- *Corresponding Author:
- H. C. Bajaj
Discipline of Inorganic Materials and Catalysis, Central Salt and Marine Chemicals, Research Institute, Council of Scientific and Industrial Research (CSIR), Gijubhai Badheka Marg, Bhavnagar-364 002, India
E-mail: hcbajaj@csmcri.org
| Date of Submission | 16 December 2012 |
| Date of Revision | 19 September 2013 |
| Date of Acceptance | 25 September 2013 |
| Indian J Pharm Sci 2013; 75(6): 722-726 |
Abstract
The research work reported in this paper is extension of our previous findings related to intercalation of procainamide hydrochloride, an antiarrythmia drug in interlayer gallery of Na+-clay (montmorillonite). The microcomposite particles prepared from procainamide-montmorillonite hybrid and poly L-lactide were characterised by scanning electron microscope and atomic force microscopy analysis. In vitro drug release study in simulated intestinal fluid showed controlled release pattern up to ~72 h and significant reduction in the drug release in gastric environment. In vivo pharmacokinetics and biodistribution in rats showed that the plasma/tissue drug levels were within therapeutic window as compared with free drug. The data from toxicity biomarker estimations and clinical biochemistry/ haematological parameters showed significant reduction in drug toxicity when formulated in montmorillonite/poly L-lactide as compared with free drug, which is of considerable value in achieving improved therapy with reduced side effects.
Keywords
Antiarrythmia, controlled release, microcomposites, procainamide, toxicity biomarker
Layered silicates are emerging as promising candidates for applications in biomedical research encompassing drug delivery [1-5], tissue engineering [6,7], protein adsorption [8-11], gene reservoirs and delivery [12,13] and nanoclay composites due to their ultra fine sizes are useful in tissue engineering applications [14-16], biocompatibility and controlled release of drug [4,5,14-16]. For delivery applications, the layered silicates are perfect model for high level of controlled release of drug and biomolecules, strength and null toxicity [4,5,14-18].
The goal of this study was to use montmorillonite/ Na+-clay (MMT) as carrier for controlled releases of procainamide hydrochloride (PA) and to achieve a delivery profile that would yield a high blood level of the drug over a long period of time and nullify toxicity of drug. Herein we report intercalation of PA in clay interlayer gallery of MMT to overcome drug toxicity and to maintain peak plasma drug concentration by controlled release, measured through in vivo biomarker assessments.
For the present study, procainamide HCl, poly L-lactide (PLLA) (inherent viscosity 0.90-1.20) and cellulose acetate dialysis tube (cut-off Mw: 7014) were procured from Sigma-Aldrich, St Louis USA. Dichloromethane (DCM) and polyvinyl alcohol (Mw: 125 000) were purchased from S. D. Fine- Chem. Ltd., India. Pentobarbital sodium was purchased from National Chemicals, Vadodara, India. The MMT-rich bentonite was collected from Akli mines, Barmer district, Rajasthan, India and purified. PA-MMT sample in bulk was prepared as was reported earlier [4]. All the other reagents were of HPLC grade and were used as received.
The microcomposite particles (MPs) were prepared with the oil in water (o/w) solvent evaporation method. One gram of PLLA was dissolved in 100 ml DCM and sonicated for 20 min at 35° (VWR Ultrasonic Cleaner, VWR International, Pennsylvania, USA), after which PA-MMT hybrid (PLLA:PAShort MMT=1:0.5 w/w) was suspended in this organic phase and further sonicated for 10 min at 35°. The organic phase was added drop wise (0.5 ml/min) into the external aqueous phase containing 0.5% w/v of polyvinyl alcohol (300 ml) with stirring till DCM evaporation. The MPs were collected and lyophilised in liquid nitrogen.
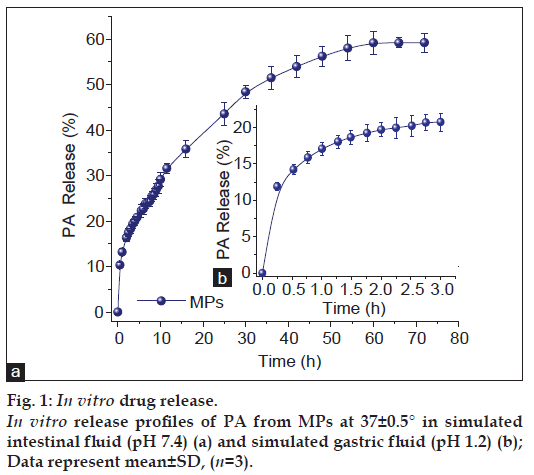
In vitro release of PA was carried out with the assistance of USP eight stage dissolution rate test apparatus (Veego, India) using dialysis bag technique at pH 1.2 and 7.4 [4]. The weighed amounts of MPs (corresponding to 10 mg of entrapped PA) were suspended in dialysis bag restraining 5 ml of the release medium followed by steps reported in our previous publication [4].
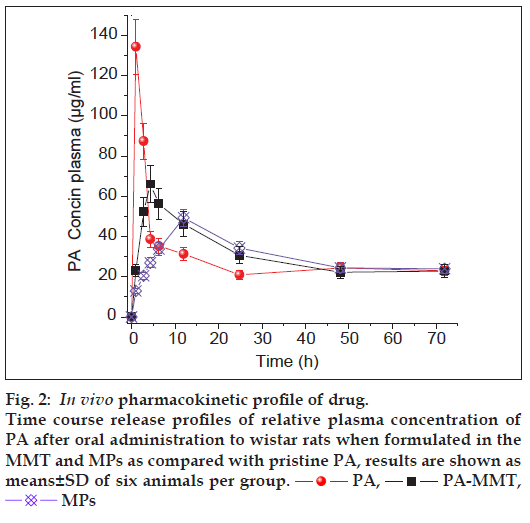
For pharmacokinetic (PK) and biodistribution study, 10-12 weeks old, 200-250 g Wistar rats (M:F::50:50) were acquired by the central animal house, Government Medical Collage, Bhavnagar, Gujarat, India and were maintained at the Animal Holding Unit at Department of Pharmacology. The animal caring, handling and the protocols were approved by the Institutional Animal Ethics Committee (IAEC), Government Medical College Bhavnagar, India (In vivo studies-protocol approval no. IAEC No. 19/2010). The animals were acclimatised at temperature of 25±2° and relative humidity of 50- 60% under natural light/dark environments for one week ahead of experiments. Each animal was fasted for 24 h prior to the studies and water was made available ad libitum. The animals were randomised into six groups of six animals each. First two groups of animals received oral pristine PA (suspension), while the second two groups of animals received PA-MMT hybrid (suspension) and third two groups received MPs (suspension). All the formulations were administered orally at a dose of 40 mg/kg body weight. For PK study, first three groups were used from each treatment and blood samples (~0.3 ml) were collected from the retro orbital plexus under mild anaesthesia into the microcentrifuge tubes containing EDTA (1.8 mg/ml blood). The blood collection time breaks were kept at 0 (predose), 1, 3, 6, 9, 12, 24, 48 and 72 h after administration of the drug. Plasma separated by centrifugation (Kubota-6500, Kubota Corporation, Japan) at 10 000 rpm for 15 min at 5° was stored at −20° for reverse-phase HPLC analysis. The distribution of formulated and pristine drug in different tissues of rat was estimated in two animals from each group, which were euthanised with an intraperitoneal injection of pentobarbital sodium (~120 mg/kg body weight) at 1, 3 and 12 h after administration of free drug and formulated drug. Instantaneously following death, carcasses were placed on ice packs and opened by bilateral thoracotomy. The heart, lung, liver, spleen, kidney, stomach and intestine were collected. Tissue samples were blotted with paper wipe, cleaned in saline, blotted to remove surplus fluid, weighed, sliced into tiny pieces and homogenised with four volumes of 0.1 M NaOH. The homogenate was centrifuged at 10 000g for 30 min at 5°, the fatty layer was discarded and supernatants were collected for quantification of drug by HPLC as described below.
The quantification of PA in plasma was done by using a validated RP-HPLC method reported in literature with slight modifications [19]. Briefly, subsequent to preparation of plasma samples, analysis by HPLC system consisting of photodiode array detector (Waters Alliance model: 2695 separation module with Waters 2996 Photodiode Array Detector) and A reverse-phase C18 column (Luna C18, Phenomenex®) was carried out. Mobile phase for analysis was the combination of 0.075 M ammonium acetate buffer (pH=4.3) and acetonitrile (80:20, by volume). The injection volume was 20 μl, retention time of PA was 20 min and detection wavelength (λmax) for PA was at 278 nm.
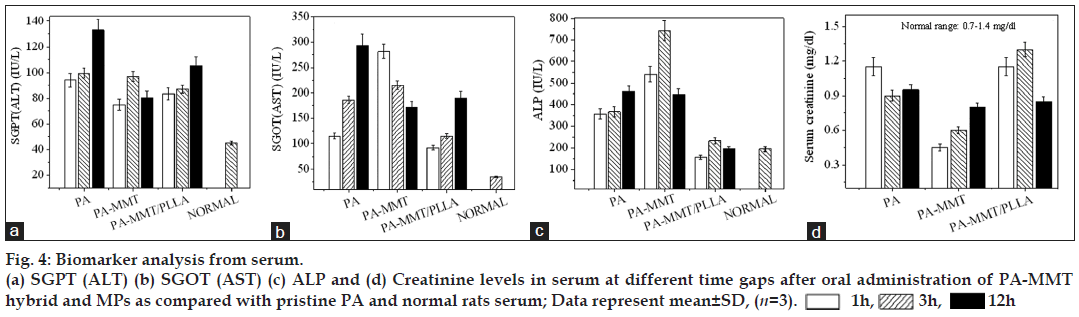
The toxicity biomarkers and clinical parameters were evaluated by separating serum from blood throughout the experiment, from animals of each group at time intervals of 1, 3 and 12 h. The drug toxicity biomarkers ALT (serum glutamate pyruvate transaminase, SGPT), AST (serum glutamate oxaloacetate transaminase, SGOT), alkaline phosphatase (ALP) and serum creatinine were estimated in serum samples by kinetic method using semi automatic biochemistry analyser-RA-50 (Bayer Inc. USA) and clinical parameters of plasma/ serum were analysed by fully automated cell counter (Nihon Kohden, Japan).
The release patterns of drug from MPs were compared with our published data (fig. 1) [4]. Approximately 20% of the intercalated PA was released up to 3 h from MPs (fig. 1b). The release profile of PA from MPs in simulated intestinal fluid is shown in fig. 1a. The formulation exhibited controlled release profile up to ~72 h. The PA from MPs had controlled release pattern with ~30% of drug released in 10 h followed by sustained release in >72 h (60%).
The key PK parameters, which include Cmax (in μg/ml) and Tmax(h) is the highest drug concentration encountered subsequent to the drug administration and the time at which Cmax is reached, MRT (h) is the mean residence time of the drug in the plasma and AUC0-∞ (μg h/ml) is the total area under the curve which represents the in vivo therapeutic effects of drug were examined (Table 1 and fig. 2). Some advantages of using MMT and PLLA can be concluded from PK data. The Cmax and MRT of pristine PA was about 134.3±13.7 μg/ml and 19.51±1.0 h, respectively. The mean values obtained for AUC0-∞ and peak plasma time (Tmax) were 1580.5±56.9 μg h/ml and 1 h, respectively. In contrast, when drug was captured inside gallery of MMT and MPs prior to oral administration to rats, higher drug concentration were detected in plasma even after 72 h (fig. 2). Upon comparing the MRT of pristine drug, it was observed that the PA-MMT hybrid and MPs increased the residence time of the drug in the plasma by 20.6±1.3 h and 23.8±2.0 h, respectively.
| PK parameters | PA | PA-MMT | MPs |
|---|---|---|---|
| Cmax(μg/ml) | 134.3 ± 13.69 | 66.05 ± 9.0 | 49.2 ± 4.3 |
| Tmax(h) | 1 | 4 | 12 |
| AUC0-∞ (μg h/ml) | 1580.46 ± 56.9 | 1730.46 ± 36.05 | 1567.64 ± 22.54 |
| MRT (h) | 19.51 ± 1.0 | 20.65 ± 1.3 | 23.84 ± 2.0 |
Table 1: Pharmacokinetic parameters
As shown in fig. 3a, drug was distributed, highest to lowest, in tissues following administration of free PA in the order; spleen (12 h)>intestine (12 h)>lung(12 h)>kidney (12 h)>stomach (12 h)>liver (3 h)>heart (1 h). The maximum concentration of PA-MMT hybrid was found in spleen, lung and kidney within 12 h, followed by medium to minor distribution of drug in intestine (12 h)>stomach (12 h)>liver (12 h)>heart (1 h) after oral administration (fig. 3b). The drug in MPs was distributed rapidly as compared with free drug and in hybrids (fig. 3c).
The results showed that when free drug was orally administered, level of the toxicity biomarkers in serum was extremely elevated as compared with normal rats. In contrast, results of rats treated with PA-MMT hybrid and MPs had significant reduction in levels of biomarkers (figs. 4a-d). Table 2 shows haematological parameters of rats treated with free PA, PA-MMT hybrids and MPs at different time intervals. Results of clinical parameters indicated that the free drug was toxic in nature as compared with drug formulated with MMT and MPs.
| Entry | Parameters | Normal values | PA | PA-MMT | MPs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 h | 3 h | 12 h | 1 h | 3 h | 12 h | 1 h | 3 h | 12 h | |||
| 1 | Hb (g/dl) | 12.95 | 14.4 | 14.45 | 13.45 | 13.85 | 14.15 | 13.45 | 13.3 | 14.05 | 13.3 |
| 2 | Total RBC (million/cmm) | 6.42 | 7.90 | 7.51 | 6.8 | 6.825 | 7.32 | 6.76 | 7.355 | 7.475 | 7.255 |
| 3 | Total WBC (cmm) | 8250 | 9550 | 11600 | 7400 | 5700 | 10650 | 8500 | 5750 | 7500 | 5850 |
| 4 | Total platelet count (cmm) | 702 000 | 576 500 | 560 500 | 654 000 | 647 500 | 628 500 | 621 000 | 599 500 | 848 000 | 831 000 |
| 5 | Polymorphs (%) | 37 | 39 | 47 | 53 | 34.5 | 37 | 58 | 36.5 | 50.5 | 37 |
| 6 | Lymphocytes (%) | 57 | 59 | 59.5 | 42.5 | 60.5 | 59 | 37.5 | 59 | 44 | 59 |
| 7 | Eosinophils (%) | 1.5 | 1 | 2.5 | 2 | 1.5 | 1.5 | 1 | 1.5 | 1.5 | 1 |
| 8 | Monocytes (%) | 2.5 | 3 | 2 | 2.5 | 3.5 | 2.5 | 3 | 3 | 4 | 2 |
| 9 | PCV (%) | 37.2 | 41.0 | 41.45 | 35.4 | 38.05 | 40.4 | 39.2 | 40.5 | 40.35 | 37.7 |
| 10 | MCV (Femtoliter) | 58.105 | 51.80 | 55.195 | 52.065 | 55.58 | 55.175 | 58 | 55.265 | 54.31 | 52.205 |
| 11 | MCH (Pico.g) | 20.225 | 18.21 | 19.26 | 19.78 | 20.305 | 19.345 | 19.89 | 18.15 | 18.845 | 18.455 |
| 12 | MCHC (%) | 34.81 | 35.16 | 34.89 | 37.99 | 36.575 | 35.08 | 34.3 | 32.845 | 34.67 | 35.325 |
| 13 | RDW (%) | 14.5 | 12.8 | 13.45 | 12.1 | 13.25 | 13.7 | 13.15 | 15.2 | 15.55 | 14.4 |
Table 2: The clinical parameters
The MPs were fabricated by solvent evaporation techniques from antiarrythmia drug, PA-MMT. In vitro release study showed controlled release of drug from MPs for over ~72 h at pH 7.4. PK and biodistribution study showed that plasma/tissue drug levels were within remedial limits as compared with free PA. The toxicity biomarker and clinical/ biochemical parameters showed significant reduction in drug toxicity when formulated in clay and PLLA as compared with free drug.
Acknowledgements
The authors are thankful to Directors, CSMCRI and Government Medical College, Bhavnagar for providing necessary infrastructure facilities and the Council of Scientific and Industrial Research (CSIR), Government of India, New Delhi, India, for Senior Research Fellowship to BDK and financial support under network project (SPECS CSC0135). The authors are also thankful for help and cooperation rendered by team members of the Central instrumentation facility and analytical section of the CSMCRI.
References
- Lin FH, Lee YH, Jian CH, Wong JM, Shieh MJ, Wang CY. A study ofpurified montmorillonite intercalated with 5-fluorouracil as drug carrier.Biomaterials 2002;23:1981-7.
- Baek M, Choy JH, Choi SJ. Montmorillonite intercalated withglutathione for antioxidant delivery: Synthesis, characterization, andbioavailability evaluation. Int J Pharm 2012;425:29-34.
- Madurai SL, Joseph SW, Mandal AB, Tsibouklis J, Reddy BSR.Intestine-specific, oral delivery of captopril/montmorillonite:Formulation and release kinetics. Nanoscale Res Lett 2011;6:1-8.
- Kevadiya BD, Joshi GV, Bajaj HC. Layered bionanocomposites ascarrier for procainamide.Int J Pharm 2010;388:280-6.
- Feng SS, Mei L, Anitha P, Gan CW, Zhou W. Poly(lactide)–vitamin E derivative/montmorillonite nanoparticle formulations for the oraldelivery of Docetaxel. Biomaterials 2009;30:3297-306.
- Vaiana CA, Leonard MK, Drummy LF, Singh KM, Bubulya A, VaiaRA, et al. Epidermal growth factor: Layered silicate nanocompositesfor tissue regeneration. Biomacromolecules 2011;12:3139-46.
- Mieszawska AJ, Llamas JG, Vaiana CA, Kadakia MP, Naik RR, KaplanDL. Clay enriched silk biomaterials for bone formation. ActaBiomater2011;7:3036-41.
- Barral S, Villa-Garcı MA, Rendueles M, Dıaz M. Interactions betweenwhey proteins and kaolinite surfaces. Acta Mater 2008;56:2784-90.
- Lin JJ, Wei JC, Juang TY, Tsai WC. Preparation of protein-silicatehybrids from polyamine intercalation of layered montmorillonite. Langmuir 2007;23:1995-9.
- Friebele E, Shimoyama A, Ponnamperuma C. Adsorption of protein andnon-protein amino acids on a clay mineral: A possible role of selectionin chemical evolution. J MolEvol 1980;16:269-78.
- Violante A, De Cristofaro A, Rat MA, Gianfreda L. Physicochemicalproperties of protein-smectite And protein-al (oH) x-smectitecomplexes.Clay Miner 1995;30:325-36.
- Choy JH, Kwak SY, Park JS, Jeong YJ, Portier J. Intercalativeanohybrids of nucleoside monophosphates and dna in layered metalhydroxide. J Am ChemSoc 1999;121:1399-400.
- Lin FH, Chen CH, Cheng WT, Kuo Tzang-Fu. Modified montmorilloniteas vector for gene delivery. Biomaterials 2006;27:3333-8.
- Depan D, Kumar AP, Singh RP. Cell proliferation and controlled drugrelease studies of monohybrids based on chitosan-g-lactic acid andMontmorillonite. ActaBiomater 2009;5:93-100.
- Ambre AH, Katti DR, Katti KS. Nanoclay based composite scaffoldsfor bone tissue engineering applications. J Nanotech Eng Med2010;031013:1-9.
- Katti KS, Ambre AH, Peterka N, Katti DR. Use of unnatural aminoacids for design of novel organomodified clays as components ofnanocomposite biomaterials. Phil Trans R Soc A 2010;368:1963-80.
- Choi SJ, Choi GE, Oh JM, Oh YJ, Park MC, Choy JH. Anticancer drugencapsulated in inorganic lattice can overcome drug resistance. J MaterChem 2010;20:9463-9.
- Dong Y, Feng SS. Poly (D, L-lactide-co-glycolide)/Montmorillonitenanoparticles for oral delivery of anticancer drugs. Biomaterials2005;26:6068-76.
- Sintov A, Levy RJ. Polymeric drug delivery of enzymaticallydegradable pendant agents: Peptidyl-linked procainamide model system studies. Int J Pharm 1997;146:55-62.
 PA,
PA,  PA-MMT,
PA-MMT,  MPs
MPs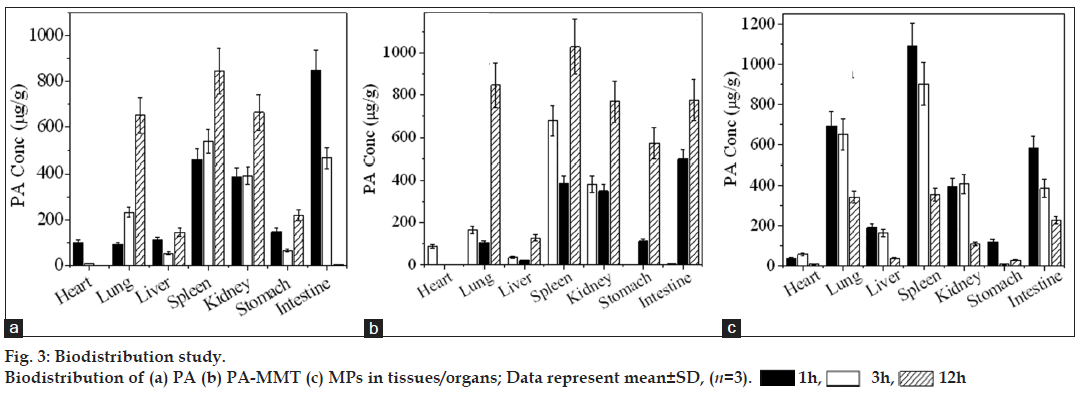
 1h,
1h,  3h,
3h,  12h
12h