- Corresponding Author:
- V. V. Dabholkar
Organic Research Laboratory, Department of Chemistry, Kishinchand Chellaram College, D. W. Road, Churchgate, Mumbai-400 020, India,
E-mail vijaydabholkar@gmail.com
| Date of Submission | 2 January 2011 |
| Date of Revision | 26 April 2011 |
| Date of Acceptance | 27 April 2011 |
| Indian J Pharm Sci, 2011, 73 (2): 199-207 |
Abstract
A series of new dicarboxylic acid derivatives of 1,3,4-thiadiazines, 1,4-benzopiperizines, 1,4-thiazines, 1,3-thiazoles, 1,3-oxazoles and 1,3-imidazoles have been synthesized in 80-87% yield by the environmentally benign microwave induced technique involving the cyclocondensation of 2,3-dibromosuccinic acid with 2-aminothiophenol, o-phenylene diamine, 1,2,4-triazole, amidinothiocarbamide, amidinocarbamide and guanidine hydrochloride. The structures of all newly synthesized compounds have been established on the basis of analytical and spectral data. Evaluation of antibacterial and antifungal activity showed that almost all compounds exhibited better results than reference drugs thus they could be promising candidates for novel drugs.
Keywords
Imidazole, thiazine, thiadiazine, thiazole, microwave, oxazole, 2,3-dibromosuccinic acid
Thiadiazine and its derivatives have found a wide range of application in medicine due to their pronounced biological activity. Many of these compounds have proved to be effective as cholecystokinin-ligands, CCK-receptor ligands [1] and active as antibacterial agent [2]. Piperazine based derivatives used as inhibitors of plasminogen activator inhibitor-1 (PAI-1) [3] and as CBI cannabinoid receptor ligands [4]. The presence of a reactive thiazine ring in compound is found to be responsible for their antimycobacterial activity [5] and broad-spectrum inhibitors of the MMPs with IC50'S against MMP-1 [6]. The compounds with the backbone of thiazoles have been reported to possess various biological activities such as cycotoxic [7] and it also act as transforming growth factor-β type 1 receptor kinase inhibitors [8]. Oxazole derivatives have attracted attention because of their potential biological activity as brain-derived Neurotrophic Factor Inducers [9]. Imidazole moiety has been reported to be associated with a variety of pharmacological activities that include antitubercular [10], antiviral [11], antimuscarinic [12], gastric H+/K+-ATPase inhibitory [13], MAP kinase and p38 inhibitory [14] and as a novel class of HIV-1 non-nucleoside reverse transcriptase inhibitors [15]. Glucuronidation of carboxylic acid containing drugs can yield reactive acyl (ester-linked) glucuronide metabolites that are able to modify endogenous macromolecules. Previous research has shown that several carboxylic acid drugs are genotoxic in isolated mouse hepatocytes, and that DNA damage is prevented by the glucuronidation inhibitor, borneol [16]. Introduction of carboxylic group will enhance the activity of a drug containing the above moiety, as it would form a Na/K-carboxylate, which would then easily get absorbed through the gastrointestinal tract (GIT).
Thus, keeping in mind the pharmacological potential of investigated moieties as well as taking advantage of biodegradability and biocompatibility of acidic group and further, in continuation of our earlier work on synthesis of bioactive heterocycles under microwave irradiation [17-25], an attempt was made towards the synthesis of titled novel series of dicarboxylic acid derivatives of the thiadiazines, piperazines, thiazines, thiazoles, oxazoles, imidazoles. The same compounds have been also synthesized by classical thermal method for comparative studies. All the new compounds were characterized by mp, elemental analyses and spectroscopic data (NMR, MS and IR). The spectral data and the elemental analysis of the new compounds reported in this study correlate with the proposed structures. The newly synthesized compounds were tested for their in vitro antimicrobial properties against Gram positive and Gram-negative bacteria and fungi.
Materials and Methods
Melting points were determined using digital meltingpoint apparatus. FT-IR spectra were recorded as KBr pellets on a Jasco FT/IR-600 Plus spectrometer. 1H-NMR and 13C-NMR spectra were recorded on Jeol NMR spectrometer (300 MHz) using CDCl3 and DMSO-d6 as a solvent and TMS as internal standard. The mass spectra were recorded on a JMS-DX 303 mass spectrometer (Jeol, Tokyo, Japan) operating at 70 eV using the electron spray ionization technique (ESI MS). The homogeneity of the compounds was checked on Aluminium backed silica gel coated TLC plates (Merck) as adsorbent and UV light was used for visualization. All the transformation was carried out in CEM microwave reactor (2.45 GHz, up to 300 W, 125-ml round-bottom flask, condenser, overhead stirring).
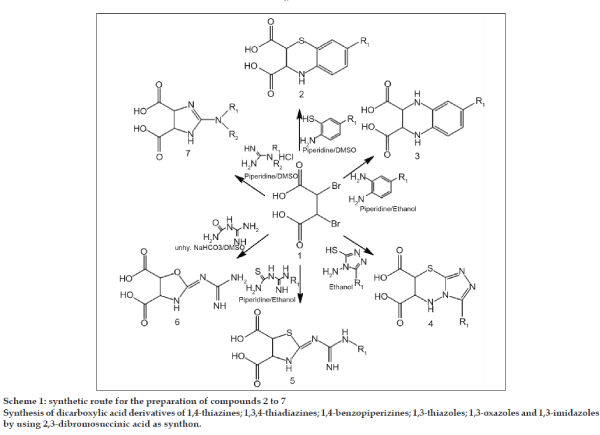
Synthesis of 2,3-dihydro-benzo[b]1,4-thiazine-2,3- dicarboxylic acid 2
A solution of 2-aminothiophenol (0.01 mol) in DMSO (15 ml) containing piperidine (0.02 mol) was irradiated with 2,3-dibromosuccinic acid 1 (0.01 mol) in microwave reactor for 5 min (Scheme 1). The reaction was monitored by TLC and after completion of the reaction; the reaction mixture was poured into cold water and extracted with ether. The organic layer was washed with DM water and dried on sodium sulphate. Ether was distilled under vacuum at 30°. The separated solid was purified by column chromatography (n-Hexane: ethyl acetate; 9:1).
2,3-dihydro-benzo[b]1,4-thiazine-2,3-dicarboxylic acid 2a; Brick red crystal; IR (cm-1): 3696 (OH), 3355 (NH), 1742 (C=O); δH (300 MHz, DMSO-d6): 2.210- 2.227 (d, 1H, HC-S, J=5.1 Hz), 3.826-3.843 (d, 1H, HC-N, J=5.1 Hz), 7.17-7.79 (m, 4H, Ar H), 9.30 (s, 1H, ring NH), 12.71 (s, 2H, 2×OH); δC (300 MHz, DMSO-d6): 60.18 (HC-S), 82.58 (HC-N), 121.59- 136.84 (Aromatic C atoms), 181.54 and 184.15 (C=O); m/z 240 M+, 215, 179, 165, 164, 163, 161, 138; Elem. Anal Calcd for C10H9NO4S: C 50.20, H 3.79, N 5.85; Found 50.13, 3.71, 5.78.
2,3-dihydro-3'-methyl benzo[b]1,4-thiazine-2,3- dicarboxylic acid 2b; Brown solid; IR (cm-1): 3698 (OH), 3356 (NH), 2973 (CH), 1741 (C=O); δH (300 MHz, DMSO-d6): 2.210-2.227 (d, 1H, HC-S, J=5.1 Hz), 2.47 (s, 3H, CH3), 3.826-3.843 (d, 1H, HC-N, J= 5.1 Hz), 7.09-7.64 (m, 3H, Ar H), 9.28 (s, 1H, ring NH), 12.53 (s, 2H, 2×OH); δC (300 MHz, DMSO-d6): 19.38 (CH3), 60.78 (HC-S), 83.09 (HCN), 122.30-139.26 (Aromatic C atoms), 181.60 and 183.56 (C=O); m/z 254 M+, 229, 193, 162, 139, 122, 107, 91; Elem. Anal Calcd for C11H11NO4S: C 52.16, H 4.38, N 5.53; Found 52.04, 4.29, 5.41.
2,3-dihydro-3'-chloro benzo[b]1,4-thiazine-2,3- dicarboxylic acid 2c; Brown solid; IR (cm-1): 3696 (OH), 3355 (NH), 2974 (CH), 1740 (C=O); δH (300 MHz, DMSO-d6): 2.209-2.226 (d, 1H, HC-S, J=5.1 Hz), 3.825-3.842 (d, 1H, HC-N, J=5.1 Hz), 7.13-7.57 (m, 3H, Ar H), 9.15 (s, 1H, ring NH), 12.60 (s, 2H, 2×OH); δC (300 MHz, DMSO-d6): 59.23 (HC-S), 82.67 (HC-N), 121.40-138.45 (Aromatic C atoms), 180.74 and 183.14 (C=O); m/z 273.5 M+, 255.5, 238, 220, 177, 151, 104, 75; Elem. Anal Calcd for C10H8ClNO4S: C 43.88, H 2.95, N 5.12; Found 43.72, 2.83, 5.02.
2,3-dihydro-3'-methoxy benzo[b]1,4-thiazine-2,3- dicarboxylic acid 2e; Brown solid; IR (cm-1): 3698 (OH), 3357 (NH), 2972 (CH), 1741 (C=O); δH (300 MHz, DMSO-d6): 2.210-2.227 (d, 1H, HC-S, J= 5.1 Hz), 3.76 (s, 3H, OCH3), 3.827-3.844 (d, 1H, HC-N, J=5.1 Hz), 7.10-7.79 (m, 3H, Ar H), 9.34 (s, 1H, ring NH), 12.48 (s, 2H, 2×OH); δC (300 MHz, DMSO-d6) 55.76 (OCH3), 60.09 (HC-S), 84.16 (HC-N), 120.43- 138.28 (Aromatic C atoms), 181.08 and 184.42 (C=O); m/z 270 M++1, 252, 239, 221, 177, 151, 104; Elem. Anal Calcd for C11H11NO5S: C, 49.06; H, 4.12; N, 5.20; Found 48.95, 4.03, 5.14.
Synthesis of 2,3-dihydro-benzo[b]1,4-piperizine-2,3- dicarboxylic acid 3
2,3-dibromosuccinic acid 1 (0.01 mol) and 1,2-diaminobenzene (0.01 mol) were taken in ethanol (20 ml) containing piperidine (0.02 mol) and the mixture was irradiated in microwave reactor (4 min). The reaction was monitored by TLC and after completion of the reaction; the ethanol was distilled out u/v. The solid mass was diluted with 15 ml of absolute alcohol and heated to 60° on water bath. 40% NaOH solution was added to it at the same temperature and stirred for one hour (pH= 10). Na salt of 3 was isolated by filtration and dissolved in 10 ml of DM water. Re-precipitated by adding 1:1 HCl (pH=7), filtered and washed with water. 2,3-dihydro-benzo[b]1,4-piperizine-2,3-dicarboxylic acid 3a; Brown solid; IR (cm-1) 3698 (OH), 3356 (NH), 1741 (C=O), δH (300 MHz, DMSO-d6): 3.815- 3.832 (dd, 2H, HC-N, J=5.1 Hz), 7.05-7.64 (m, 4H, Ar H), 8.56 (s, 2H, ring NH), 12.68 (s, 2H, 2×OH); δC (300 MHz, DMSO-d6): 81.85 (HC-N), 122.59-137.44 (Aromatic C atoms), 182.32 (C=O); m/z 222 M+, 204, 124, 98, 76; Elem. Anal Calcd for C10H10N2O4: C 54.05, H 4.45, N 12.61; Found 53.97, 4.38, 12.54.
2,3-dihydro-3'-methyl benzo[b]1,4-piperizine-2,3- dicarboxylic acid 3b; Brown solid; IR (cm-1): 3698 (OH), 3355 (NH), 1742 (C=O); δH (300 MHz, DMSO-d6): 2.42 (s, 3H, CH3), 3.824-3.841 (d, 1H, HC-N, J=5.1 Hz), 7.14-7.71 (m, 3H, Ar H), 9.29 (s, 1H, ring NH), 12.70 (s, 1H, 2×OH); δC (300 MHz, DMSO-d6): 18.23 (CH3), 81.48 (HC-N), 122.12- 136.52 (Aromatic C atoms), 181.86 (C=O); m/z 236 M+, 218, 118, 116, 91; Elem. Anal Calcd for C11H12N2O4: C 55.93, H 5.12, N 11.86; Found 55.81, 5.01, 11.75.
Synthesis of 5'-substituted-1',2',4'-triazolo[3',4'- b]4H-2,3-dihydro-1,4-thiadiazine-2,3-dicarboxylic acid 4
4-amino-5-methyl-3-mercapto-1,2,4-triazole (0.01 mol) and 2,3-dibromosuccinic acid 1 (0.01 mol) were taken in methanol (25 ml) and irradiated in microwave reactor for 4 min. The reaction was monitored by TLC and after completion of the reaction; reaction mixture was filtered in hot condition. Filtrate was concentrated u/v, which yielded compound 4.
5'-methyl-1',2',4'-triazolo[3',4'-b]4H-2,3-dihydro-1,4- thiadiazine-2,3-dicarboxylic acid 4a; Yellow crystal; IR (cm-1): 3697 (OH), 3356 (NH), 1740 (C=O), 1657, 1649 (C=N); δH (300 MHz, DMSO-d6): 2.14 (s, 3H, CH3), 2.209-2.226 (d, 1H, HC-S, J= 5.1 Hz), 3.825- 3.842 (d, 1H, HC-N, J= 5.1 Hz), 9.46 (s, 1H, ring NH), 12.72 (s, 2H, 2×OH); δC (300 MHz, DMSO-d6): 10.56 (CH3), 59.45 (HC-S), 83.72 (HC-N), 151.36 and 153.84 (C=N), 182.45 and 185.25 (C=O); m/z 246 M++2, 216, 182, 170, 155, 116, 96, 82; Elem. Anal Calcd for C7H8N4O4S: C 34.42; H 3.30; N 22.94; Found 34.29, 3.18, 22.83.
5'-ethyl-1',2',4'-triazolo[3',4'-b]4H-2,3-dihydro-1,4- thiadiazine-2,3-dicarboxylic acid 4b; Pale yellow crystal; IR (cm-1): 3698 (OH), 3356 (NH), 1741 (C=O), 1658, 1648 (C=N); δH (300 MHz, DMSO-d6): 1.16-1.20 (t, 3H, CH3), 2.210-2.227 (d, 1H, HC-S, J= 5.1 Hz), 3.75-3.85 (q, 2H, CH2), 3.827-3.844 (d, 1H, HC-N, J=5.1 Hz), 9.39 (s, 1H, ring NH), 12.73 (s, 2H, 2×OH); δC (300 MHz, DMSO-d6): 9.26 (CH3), 19.25 (CH2), 59.89 (HC-S), 83.24 (HC-N), 152.35 and 154.59 (2×C=N), 181.45 and 184.26 (C=O); m/z 260 M++2, 236, 196, 184, 169, 142, 110, 96; Elem. Anal Calcd for C8H10N4O4S: C 37.21; H 3.90; N 21.69; Found 37.12, 3.88, 21.58.
Synthesis of 3H-2-guanidino/substituted guanidino- 4,5-dihydro-1,3-thiazole-4,5-dicarboxylic acid 5
Compound 1 (0.01 mol) was allowed to interact with substituted amidinothiocarbamide (0.01 mol) in presence of piperidine (0.02 mol) and ethanol (20 ml) under MWI for 5 min. Monitored by TLC, reaction mixture cooled to RT and diluted by 30 ml ice-cold water. Solid obtained was filtered, washed with 2×10 ml of DM water, dried and purified by Column chromatography (n-Hexane: ethyl acetate; 9.5:0.5).
3H-2-guanidino-4,5-dihydro-1,3-thiazole-4, 5-dicarboxylic acid 5a; Black Crystal; IR (cm-1): 3697 (OH), 3429 (NH2), 3360, 3357 (NH), 1740 (C=O), 1656, 1648 (C=N); δH (300 MHz, DMSO-d6): 2.211- 2.228 (d, 1H, HC-S, J=5.1 Hz), 3.825-3.842 (d, 1H, HC-N, J=5.1 Hz), 5.12 (s, 2H, NH2), 6.82 (s, 1H, NH), 9.31 (s, 1H, ring NH), 12.70 (s, 2H, 2×OH); δC (300 MHz, DMSO-d6): 59.68 (HC-S), 83.82 (HC-N), 151.26 and 154.59 (C=N), 182.50 and 184.56 (C=O); m/z 232 M+, 216, 207, 144, 115, 81; Elem. Anal Calcd for C6H8N4O4S: C 31.03, H 3.47, N 24.13; Found 30.96, 3.41, 24.07.
3H-2-(phenyl)guanidino-4,5-dihydro-1,3-thiazole-4,5- dicarboxylic acid 5b; Black Crystal; IR (cm-1): 3697 (OH), 3364-3357 (NH), 1741 (C=O), 1655, 1647 (C=N); δH (300 MHz, DMSO-d6): 2.211-2.228 (d, 1H, HC-S, J=5.1 Hz), 3.825-3.842 (d, 1H, HC-N, J=5.1 Hz), 6.78 (s, 1H, NH), 7.26-7.89 (m, 5H, Ar H), 8.56 (s, 1H, NH), 9.29 (s, 1H, ring NH), 12.68 (s, 2H, 2×OH); δC (300 MHz, DMSO-d6): 59.63 (HC-S), 83.75 (HC-N), 123.56-138.25 (Aromatic C atoms), 151.45 and 154.05 (C=N), 181.45 and 183.18 (C=O); m/z 308 M+, 292, 283, 220, 219, 193, 151, 117; Elem. Anal Calcd for C12H12N4O4S: C 46.75, H 3.92, N 18.17; Found 46.68, 3.85, 18.12.
3H-2-(3'-methylphenyl)guanidino-4,5-dihydro-1,3- thiazole-4,5-dicarboxylic acid 5d; Black crystal; IR (cm-1): 3696 (OH), 3361-3356 (NH), 1741 (C=O), 1655, 1648 (C=N); δH (300 MHz, DMSO-d6): 2.210- 2.227 (d, 1H, HC-S, J=5.1 Hz), 2.42 (s, 3H, CH3), 3.825-3.842 (d, 1H, HC-N, J=5.1 Hz), 6.80 (s, 1H, NH), 7.19-7.61 (dd, 4H, Ar H), 8.53 (s, 1H, NH), 9.31 (s, 1H, ring NH), 12.65 (s, 2H, 2×OH); δC (300 MHz, DMSo-d6): 19.24 (CH3), 59.71 (HC-S), 83.69 (HC-N), 122.16-138.46 (Aromatic C atoms), 151.86 and 154.53 (C=N), 182.82 and 185.37 (C=O); m/z 322 M+, 306, 297, 234, 233, 207, 165, 131; Elem. Anal Calcd for C13H14N4O4S: C 48.44; H 4.38; N 17.38; Found 48.31, 4.28, 17.22.
Synthesis of 3H-2-guanidino-4,5-dihydro-1,3- oxazole-4,5-dicarboxylic acid 6
2,3-dibromosuccinic acid 1 (0.01 mol), amidinocarbamide (0.01 mol), fused NaOAc (0.02 mol) and DMSO (15 ml) were exposed to microwave irradiation for 6 min. Progress of the reaction was monitored by TLC and the product was isolated in a similar manner as described above for compound 5 to yield 6.
White amorphous solid; IR (cm-1): 3698 (OH), 3428 (NH2), 3361, 3356 (NH), 1742 (C=O), 1657, 1649 (C=N); δH (300 MHz, DMSO-d6): 2.210-2.227 (d, 1H, HC-S, J=5.1 Hz), 3.824-3.841 (d, 1H, HC-N, J=5.1 Hz), 5.08 (s, 2H, NH2), 6.75 (s, 1H, NH), 9.35 (s, 1H, ring NH), 12.72 (s, 2H, 2×OH); δC (300 MHz, DMSO-d6): 64.89 (HC-O), 83.80 (HC-N), 151.82 and 154.61 (C=N), 182.26 and184.51 (C=O); m/z 216 M+, 198, 173, 168, 159, 141, 99; Elem. Anal Calcd for C6H8N4O5: C 33.34, H 3.73, N 25.92; Found 33.27, 3.67, 25.86.
Synthesis of 3H-2-amino/substituted amino-4,5- dihydro-1,3-imidazole-4,5-dicarboxylic acid 7
Guanidine hydrochloride (0.01 mol) was added to the solution of 2,3-dibromosuccinic acid 1 (0.01 mol) in DMSO (15 ml) in microwave reactor. A catalytic amount of piperidine (0.02 mol) was added to it and the mass subjected to microwave irradiation for 4 min. Progress of the reaction was monitored by TLC. Upon completion of the reaction, the reaction mixture was poured onto crushed ice, thus 7 was obtained. It was filtered, washed with water purified by column chromatography (n-hexane:ethyl acetate; 9:1)
3H-2-amino-4,5-dihydro-1,3-imidazole-4,5-dicarboxylic acid 7a; Light brown crystal; IR (cm-1): 3698 (OH), 3428 (NH2), 3358 (NH), 1741 (C=O), 1645 (C=N); δH (300 MHz, DMSO-d6): 2.209-2.226 (d, 1H, HC-S, J=5.1 Hz), 3.62 (s, 2H, NH2), 3.824-3.841 (d, 1H, HC-N, J=5.1 Hz), 6.42 (s, 1H, ring NH), 12.56 (s, 2H, 2×OH); δC (300 MHz, DMSO-d6): 78.82 (HC-N), 83.69 (HC-N), 155.27 (C=N), 181.50 and 184.35 (C=O); m/z 173 M·, 157, 155, 140, 98; Elem. Anal Calcd for C5H7N3O4: C 34.69; H 4.08; N 24.27; Found 34.62, 4.01, 24.22.
3H-2-(N-methyl)amino-4,5-dihydro-1,3-imidazole-4,5- dicarboxylic acid 7b; Light brown crystal; IR (cm-1): 3698 (OH), 3362, 3358 (NH), 2974 (CH3), 1740 (C=O), 1644 (C=N); δH (300 MHz, DMSO-d6): 2.208- 2.225 (d, 1H, HC-S, J=5.1 Hz), 2.75 (s, 3H, CH3), 3.825-3.842 (d, 1H, HC-N, J=5.1 Hz), 5.73 (s, 1H, NH), 6.56 (s, 1H, ring NH), 12.59 (s, 2H, 2×OH); δC (300 MHz, DMSO-d6): 26.35 (CH3), 78.36 (HC-N), 82.17 (HC-N), 154.64 (C=N), 181.34 and 184.97 (C=O); m/z 187 M+, 172, 169, 154, 140, 116, 101; Elem. Anal Calcd for C6H9N3O4: C 38.51, H 4.85, N 22.45; Found 38.42, 4.71, 22.32.
Antifungal activity
For the antifungal bioassays, eight fungi were used: Aspergillus flavus (ATCC 9643), Aspergillus fumigatus (plant isolate), Aspergillus niger (ATCC 6275), Aspergillus versicolor (ATCC 11730), Fulvia fulvum (TK 5318), Penicillium funiculosum (ATCC 36839), Penicillium ochrochloron (ATCC 9112) and Trichoderma viride (IAM 5061). The organisms were obtained from the Department of Microbiology and Biotechnology, K. C. College, Mumbai.
The micromycetes were maintained on malt agar and the cultures stored at 4° and sub-cultured once a month [26]. In order to investigate the antifungal activity of the extracts, a modified micro dilution technique was used [27-29]. The fungal spores were washed from the surface of agar plates with sterile 0.85% saline containing 0.1% Tween 80 (v/v). The spore suspension was adjusted with sterile saline to a concentration of approximately 1.0×105 in a final volume of 100 μl per well. The inocula were stored at 4° for further use. Dilutions of the inocula were cultured on solid malt agar to verify the absence of contamination and to check the validity of the inoculum.
Minimum inhibitory concentration (MIC) determinations were performed by a serial dilution technique using 96-well microtiter plates. The compounds investigated were dissolved in DMSO (1 mg/ml) and added in broth Malt medium with inoculum. The microplates were incubated for 72 h at 28°, respectively. The lowest concentrations without visible growth (at the binocular microscope) were defined as MICs.
The fungicidal concentrations (MFCs) were determined by serial sub-cultivation of a 2 μl into microtiter plates containing 100 μl of broth per well and further incubation for 72 h at 28°. The lowest concentration with no visible growth was defined as MFC indicating 99.5% killing of the original inoculum. DMSO was used as a negative control, commercial fungicides, bifonazole and ketoconazole were used as positive controls (1-3000 μg/ml). All experiments were performed in duplicate and repeated three times.
Test for antibacterial activity
The following Gram negative bacteria were used: Escherichia coli (ATCC 35210), Pseudomonas aeruginosa (ATCC 27853), Salmonell typhimurium (ATCC 13311), Proteus mirabilis (human isolate) and the following Gram positive bacteria: Bacillus cereus (clinical isolate), M. flavus (ATCC 10240), Listeria monocytogenes (NCTC 7973), and Staphylococcus aureus (ATCC 6538). The organisms were obtained from the Department of Microbiology and Biotechnology, K. C. College, Mumbai.
The antibacterial assay was carried out by microdilution method [27-29] in order to determine the antibacterial activity of compounds tested against the human pathogenic bacteria. The bacterial suspensions were adjusted with sterile saline to a concentration of 1.0×105 CFU/ml. The inocula were prepared daily and stored at +4° until use. Dilutions of the inocula were cultured on solid medium to verify the absence of contamination and to check the validity of the inoculum. All experiments were performed in duplicate and repeated three times.
Microdilution test
The minimum inhibitory and bactericidal concentrations (MICs and MBCs) were determined using 96-well microtitreplates. The bacterial suspension was adjusted with sterile saline to a concentration of 1.0×105 cfu/ml. Compounds to be investigated were dissolved in broth LB medium (100 μl) with bacterial inoculum (1.0×104 cfu per well) to achieve the wanted concentrations (1 mg/ml). The microplates were incubated for 24 h at 48°. The lowest concentrations without visible growth (at the binocular microscope) were defined as concentrations that completely inhibited bacterial growth (MICs). The MBCs were determined by serial sub-cultivation of 2 μl into microtitre plates containing 100 μl of broth per well and further incubation for 72 h. The lowest concentration with no visible growth was defined as the MBC, indicating 99.5% killing of the original inoculum. The optical density of each well was measured at a wavelength of 655 nm by Microplate manager 4.0 and compared with a blank and the positive control. Streptomycin and Ampicillin were used as a positive control (1 mg/ml DMSO). All experiments were performed in duplicate and repeated three times.
Results And Discussion
The key intermediate in the synthesis of 2–7 was the 2,3-dibromosuccinic acid 1 which was prepared by reported procedure [30]. Compound 1 on condensation with 2-aminothiaphenol, o-phenelynediamine, 1,2,4-triazole, amidinothiocarbamide, amidinocarbamide and guanidine hydrochloride under different condition, exclusive formation of thiazine (2), benzopiperizine (3), 1,3,4-thiadiazino-s-triazole (4), 2-guanidino-1,3- thiazole (5), 2-guanidino-1,3-oxazole (6) and 2-amino- 1,3-imidazole (7) were achieved respectively (Scheme 1) and confirmed by spectral studies such as IR, 1H NMR, 13C NMR and MASS. Comparisons of the two steps by conventional and microwave methods are depicted in Table 1. Formation of the desired compounds was achieved by microwave irradiation being obtained in 2-4 min with higher yields as compared with the conventional method.
The results of antifungal activity of derivatives of compounds 2–7 against a panel of selected fungi are presented in Table 2 and antibacterial activity against Gram positive, Gram negative bacteria are presented in Table 3 in comparison with those of the reference drugs bifonazole and ketoconazole, ampicillin and streptomycin, respectively.
Compound 2a inhibited fungal growth at 0.60- 2.38×10-2 μmol/ml while fungicidal activity was achieved at 2.38-3.35×10-2 μmol/ml. This compound showed the lowest antifungal activity, expressed as minimal inhibitory concentration (MIC) against Penicillium funiculosum (MIC 1.79×10-2 μmol/ml), Aspergillus flavus (MIC 1.79×10-2 μmol/ml) and Aspergillus versicolor (MIC 2.38×10-2 μmol/ml), moderate activity against Trichoderma viride, Aspergillus fumigatus, Aspergillus niger with MIC 1.19×10-2 μmol/ml, whereas it exhibited a strong effectiveness towards Penicillium ochrochloron and Fulvia fulvum (MIC 0.60×10-2 μmol/ml). In all cases activity of compound 2a was better than activity of two reference drugs, bifonazole and ketoconazole.
| Comp | R1 | R2 | mp | MWI | Conventional synthesis | ||||
|---|---|---|---|---|---|---|---|---|---|
| (°) | Temp | Power | Time | Yield | Time | Yield | |||
| (°) | watts | (min) | (%) | (h) | (%) | ||||
| 2a | H | - | 150-153 | 145 | 200 | 5 | 83 | 6 | 72 |
| 2b | CH3 | - | 172-174 | 145 | 200 | 5 | 81 | 6 | 70 |
| 2c | Cl | - | 162-165 | 145 | 200 | 5 | 80 | 6 | 72 |
| 2d | OCH3 | - | 177-180 | 145 | 200 | 5 | 82 | 6 | 73 |
| 3a | H | - | 168-170 | 110 | 170 | 4 | 76 | 4 | 65 |
| 3b | CH3 | - | 159-162 | 110 | 170 | 4 | 78 | 4 | 67 |
| 4a | CH3 | - | Semisolid | 120 | 170 | 4 | 84 | 5 | 71 |
| 4b | C2H5 | - | Semisolid | 120 | 170 | 4 | 81 | 5 | 70 |
| 5a | H | - | <250 | 135 | 230 | 5 | 83 | 6 | 72 |
| 5b | C6H5 | - | 184-187 | 135 | 230 | 5 | 82 | 6 | 70 |
| 5c | p-Cl-C6H4 | - | 189-193 | 135 | 230 | 5 | 88 | 6 | 75 |
| 5d | p-CH3 -C6H4 | - | 186-188 | 135 | 230 | 5 | 83 | 6 | 73 |
| 6 | - | - | 170-174 | 145 | 230 | 6 | 69 | 8 | 61 |
| 7a | H | H | 150-153 | 145 | 230 | 4 | 87 | 5 | 78 |
| 7b | CH3 | H | 145-147 | 145 | 230 | 4 | 84 | 5 | 76 |
Table 1 Physical and comparative study data of compounds (2-7)
| Comp | P. funiculosum | P. ochrochloron | T. viride | A. fumigates | A. niger | A. flavus | A. versicolor | F. fulvum | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | |
| 2a | 1.10 | 2.19 | 1.64 | 2.19 | 1.10 | 1.64 | 1.10 | 2.19 | 1.10 | 2.19 | 1.10 | 2.19 | 1.64 | 2.19 | 1.10 | 1.64 |
| 2b | 2.19 | 2.19 | 0.55 | 1.10 | 1.10 | 2.19 | 1.10 | 2.19 | 1.10 | 2.19 | 1.64 | 2.19 | 1.10 | 2.19 | 1.64 | 2.19 |
| 2c | 2.19 | 3.23 | 1.10 | 2.19 | 1.10 | 2.19 | 1.64 | 2.19 | 1.10 | 2.19 | 1.10 | 2.19 | 1.64 | 2.19 | 1.10 | 1.64 |
| 2d | 2.15 | 2.15 | 1.61 | 2.15 | 1.08 | 2.15 | 1.61 | 2.15 | 1.08 | 2.15 | 1.61 | 2.15 | 1.61 | 2.15 | 1.61 | 2.15 |
| 3a | 0.54 | 1.08 | 1.08 | 2.15 | 1.08 | 1.61 | 1.61 | 2.15 | 1.10 | 2.19 | 1.08 | 2.15 | 0.54 | 1.08 | 1.08 | 2.15 |
| 3b | 1.61 | 2.15 | 1.10 | 2.19 | 1.08 | 2.15 | 0.54 | 1.08 | 0.55 | 1.19 | 1.10 | 2.19 | 1.08 | 2.15 | 1.08 | 1.61 |
| 4a | 1.79 | 2.38 | 0.60 | 2.38 | 1.19 | 2.38 | 1.19 | 2.38 | 1.19 | 2.38 | 1.79 | 2.38 | 2.38 | 3.35 | 0.60 | 1.19 |
| 4b | 1.82 | 2.19 | 0.55 | 1.10 | 1.79 | 2.38 | 1.79 | 2.38 | 1.08 | 2.15 | 0.60 | 1.19 | 1.10 | 2.19 | 0.54 | 1.19 |
| 5a | 1.72 | 2.29 | 1.72 | 2.29 | 1.15 | 2.29 | 1.15 | 2.29 | 1.15 | 2.29 | 1.15 | 2.29 | 1.72 | 2.29 | 1.15 | 2.29 |
| 5b | 1.64 | 2.19 | 1.64 | 2.19 | 1.10 | 2.19 | 1.64 | 2.19 | 1.64 | 2.19 | 1.64 | 2.19 | 1.64 | 2.19 | 1.10 | 2.19 |
| 5c | 1.05 | 2.09 | 0.52 | 1.05 | 1.05 | 2.09 | 1.57 | 2.09 | 1.05 | 2.09 | 1.05 | 2.09 | 1.57 | 2.09 | 0.52 | 2.09 |
| 5d | 1.16 | 2.31 | 1.16 | 2.31 | 1.16 | 2.31 | 1.73 | 2.31 | 1.73 | 2.31 | 1.73 | 2.31 | 1.73 | 2.31 | 0.58 | 2.31 |
| 6 | 1.08 | 2.15 | 1.08 | 2.15 | 0.54 | 1.08 | 1.61 | 2.15 | 1.08 | 2.15 | 1.61 | 2.15 | 1.61 | 2.15 | 1.08 | 1.61 |
| 7a | 1.08 | 2.15 | 1.08 | 2.15 | 1.08 | 2.15 | 1.08 | 2.15 | 1.61 | 2.15 | 1.61 | 2.15 | 1.08 | 1.61 | 0.54 | 1.08 |
| 7b | 1.08 | 2.15 | 1.08 | 2.15 | 1.08 | 2.15 | 1.08 | 2.15 | 1.61 | 2.15 | 1.61 | 2.15 | 1.08 | 1.61 | 0.54 | 1.08 |
| Bif | 64.0 | 80.0 | 48.0 | 64.0 | 64.0 | 80.0 | 48.0 | 64.0 | 48.0 | 64.0 | 48.0 | 64.0 | 32.0 | 64.0 | 32.0 | 64.0 |
| Ket | 38.0 | 95.0 | 380.0 | 380.0 | 475.0 | 570 | 38.0 | 95.0 | 38.0 | 95.0 | 285 | 380 | 38.0 | 95.0 | 38.0 | 95.0 |
Table 2 Antifungal screening results of compounds (2-7)
| Comp | B. cereus | M. flavus | S. aureus | E. coli | P. aeruginosa | P. mirabilis | S. typhimuirium | L. monocyto | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| 2a | 1.64 | 2.19 | 1.10 | 2.19 | 2.19 | 4.38 | 2.19 | 4.38 | 2.19 | 4.38 | 4.38 | 6.57 | 2.19 | 4.38 | 2.19 | 4.38 |
| 2b | 1.64 | 2.19 | 1.10 | 2.19 | 1.64 | 4.38 | 2.19 | 4.38 | 2.19 | 4.38 | 4.38 | 6.57 | 2.19 | 4.38 | 2.19 | 4.38 |
| 2c | 2.19 | 4.38 | 1.64 | 2.38 | 2.19 | 4.38 | 2.19 | 4.38 | 2.19 | 4.38 | 4.38 | 6.37 | 2.19 | 4.38 | 2.19 | 4.38 |
| 2d | 1.64 | 2.19 | 1.10 | 2.19 | 1.64 | 2.19 | 1.10 | 2.19 | 1.64 | 4.38 | 4.38 | 6.37 | 2.19 | 4.38 | 1.64 | 2.19 |
| 3a | 1.72 | 2.29 | 2.29 | 4.58 | 1.72 | 4.58 | 2.29 | 4.58 | 2.29 | 4.58 | 4.58 | 6.87 | 1.15 | 1.72 | 2.29 | 4.58 |
| 3b | 1.72 | 2.29 | 2.29 | 4.58 | 1.72 | 4.58 | 2.29 | 4.58 | 2.29 | 4.58 | 4.58 | 6.87 | 1.15 | 1.72 | 2.29 | 4.58 |
| 4a | 2.09 | 2.09 | 1.05 | 2.09 | 1.05 | 1.57 | 2.09 | 4.18 | 1.57 | 4.18 | 4.18 | 6.27 | 1.05 | 1.57 | 2.09 | 4.18 |
| 4b | 2.31 | 2.31 | 2.31 | 4.62 | 2.31 | 4.62 | 1.16 | 2.31 | 1.16 | 2.31 | 4.62 | 6.93 | 1.16 | 2.31 | 2.31 | 4.62 |
| 5a | 2.15 | 2.15 | 2.15 | 2.15 | 1.61 | 4.30 | 2.15 | 4.30 | 2.15 | 4.30 | 4.30 | 6.45 | 2.15 | 2.15 | 2.15 | 4.30 |
| 5b | 1.61 | 2.15 | 2.15 | 4.30 | 2.15 | 4.30 | 2.15 | 2.15 | 2.15 | 4.30 | 4.30 | 6.45 | 1.08 | 2.15 | 2.15 | 4.30 |
| 5c | 1.08 | 2.15 | 2.15 | 4.30 | 1.61 | 2.15 | 2.15 | 4.30 | 2.15 | 4.30 | 4.30 | 6.45 | 2.15 | 4.30 | 2.15 | 4.30 |
| 5d | 1.61 | 4.30 | 2.09 | 4.18 | 2.15 | 4.30 | 1.61 | 4.30 | 1.08 | 2.15 | 4.24 | 6.36 | 2.15 | 4.30 | 2.15 | 4.30 |
| 6 | 1.79 | 2.38 | 2.38 | 4.76 | 2.38 | 4.76 | 2.38 | 4.76 | 1.79 | 4.76 | 4.76 | 7.17 | 2.38 | 4.76 | 2.38 | 4.76 |
| 7a | 1.10 | 2.19 | 1.10 | 2.19 | 2.19 | 4.38 | 1.10 | 1.64 | 1.64 | 4.38 | 4.38 | 6.57 | 2.19 | 4.38 | 1.10 | 2.19 |
| 7b | 1.10 | 2.19 | 1.10 | 2.19 | 2.19 | 4.38 | 1.10 | 1.64 | 1.64 | 4.38 | 4.38 | 6.57 | 2.19 | 4.38 | 1.10 | 2.19 |
| Str | 4.3 | 8.6 | 8.6 | 17.2 | 17.2 | 34.4 | 17.2 | 34.4 | 17.2 | 34.4 | 17.2 | 34.4 | 17.2 | 34.4 | 25.8 | 51.6 |
| Amp | 24.8 | 37.2 | 24.8 | 37.2 | 24.8 | 37.2 | 37.2 | 49.2 | 74.4 | 124.0 | 37.2 | 49.2 | 24.8 | 49.2 | 37.2 | 74.4 |
Table 3Antibacterial screening results of compounds (2-7) (MIC AND MBC IN ΜMOL/ML ×10−2)
Derivatives 2b, 2d exhibited fungistatic effect at 0.55–2.19×10-2 μmol/ml and fungicidal activity was observed at 1.64–3.23×10-2 μmol/ml. In this group compound 4a showed the best antifungal potential. Compounds 5a, 5c possessed almost the same activity, MIC at 0.54–2.15×10-2 μmol/ml, and MFC 1.08– 2.15×10-2 μmol/ml. Derivatives 5a–5d showed MIC at 0.52–1.73×10-2 μmol/ml and MFC at 1.05–2.31×10-2 μmol/ml, where compound 5c exhibited the highest antifungal potential with MIC at 0.52–1.57×10-2 μmol/ ml and MFC at 1.05–2.09. This compound showed the best antifungal effect among all the tested.
The majority of compounds showed the worst activity against A. versicolor, while F. fulvum is the most sensitive species. The most active compounds against this species (F. fulvum) among all tested are compounds 5c (0.52×10-2 μmol/ml), followed by 7a and 4a. Taking into account that almost all compounds exhibited activity better than reference drugs, they could be promising candidates for antifungal drugs.
In addition compound 2-7 were evaluated for antibacterial activity against a wide number of Gram positive bacteria, as well as Gram negative bacteria. The antibacterial activity of compounds tested by micro dilution method, are presented in Table 3. The kind of the exerted antibacterial activity was investigated by determining the minimal bactericidal concentrations (MBCs). The experimental data (second values) presented in Table 3 show that 2-7 possess bacteriostatic properties, being MBCs almost twofold higher than the corresponding MICs.
All compounds showed strong antibacterial activity against all bacterial species. Compound 6 exhibited the lowest antibacterial activity among all the other tested, with MIC 1.79-2.38×10-2 μmol/ml and MBC at 2.38-7.17×10-2 μmol/ml. Group of compounds 2a–2d showed MIC at 1.10-4.38×10-2 μmol/ml and MBC 2.19-6.57×10-2 μmol/ml, where 2b had the best activity. MIC of compounds 5a–5d is at 1.08-4.30×10-2 μmol/ml and MBC at 2.15-6.45×10-2 μmol/ml. These compounds showed almost the same activity. Among derivatives 4a-b (MIC at 1.05-4.62×10-2 μmol/ml and MBC 1.57-6.93×10-2 μmol/ml) compound 4b possessed the best antibacterial activity, MIC 1.05-4.18×10-2 μmol/ml and MBC at 2.09–6.27×10-2 μmol/ml. It can be seen that this compound in general showed the highest antibacterial activity. More significant inhibitory properties were detected for compound 4b against Micrococcus flavus (ATCC 10240), Staphylococcus aureus as well as towards Salmonella typhimurium (MICs 1.05×10-2 μmol/ml).
The most sensitive bacterial species on compounds tested is Gram positive bacteria, especially, Bacillus cereus, while Gram negative bacteria Pseudomonas mirabilis is the most resistant species. It can be seen that all the compounds tested on antibacterial activity showed much better effect than commercial antibiotics, streptomycin and ampicillin (MIC 4.3- 25.8×10-2 μmol/m and MBC 8.6-51.6×10-2 μmol/ml for streptomycin, and MIC 24.8-74.4×10-2 μmol/ml and MBC 37.2-124.0×10-2 μmol/ml for ampicillin). Thus all these compounds could be used as lead compounds for new antibacterial drugs. Compounds 5c in general showed the highest antibacterial as well as antifungal activity, while compound 6 exhibited the lowest antimicrobial potential.
As a conclusion it can be noticed that fungi were in general more sensitive than bacterial species on these compounds. The title compounds were obtained in good yields via condensation reactions. The significant advantages offered by microwave procedure are operational simplicity, fast reaction, high selectivity, excellent yields of products when it’s compared to conventional method, providing 7-11% additional yields. The newly synthesized compounds 2-7, exhibit a remarkable inhibition of the growth of a wide spectrum of Gram positive bacteria, Gram negative bacteria and fungi. The most sensitive bacterial species on compounds tested is Gram positive bacteria, B. cereus, while Gram negative bacteria Proteus mirabilis is the most resistant species. As far as concern the fungi, the majority of compounds showed the worst activity against A. versicolor, while F. flavum is the most sensitive species. It should be noticed that all compounds tested exhibited better activity than commercial antimicrobial agents used as reference drugs and few times higher activity than ketoconazole.
Acknowledgements
We wish to thank University Grants Commission, New Delhi for financial support. The authors thank the Principal, Ms. Manju J. Nichani and the Management of K. C. College for encouragement and providing necessary facilities. We gratefully acknowledge the Director, Institute of Science, Mumbai (India) for spectral analysis. Also, thanks are due to the Head, Microbiology department for biological testing.
References
- De Tullio P, Pirotte B, Neven P, Masereel B, Dewalque D, Diouf O. Synthesis and biological evaluation of new 3-aralkylamino-2-aryl-2H-1, 2,4-pyridothiadizine-1,1-dioxide as potential CCK-receptor ligands. J Pharm Pharmacol 1997;49:463-71.
- Ahmed K, Ahmed SK, Reddy KS, Ahmed Khan MN, Shetty RV,Siddhardha B. et al. Synthesis and Biological evaluation of a new series Benzothiazole-Benzothiadiazine conjugate as Antibacterial agents. Lett Drug Des Discov 2007;4:550-6.
- Ye B, Chon Y, Karanjawala R, Lee W, Lu S, Shaw KJ, et al. Synthesis and biological evaluation of piperazine-based derivatives as inhibitors of plasminogen activator inhibitor-1 (PAI-1). Bioorg Med Chem Lett 2004;14:761-5.
- Song K, Lee S, Hyun JC, Jong YK, Myung EJ, Ahn K, et al. Design, synthesis and biological evaluation of piperazine analogues as CBI cannabinoid receptor ligands. Bioorg Med Chem 2008;16:4035-51.
- Koketsu M, Tanaka K, Takenaka Y, Kwong CD, Ishihara H. Synthesis of 1,3-thiazine derivatives and their evaluation as potential antimycobacterial agents. Eur J Pharm Sci 2002;15:307-10.
- Almatead NG, Bradley RS, Pikul S, De B, Natchus MG, Taiwo YO. Design, synthesis and biological evaluation of potent thiazine- and thiazepine-based matrix metalloproteinase inhibitors. J Med Chem 1999;42:4547-53.
- Popsavin M, Torovic L, Kojic V, Bogdanovic G, Popsavin V. Synthesis and biological evaluation of two novel 2'-substituted tiazofurin analogues. Tetrahedron Lett 2004;45:7125-32.
- Kim D, Choi JH, An YJ, Lee HS. Synthesis and biological evaluationof 5-(pyridin-2-yl)thiazoles as transforming growth factor-β type1 receptor kinase inhibitors. Bioorg Med Chem Lett 2008;18:2122-8.
- Maekawa T, Sakai N, Tawada H, Murase K, Hazama M, Sugiyama Y, et al. Synthesis and biological activity of novel 5-(ω-Aryloxyalkyl) oxazole derivatives as brain-derived neurotrophic factor inducers. Chem Pharm Bull 2003;51:565-73.
- Gupta P, Hameed S, Jain R. Ring-substituted imidazoles as a new class of anti-tuberculosis agents. Eur J Med Chem 2004;39:805-14.
- Wang Y, Inguaggiato G, Jasamai M, Shah M, Hughes D, Slater M, et al. Synthesis and biological evaluation of novel 2′-deoxy-4′-thio-imidazole nucleoside. Bioorg Med Chem 1999;7:481-8.
- Miyachi H, Kiyota H, Segawa M. Novel imidazole derivatives with subtype-selective antimuscarinic activity. Bioorg Med Chem Lett 1998;8:2163-8.
- Yamada M, Yura T, Morimoto M, Harada T, Yamada K, Honma Y, et al. 2-[(2-Aminobenzyl)sulfinyl]-1-(2-pyridyl)-1,4,5,6-tetrahydrocyclopent[d]imidazoles as a novel class of gastric H+/K+-ATPase inhibitors. J Med Chem 1996;39:596-604.
- Dobler MR. Design and novel synthesis of aryl-heteroaryl-imidazole MAP kinase inhibitors. Tetrahedron Lett 2003;44:7115-7.
- Hirohiko S, Itsuo M, Ken'ichi S, Hitoshi M, Hiroshi M, Eiichi O. Synthesis and biological activity of imidazole derivatives as a novel class of HIV-1 non-nucleoside reverse transcriptase inhibitors. Medi Kemi Shin 1999;19:105-12.
- Southwood HT, DeGraaf YC, Mackenzie PI, Miners JO, Burcham PC, Sallustio BC. Carboxylic acid drug-induced DNA nicking in HEK293cells expressing human UDP-Glucuronosyltransferases: Role of acyl glucuronide metabolites and glycation pathways. Chem Res Toxicol 2007;20:1520-7.
- Dabholkar VV, Parab SD. Synthesis of novel triazole, quinoline, oxazole and imidazole annulated carbostyrils by microwave irradiation. Ind J Chem 2007;46B:344-9.
- Dabholkar VV, Parab SD. Microwave accelerated synthesis of novel spiro heterocycles. Ind J Chem 2007;46B:195-9.
- Dabholkar VV, Mishra SK. Microwave-mediated synthesis of some novel heterocycles containing thiazole, oxazole, thiazine, oxazine, thiadiazine and triazolo-thiadiazine moiety. Ind J Chem 2006;45B:2112-6.
- Dabholkar VV, Mishra SK. Efficient synthesis of some novel spiro heterocycles containing thiazole, oxazole, thiadiazole and triazolo-thiadiazole moiety under microwave irradiation. Het Comm 2006;12:241-5.
- Dabholkar VV, Gavande RP. Synthesis of fused 1,3,4-thiadiazines. Ind J Het Chem 2006;16:101-5.
- Dabholkar VV, Sanghvi AS. Synthesis of oxazoles, thiazoles and Benzothiazines by microwave technique. Ind J Het Chem 2006;16: 105-9.
- Dabholkar VV, More GD. Synthesis of 4,10-dihydro-5/7-substituted- 9-oxo-quinolino[2, 3-e]-2-amino-1,3,4-thiadiazine and Schiff base by microwave irradiation. Ind J Chem 2004; 43B:682-6.
- Dabholkar VV, Ahmed SS. Microwave induced synthesis of 3/4-substituted-2-thioxo/2-oxo-6-thioxo-1,2,3,4,6,7-hexahydrothiazolo [4,5-d]pyrimidine and thiazole derivatives. Ind J Chem 2004;43B: 2646-50.
- Dabholkar VV, Gavande RP. Microwave-catalyzed rapid, efficient and ecofriendly synthesis of substituted pyrazol-5-ones. J Ser Chem Soc 2003;68:723-8.
- Booth C. Fungal Culture Media. In: Norris JR, Ribbons DW, editors. Methods in Microbiology IV. New York: Academic Press; 1971.
- Daouk KD, Dagher MS, Sattout JE. Antifungal activity of the essentialoil of Origanum syriacum L. J Food Prot 1995;58:1147-9.
- Hanel H, Raether W. A more sophisticated method of determining the fungicidal effect of water-insoluble preparations with a cell harvester, using miconazole as an example. Mycoses 1988;31:148-54.
- Espinel-Ingroff A. Comparison of the E-test with the NCCLS M38-P method for antifungal susceptibility testing of common and emerging pathogenic filamentous fungi. J Clin Microbiol 2001;39:1360-7.
- Rhinesmith HS, α,β-dibromosuccinic acid. Organic Synthesis; 1943;2:177-8.