate for another 30 s in a similar way to the first stage. This was then lubricated with help of talc and magnesium stearate and final blend was evaluated for precompression parameter. Prepared liquisolid formulation blend was compressed using rotary tablet press machine [19,20].
Flow properties of liquisolid powder blend
Flow properties are the important concern in the formulation and industrial production of tablet dosage form. Angle of repose is characteristic to the flow rate of powder. Flow properties of the drug and prepared blend were studied by determining the bulk density, tapped density, Carr’s Index and Hausner ratio.
Angle of repose was determined by fixed funnel and freestanding cone method [21,22].
Infrared spectra analysis
FTIR spectra of prepared blend were recorded on FTIR?8400 spectrophotometer. Potassium bromide pellet method was employed and background spectrum was collected under identical situation. Each spectrum was derived from single average scans collected in the region 400?4000 cm?1 at spectral resolution of 2 cm?1 and ratio against background interfere gram. Spectra will be analyzed by software. FTIR was performed for the pure drug and liquisolid powders to detect any sign of interaction which would be reflected by a change in the position or disappearance of any characteristic stretching vibration of the compound [23].
Drug content
Twenty tablets were selected randomly and average weight was calculated. Tablets were crushed in a mortar and accurately weighed amount of average tablet was taken from the crushed blend. Then, the samples were transferred to 100 ml volumetric flasks and were diluted up to the mark with methanol. The content was shaken periodically and kept for one hour to dissolve of the drug completely. The mixtures were filtered and appropriate dilutions were made. The drug content in each tablet was estimated at λmax238 nm against blank reference and reported.
Hardness
It is a measure of the mechanical strength of a tablet. The mechanical strength of a tablet is associated with the resistance of a tablet to fracture or attrition. It was determined using Monsanto hardness tester [24,25].
Friability
Roche friabilator was used to measure the friability of the tablets. Ten tablets were weighed collectively and placed in the chamber of the friabilator. In the friabilator, the tablets were exposed to rolling, resulting from free fall of tablets within the chamber of the friabilator. It was rotated for 4 min at a rate of 25 rpm. The tablets were taken out from the friabilator and intact tablets were again weighed collectively. The percent friability was determined using the formula, friability=(W1?W2/W1)×100, where, W1 and W2 are weight of the tablet before test and weight of the tablets after test, respectively [24,25].
Weight variation test
Twenty tablets were weighed individually and then all together. Average weight was calculated from the total weight of all tablets. The individual weights were compared with the average weight. The percentage difference in the weight variation should be within the permissible limits as specified in IP. The percent deviation was calculated using the formula, percent deviation=(individual weight–average weight/average weight)×100. Any variation in the weight of tablet (for any reason) leads to either under medication or over medication. So, every tablet in each batch should have a uniform weight. Corrections were made during the compression of tablets to get uniform weight [24,25].
In vitro disintegration time
The process of breakdown of a tablet into smaller particles is called as disintegration. The in vitro disintegration time of a tablet is determined using disintegration test apparatus as per IP specifications. Place one tablet in each of the 6 tubes of the basket. Add a disc to each tube and run the apparatus using pH 7 maintained at 37±2° as the immersion liquid. The assembly should be raised and lowered between 30 cycles per minute in the pH 7 maintained at 37±2°. The time in seconds taken for complete disintegration of the tablet with no palpable mass remaining in the apparatus was measured and recorded [24,25].
In vitro dissolution studies
The USP dissolution apparatus II was used with 900 ml 0.1 N HCl (2% SLS) as dissolution medium [26]. The apparatus was run at 50 rpm. Samples of the dissolution medium was withdrawn at specified time intervals and compensated by fresh dissolution medium. Samples were properly diluted and drug concentrations were analyzed spectrophotometrically. The cumulative percentage drug released at each time interval was calculated and plotted against time.
Comparison of dissolution profile of liquisolid tablet and conventional tablet
Dissolution was carried out in 0.1 N HCl (2% SLS) and phosphate buffer pH 7 (2% SLS). Percentage cumulative percentage drug released at each time interval was calculated and plotted against time.
Stability study of optimized batch
The optimized formulation of lovastatin tablets was selected for the stability studies. The accelerated stability studies were carried out according to ICH guidelines by storing the samples at 40±2o and 75±5% RH for 6 months. The tablets were evaluated for hardness, drug content, and dissolution study and compared with room temperature samples (25±2º/60±5% RH).
Results and Discussion
Solubility of the drug was determined in 4 different solvents and results are recorded in Table 2. It was found that drug is insoluble in water and freely soluble in propylene glycol, PEG 200, PEG 400. Maximum solubility was found in PEG 400, so PEG 400 was chosen as liquid vehicle in liquisolid formulation.
| Solvent | Concentration (mg/ml) |
| Water | 0.002 ± 0.001 |
| Propylene glycol | 7.8 ± 0.6 |
| PEG 200 | 13.6 ± 0.5 |
| PEG 400 | 15.155 ± 0.8 |
Table 2: Solubility Of Lovastatin.
The ?CA?value and ?CO?value decide the amount of carrier and coating materials required to produce dry?looking, nonadherent, free?? owing and readily compactible liquisolid formulations. Hence, determination of the ?ow properties of powder excipients and liquid/powder admixtures is an important step to produce a successful liquisolid formulation.
The obtained ??values were plotted against the corresponding angle of repose. The ??value which corresponded to an angle of repose of 33° represents the flowable liquid retention potentials of powder. In cases where the ??value did not correspond to 33°, the highest ??value reached was chosen as the flowable liquid retention potential. The results are shown in figs. 1 and 2. ?CA?value for Avicel® PH 200 and ?CO?value for Cab?O?Sil were found to be 0.15 and 1.975, respectively.
Liquid loading factor was calculated using determined ?CA?value and ?CO?value. Then, Liquisolid powder blend was prepared as mentioned in methodology and evaluated for following precompression parameters. The angle of repose, Carr’s index (compressibility index), and Hausner’s ratio were determined and their results are presented in Table 3.
| Formulation | Angle of | Carr’s | Hausner’s |
|---|---|---|---|
| code | repose (θ) | index (%) | ratio |
| F1 | 28.8 ± 0.5 | 32.1 ± 0.8 | 1.47 ± 0.02 |
| F2 | 29.4 ± 0.2 | 33.3 ± 0.5 | 1.5 ± 0.12 |
| F3 | 29.6 ± 0.8 | 25 ± 0.5 | 1.33 ± 0.03 |
| F4 | 29.9 ± 0.5 | 19.2 ± 0.4 | 1.23 ± 0.05 |
| F5 | 31.9 ± 1.2 | 19 ± 0.5 | 1.25 ± 0.08 |
| F6 | 33 ± 1.0 | 28.1 ± 0.8 | 1.29 ± 0.06 |
| F7 | 32.2 ± 1.0 | 20.2 ± 0.5 | 1.25 ± 0.05 |
| F8 | 33.7 ± 1.3 | 30.9 ± 1.2 | 1.44 ± 0.02 |
| F9 | 35.6 ± 1.2 | 31.7 ± 1.0 | 1.46 ± 0.06 |
Table 3: Flowability Parameters Of Lovastatin Liquisolid Powder Systems.
As the angle of repose (Φ) is a characteristic of the internal friction or cohesion of the particles, the value of the angle of repose will be high if the powder is cohesive and low if the powder is noncohesive. As presented in Table 3, formulation F1?F5, F7 showed acceptable flowability according to the angle of repose measurements, while those having higher angles of repose were considered as nonacceptable.
Powders showing Carr’s index (Ci) up to 25 are considered of acceptable flow properties. In addition to Carr’s index, Hausner’s ratio found that the ratio was related to the inter particle friction, so that powders with low interparticle friction, had ratios of approximately 1.25 indicating good flow. Therefore, formulation F3?F5 and F7 showed acceptable Carr’s index and formulation F4?F7 showed acceptable Hausner’s ratio.
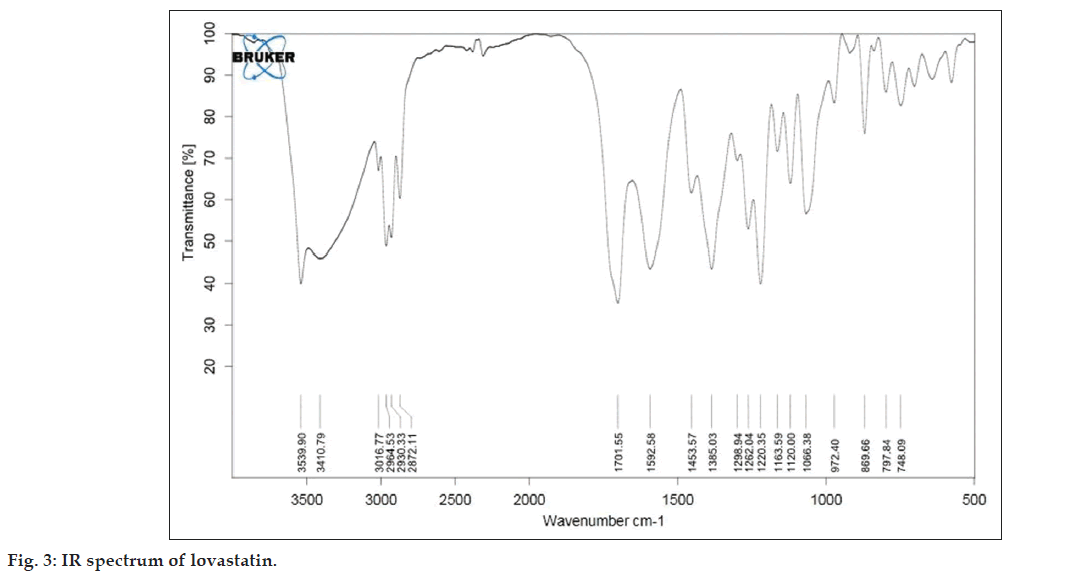
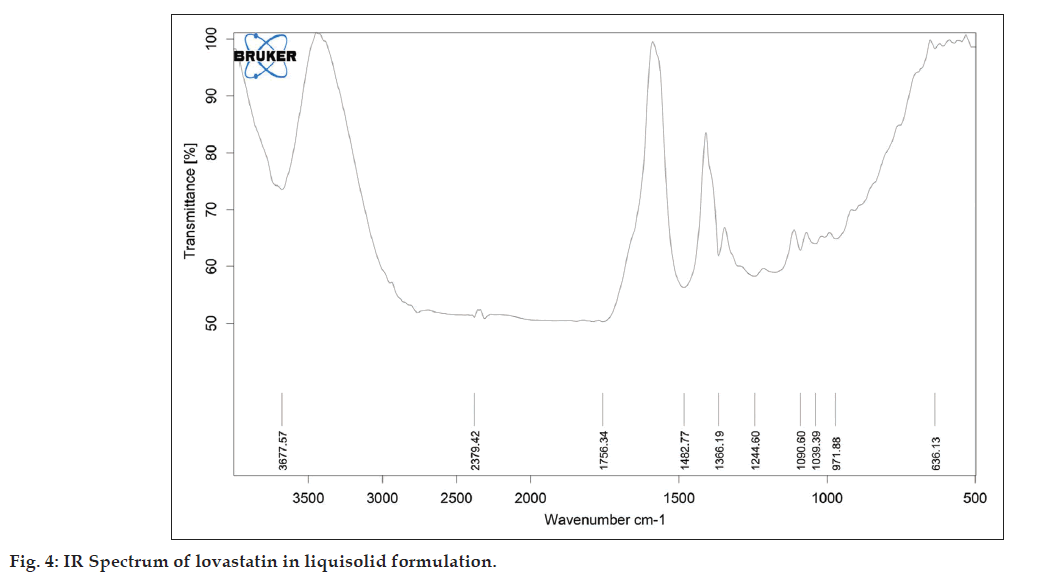
As described in the methodology section the FTIR was performed to detect any sign of interaction, which would be reflected by a change in the position or disappearance of any characteristic stretching vibration of lovastatin. IR spectrum of pure lovastatin and liquisolid formulation was shown in the figs. 3 and 4, respectively.
From the infrared spectrum of pure lovastatin, absorption bands are observed at various frequencies. The 3539.90 cm?1 band is assigned to the –OH stretching vibration and 1701.55 cm?1 band is assigned to the –C=O stretching vibration of saturated 6?ring lactones. The 1592 cm?1 band is due to the asymmetric stretching vibration of the carbonyl group and the 1385 cm?1 band is assigned to the stretching vibration of the –C?O group in the lovastatin structure.
It is evident from fig. 4 that the characteristic absorption band of lovastatin (3539.90 and 1592.58 cm?1) has disappeared and has been replaced by broad peak. This might be attributed to change in crystalline form into molecularly dispersed lovastatin into PEG 400.
The liquisolid tablets were evaluated for drug content, hardness, friability, weight variation and disintegration time. All the lovastatin liquisolid tablets had acceptable friability as none of the tested formula had percentage loss in tablets weights that exceeded 1%; also, none of the tablets cracked, split or broke. Since all the prepared formula met the standard friability criteria, they are expected to show acceptable durability and withstand abrasion in handling, packaging and shipment. The results are recorded in Table 4. The mean hardness of each liquisolid formula was determined and is presented in Table 4 proving that all the liquisolid tablet formula had acceptable hardness.
| Formulation code |
Hardness (kg/cm2) |
Friability(%) | Uniformity ofweight (mg) | Drugcontent (%) | Disintegration time (sec) |
%CDR 0.1NHCl (60 min) |
|---|---|---|---|---|---|---|
| F1 | 3 ± 0.5 | 0.60 ± 0.08 | 279.4 ± 3.78 | 96.2 ± 1.2 | 331 ± 3 | 78.64 ± 1.8 |
| F2 | 3.5 ± 0.2 | 0.43 ± 0.1 | 318 ± 2.44 | 97.3 ± 1.8 | 348 ± 5 | 83.02 ± 2.0 |
| F3 | 4 ± 0.2 | 0.29 ± 0.06 | 341.8 ± 1.78 | 100.2 ± 1.5 | 352 ± 7 | 80.57 ± 1.5 |
| F4 | 4.5 ± 0.5 | 0.40 ± 0.05 | 454.6 ± 2.96 | 97.2 ± 2.0 | 300 ± 5 | 80.52 ± 0.5 |
| F5 | 5.5 ± 0.2 | 0.13 ± 0.02 | 520.4 ± 4.39 | 97.8 ± 1.5 | 198 ± 2 | 84.46 ± 1.8 |
| F6 | 6 ± 1.0 | 0.053 ± 0.05 | 559.2 ± 2.28 | 99.4 ± 1.0 | 521 ± 5 | 74.13 ± 2.0 |
| F7 | 5 ± 0.6 | 0.225 ± 0.018 | 628.6 ± 1.81 | 102.3 ± 1.8 | 365 ± 8 | 79.88 ± 2.5 |
| F8 | 2.5 ± 1.0 | 0.499 ± 0.05 | 718.4 ± 2.3 | 104.3 ± 0.5 | 515 ± 4 | 72.95 ± 2.5 |
| F9 | 3 ± 0.5 | 0.458 ± 0.08 | 784.2 ± 2.28 | 97.3 ± 1.5 | 373 ± 4 | 65.48 ± 1.6 |
CDR is cumulative drug release. SD is standard deviation for n=3 observations
Table 4: Postcompression Evaluation Parameters Of The Liquisolid Tablets.
Hardness was found to be increase from formulation F1 to F7. This may be due to hydrogen bonds between hydrogen groups on adjacent cellulose molecules in Avicel PH 200 may account almost exclusively for the strength and cohesiveness of compacts. In addition, PEG 400 molecule contain more hydroxyl groups, thus there is also a probability of forming hydrogen bonds with Avicel PH 200. Weight variation and drug content test was determined for all formulations and results are mentioned in Table 4. All formulations met the standard criteria for weight variation and drug content.
The disintegration time test revealed that the liquisolid tablet formulation F1?F5 and F7 disintegrated in less time. F4, F5 show less disintegration time compare to F1?F3, this may be due to increase amount of liquid, which may help in disintegration. Since our aim was to improve Lovastatin bioavailability via improving the tablets’ physical characteristics, the long disintegration time of might retard the drug release and therefore bioavailability.
The dissolution tests were carried out for all batches as per USP and drug release obtained after 60 min are mentioned in Table 4. Formulae F4, F5 and F6 showed better release profile compared to all other formula. F5 was selected as optimized batch because it showed good flow properties, hardness, friability and minimum disintegration time compared to all other batches.
Dissolution studies for the liquisolid formulation (F5) and conventional (direct compression) product were conducted in different media and the percent drug release at different time intervals is recorded in Table 5.
| Formulation | % CDR | |
|---|---|---|
| F5 | CDT | |
| 0.1 N HCl (2% SLS) | ||
| 20 min | 47.2 ± 1.5 | 41.20 ± 2.5 |
| 40 min | 74.71 ± 2.0 | 63.41 ± 1.6 |
| 60 min | 84.46 ± 1.8 | 68.92 ± 2.0 |
| Buffer pH 7 (2% SLS) | ||
| 10 min | 55.03 ± 1.5 | 42.63 ± 1.5 |
| 30 min | 98.98 ± 1.2 | 82.49 ± 1.0 |
Comparison of dissolution of liquisolid tablets and conventional tablet in different dissolution medium at different time interval. CDT is conventional tablet, CDR is cumulative drug release, SLS is sodium lauryl sulphate.
Table 5: Comparison Of Dissolution Of Liquisolid Tablets And Conventional Tablets.
The dissolution studies revealed that in 0.1N HCl, liquisolid formulation showed more than 80% of drug release in 60 min. But, CDT failed to achieve more than 80% of drug release in 60 min. However in phosphate buffer (pH 7) more than 80% of the drug was released from both liquisolid and CDT formulation within 30 min. ANOVA was applied to compare the dissolution data of LS and CDT. P value was found to be less than 0.05, hence, it can be concluded that there was significant enhancement in dissolution of drug when formulated as liquisolid formulation, as compared to CDT.
The most important observation Table 5 signifies is F5 had higher drug dissolution rate and larger amounts of drug dissolved in the ?rst 10 min in phosphate buffer pH 7 and first 40 min in 0.1N HCl than the conventional, directly compressed lovastatin tables. This could be explained according to the Noyes–Whitney equation and the diffusion layer model dissolution theories, the dissolution rate of a drug is equal to DR=(D/h) S (CS?C), where DR is the dissolution rate of the drug particles, D is the diffusion coefficient of the dissolved drug particles, which affected by the viscosity of the dissolution medium; S is the surface area exposed to dissolution; h is the thickness of the diffusion layer, and it is affected by agitation; Cs is the saturation solubility of the drug in solution in the diffusion layer, and C is the concentration of the drug in the dissolution medium. All the dissolution tests were stirred under the same paddle speed (50 rpm) and dissolution media with same viscosity; therefore, h and D were assumed to be constant. Therefore, this leaves S and (Cs?C) as the factors affecting dissolution rates of liquisolid formulations.
The drug particles in liquisolid formulations were dispersed in selected hydrophilic liquid vehicle (lovastatin in PEG 400), which means the wetting properties of the drug particles were increased; hence, the surface area of drug particles available for dissolution increased tremendously. After liquisolid tablet was disintegrated, the primary particles of liquisolid suspended in the dissolution medium contained drug particles in a state of molecular dispersion.
For conventional tablet, the surface exposed for dissolution is very limited, due to the hydrophobicity of the drug particles. Accordingly, the higher dissolution rates observed in liquisolid formulations may be attributed to significantly larger surface area of the molecularly dispersed drug particles.
Since the drug particles in liquisolid formulations are in a state molecular dispersion, its saturation solubility (Cs) might be increased. The small amount of liquid vehicle in liquisolid tablet might not be adequate to increase the overall saturation solubility of drug particles in the dissolution medium.
Nevertheless, in the diffusion layer (the solid/liquid interface between primary liquisolid particles and dissolution medium), in such a micro?environment, it is highly possible that infinite amounts of PEG 400 diffuse with the drug particles away from the primary liquisolid particles. In this case, small amount of liquid vehicle might be sufficient to improve the solubility of drug particles by acting as a co?solvent with the dissolution medium of the diffusion layer. As a consequence of increase in Cs, the concentration gradient (Cs?C) of the drug will be increased, and hence, the drug dissolution rate increased.
According to ICH guidelines stability studies were conducted by storing the optimized batch sample at room temperature (25±2º/60±5% RH) and accelerated condition (40±2º/75±5% RH) for 6 months. Results after 6 months studies, shown in Table 6, revealed that prepared liquisolid tablet of lovastatin stable for long period of time.
| Parameter | Room temperature (25 ± 2º/60% ± 5% RH) |
Accelerated condition (40 ± 2º/75 ± 5% RH) |
|---|---|---|
| Hardness (kg/cm2) | 5.5 ± 0.28 | 5.5 ± 0.50 |
| Drug content (%) | 98.2 ± 0.17 | 97.9 ± 0.5 |
| Disintegration time (sec) | 213 ± 2 | 220 ± 3 |
| %CDR* (0.1N HCl, 60 min) | 84.1 ± 2.5 | 83.79 ± 1.8 |
CDR is cumulative drug release. SD is standard deviation for n=3 observations.
Table 6: Stability Studies.
Lovastatin exhibits high permeability through biological membranes, but its absorption after oral administration is limited by its low dissolution rate due to its very low aqueous solubility. Hence, the use of the liquisolid technique was chosen to enhance the dissolution properties of Lovastatin.
From the results obtained during investigation, it was found that because of higher solubility in PEG 400, it could be selected as liquid vehicle for the drug. Avicel PH 200 and Cab?O?Sil were suitable excipient as carrier and coating material, respectively, because they showed good flow properties compared to other material evaluated for flow properties. Liquisolid tablet with 1:4 drug:liquid ratio and 15:1 carrier:coating ratio was found to be the best formulation in terms of acceptable flow property, sufficient hardness and friability, faster disintegrate and enhanced dissolution. The in vitro dissolution study confirmed enhanced drug release from liquisolid compacts compared with directly compressed tablets.
Liquisolid technique changes the properties of lovastatin by simply dispersing the drug particles in a nonvolatile hydrophilic liquid vehicle, which in turn increases the wetting properties and surface area of drug particles, and hence improves the dissolution. The improved dissolution may enhance the oral bioavailability of the drug. Thus, the liquisolid technique was found to be a promising approach for improving the dissolution of a poorly soluble drug like lovastatin.
Acknowledgements
The authors thank Emcure Pharmaceuticals, Pune, India, for providing lovastatin as a gift sample for this work.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Wong SM, Kellaway IW, Murdan S. Enhancement of the dissolution rate and oral absorption of a poorly water soluble drug by formation of surfactant?containing microparticles. Int J Pharm 2006;317:61?8.
- Ajit SK, Nagesh H, Madhav S. Liquisolid systems. Int J Pharm Sci Nano 2010;3:795?802.
- Horter D, Dressman JB. Inflluence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv Drug Deliv Rev 1997;46:75?87.
- Lobenberg R, Amidon GL. Modern bioavailability, bioequivalence and biopharmaceuticsclassifiication system. New scientifiic approaches to international regulatory standards. Eur J Pharm Biopharm 2000;50:3?12.
- Lipinski C. Poor Aqueous Solubility ? An Industry wide problem in Drug Discovery. Am Pharm Rev 2002;5:82?5.
- Sharma A, Jain CP. Techniques to enhance solubility of poorly soluble drugs: A review. J Global Pharm Tech 2010;2:18?28.
- Saharan VA, Kukkar V. Dissolution enhancement of Drugs. Part II: Effect of Carriers. Int J Health Res 2009;2:207?23.
- Hentzschel CM, Sakmann A, Leopold CS. Suitability of Various Tableting Excipients as Carriers for Liquisolid Systems. 7th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology 2010, Malta.
- Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins. Drug solubilization and stabilization. J Pharm Sci 1996;85:1017?25.
- Valizadeh H, Zakeri?Milani P, Barzegar?Jalali M, Mohammadi G, Danesh?Bahreini MA, Adibkia K, et al. Preparation and characterizationof solid dispersions of piroxicam with hydrophilic carriers. Drug DevInd Pharm 2007;33:45?56.
- Bindu MB, Kusum B. Novel strategies for poorly water soluble drugs. Int J Pharm Sci Rev Res 2010;4:76?84.
- Nokhodchi A, Javadzadeh Y, Siahi?Shadbad MR, Barzegar?Jalali M. The effect of type and concentration of vehicles on the dissolution rate of a poorly soluble drug (Indomethacin) From Liquisolid Compacts. Int J Pharm Sci 2005;8:18?25.
- Khaled KA, Asiri YA, El ?Sayed YM. In vivoevaluation of hydrochlorothiazide liquisolid tablets in Beagle dogs. Int J Pharm 2001;222:1?6.
- Spireas S, Sadu S. Enhancement of prednisolone dissolution properties using liquisolid compacts. Int J Pharm 1998;166:177?88.
- Javadzadeh Y, Shariati H, Movahhed? Danesh E, Nokhodchi A. Effects of different grades of microcrystalline cellulose on flowability, compressibility and dissolution of liquisolid systems. Drug DevInd Pharm 2009;35243?51.
- Javadzadeh Y, Siahi?Shadbad MR, Barzegar?Jalali M, Nokhodchi A. Enhancement of dissolution rate of piroxicam using liquisolid compacts. Farmaco 2005;60:361?5.
- Enhancement of dissolution rate of piroxicam using liquisolid compacts. Farmaco 2005;60:361?5.
- Emmadi S, Sanka K, Potu AR, Jukanti R, Bandari S, Reddy VR. Formulation and Pharmacodynamic Evaluation of Meloxicam Liquisolid Compacts. Lat Am J Pharm 2010;29:1303?10.
- Akinlade B, Elkordy AA, Essa EA, Elhagar S. Liquisolid system to improve the dissolution of furosemide. SciPharma 2010;78:325?44.
- Spireas S, Bolton M. Liquisolid systems and methods of preparing same. 1999, U.S. Patent 5,968,550. U.S. Patent 6,423,339 B1.
- Spireas S, Wang T, Grover R. Effect of Powder substrate on dissolution properties of MethylclothiazideLiquisolid compacts. Drug DevInd Pharm 1999;25:163?8.
- Patric JS. Martin?s Physical Pharmacy and Pharmaceutical Sciences. 2nd ed. Indian reprint. New Delhi: Wolters Kluwer (India) Pvt. Ltd.; 2008. p. 233?45.
- Fahmy RH, Kassem MA. Enhancement of famotidine dissolution rate through liquisolid tablet formulation: In vitroandIn vivoEvaluation.Eur J Pharm Biopharm 2008;69:993?1003.
- Lachman L, Liberman HA, Kanig JL. The Theory and Practice of Industrial Pharmacy. 3rd ed. Mumbai, India: Varghese Publishing House; 1987. p. 293?345.
- Gubbi SR, Jarag R. Formulation and characterisation of atorvastatin calcium liquisolid compacts. Asian J Pharm Sci 2010;5:50?60.
- United States Pharmacopeia/National Formulary. 19th ed, Rockville, MD: Pharmacopoeial Convention; 2000. p. 292?3.S