- Corresponding Author:
- H. C. Bajaj
Discipline of Inorganic Materials and Catalysis, Central Salt and Marine Chemicals Research Institute, Council of Scientific and Industrial Research, G. B. Marg, Bhavnagar - 364 021, India
E-mail: hcbajaj@csmcri.org
| Date of Submission | 11 August 2009 |
| Date of Revision | 27 October 2010 |
| Date of Acceptance | 15 November 2010 |
| Indian J Pharm Sci, 2010, 72 (6): 732-737 |
Abstract
Diclofenac sodium and alginate was intercalated into montmorillonite to form uniform sized beads by gelation method. The structure and surface morphology of the synthesized composite beads were characterized by powdered X-ray diffraction, Fourier transform infrared spectroscopy, thermo gravimetric analysis and scanning electron microscopy. Diclofenac release kinetics of the composite in simulated intestinal fluid medium (pH 7.4) and effect of montmorillonite content on the in vitro release of diclofenac from diclofenac-montmorillonite-alginate composites bead was investigated by UV/Vis spectrophotometer. Diclofenac encapsulation efficiency in the montmorillonite-alginate composites bead increases with an increase in the montmorillonite content. The control release of diclofenac from diclofenac-montmorillonite-alginate composites beads was observed to be better as compared to diclofenac-alginate beads.
Keywords
Control release, composites, diclofenac sodium, montmorillonite
The controlled drug delivery systems in the field of pharmaceuticals have been of great interest for the past few decades to realize the effective and targeted drug delivery and to reduce the side effects. A particular attention has been paid to find a way to regulate the rate of drug release by a carrier where the drug is dispersed or incorporated in an inert matrix [1,2]. To develop such carriers much attention has been paid to study the interaction between the host and the guest (drugs) compounds. It is important to note here that the host material must be harmless to the human body, should disintegrate and eliminated from the body once its drug delivery job is over [3]. Various organic and inorganic carriers have been used in the past. Among the promising inorganic materials, montmorillonite (MMT) which belongs to smectites clay family is of particular interest. The smectites clay is a layered alumino silicates derived from stacks of tetrahedral sheets of [SiO4]4- and octahedral sheets of [AlO3(OH)3]6-. The isomorphous substitution of Al3+ with Mg2+ or Fe2+ from octahedral sheets or replacement of Si4+ with Al3+ from tetrahedral sheets generate surface with negative charge [4-5].
Due to the high charge and flexible interlayer space smectites clays constitute vary good material as host to many targeted guest molecules. Among organic hosts alginate (AL) have been extensively used a vehicle to numerous drug molecules. AL, obtained from brown sea algae is a linear, naturally occurring polysaccharide. Importantly, Ca-cross linked AL is pH sensitive. It is stable in low pH but swells and disintegrates in weakly basic solutions. AL beads stay intact in gastric fluid (pH 1.2) and rapidly disintegrate in intestinal fluids (pH 7.4) [6,7]. Due to this property and attributes like, biodegradability, biocompatibility and avirulence, AL has been used in many biomedical applications, mostly in the form of beads or matrix. However, the tensile properties of pristine AL beads are poor. To improve physical properties of the AL several chemical modifications like intercalation and reinforcement with inert organic and inorganic have been reported [8].
Diclofenac (sodium [o-[(2,6-dichlorophenyl) amino] phenyl] acetate, DS), is a well-known drug to relive pain and swelling of the joints; and connective tissues in the body typically occur in rheumatic disorders. However, its use in conventional formulations is limited due to the side effects caused by high plasma levels following its administration [9]. Controlled delivery systems have been designed with the objective of maintaining the desired concentration of drug thereby avoiding the build-up of higher drug concentrations that cause toxicity. At the same time controlled release also maintains minimum effective level of the drug for its requisite drug action. In view of the above we have prepared alginatemontmorillonite hybrid beads as host to DS. The main significance of the present work is to improve drug carrying capacity, tensile strength and controlled drug-release properties of the host [10].
Herein we report the facile preparation of DS-MMTAL composites by gelation method. DS-MMT-AL composites beads were characterized by powdered X-ray diffraction (p-XRD), Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM) and thermogravimetric analysis (TGA). The DS release profile of the composites was studied in vitro by simulating gastric fluid (pH 1.2) and simulating intestinal fluid (pH 7.4).
Materials and Methods
Alginic acid sodium salt (AL) (viscosity: 20.0-40.0 cP in 1% water, MW: 7334), diclofenac sodium salt and cellulose acetate dialysis tube (MW: 7014) was procure from Sigma–Aldrich, St. Louis, USA. Calcium chloride, hydrochloric acid, potassium chloride, potassium dihydrogen orthophosphate, sodium chloride and sodium hydroxide were purchased from S. D. Fine Chemicals, Mumbai, India. MMT rich bentonite clay was collected from Akli mines, Barmer district, Rajasthan, India. De-ionized water was obtained from Milli-Q Gradient A10 water purification system.
Purification of MMT
To obtain MMT in Na-form, MMT slurry was treated with NaCl solution. The raw bentonite (300 g) was dispersed in 3 l solution of 0.1 M NaCl and stirred for 12 h. Finally, the slurry was centrifuged and washed with de-ionized water until the supernatant is free from chloride ion as tested by AgNO3 solution [11]. The above procedure is repeated thrice to have 100% Na-MMT. The raw bentonite was purified by sedimentation technique as described earlier [12].
According to the Stoke’s law of sedimentation, the purified clay was obtained by dispersing 150 g of raw bentonite in 10 l of de-ionized water and clay particles of < 2 μm size were collected from 15 cm height after 10 h at 30°. The desired particles thus obtained was dried at 90-100°, ground and sieved through 200 mesh sieve (ASTM). The cation exchange capacity (CEC) of clay was measured by the standard ammonium acetate method at pH 7 [11] and was found to be 91 meq/100 g of MMT on dry basis (dried at 110°).
Preparation of DS-MMT-AL composite beads
The suitable amount of AL dissolved in 25 ml of Milli-Q water with stirred till complete homogeneous solution was obtained. The desired quantity of DS was added into AL and solution was kept at 35° into shaking water bath (100 rpm) for 3 h. To this MMT (200 mesh size) powder was added and the emulsion was stirred overnight at 40° [13]. This emulsion was dropped using a peristaltic pump (Master flex L/S 7518-00, Cole-Parmer, USA) with helps of a 20-guage hypodermic needle fitted with a rubber tubing (falling distance 2 cm, pumping rate 1.5 ml/min) into 200 ml of 100 mM calcium chloride solution, gently agitated with a magnetic stirrer. The formed beads were allowed to stand in solution for 20 min for curing, then collected by filtration and were washed thrice with Milli-Q water [14]. The mean diameter of the beads was determined by measuring 50 beads, using micrometer screw (Mitutoyo, Japan). The formulation code, content for the formulation, encapsulation efficiency and bead diameter are given in Table 1.
| Code | DS:AL:MMT(W/W) | Bead diameter (mm) | EE(%) |
|---|---|---|---|
| DS-AL | 1:1:0 | 0.55 | 35.33 |
| DS-MMT-AL-i | 1:1:1.0 | 0.62 | 56.35 |
| DS-MMT-AL-ii | 1:1:1.5 | 0.67 | 65.01 |
| DS-MMT-AL-iii | 1:1:2.0 | 0.73 | 76.48 |
| DS-MMT-AL-iv | 1:1:2.5 | 0.85 | 82.84 |
Table 1: Formulation Codes and Different Characteristic of Ds-Mmt-Al Composites
Encapsulation efficiency
The DS content in beads was determined by a digestion method. The DS-loaded beads (900 mg) were smashed and incubated in 50 ml of phosphate buffer (pH 7.4). The resulting suspension was stirred to promote swelling and break-up of the cross linked structure to facilitate liberation of DS from DS-MMT-AL composite into buffer media. The DS was quantified by centrifuging the suspension at 10,000 rpm for 30 min (Kubota-6500, Kubota Corporation, Japan) and DS was determined from the clear supernatant by UV-spectrophotometer at λmax=276 nm. These studies were performed in triplicate for each sample and the average values were used in data analysis. The DS encapsulation efficiency, EE (%) was determined by using equation; EE = (AQ/TQ)×100, where AQ is the actual quantity of DS present in DS-MMT-AL composite beads and TQ is 100 % theoretical quantity of DS present in DS-MMT-AL composite beads [14].
Characterization
The intercalation of DS and AL into interlayer of MMT was examined by powder X-ray diffraction (p-XRD) analysis. p-XRD was carried out on a Phillips powder diffractometer X’ Pert MPD using PW3123/00 curved Cu-filtered Cu-Ka (λ=1.54056 Ǻ) radiation with slow scan of 0.3 deg/s in 2θ range of 2-10°. Fourier transform infrared spectra (FT-IR) were measured with Perkin-Elmer, GX-FTIR as KBr pellet over the wavelength range 4000–400 cm-1. Thermogravimetric analysis was carried out within 30-800° at the rate 10°/ min in the nitrogen flow of 60 ml/min using Mettler- Toledo, TGA/SDTA 851e. UV/Vis absorbance of DS solutions was measured at a characteristic λmax = 276 nm by UV/Vis spectrophotometer (Cary 500, Varian) equipped with a quartz cell having a path length of 1 cm. The surface morphology of composite was observed under scanning electron microscope (SEM), LEO-1430VP, UK.
In vitro release studies:
Buffer solution of pH 7.4 (simulated intestinal fluid) was prepared by mixing 500 ml of 0.1 M KH2PO4 and 391 ml of 0.1 M NaOH. In vitro release studies were performed using USP six stage dissolution rate test apparatus (Thermonik, Campbell Electronics, Mumbai, India) by using dialysis bag technique [15]. The dialysis sac was equilibrated with the dissolution medium for few hours prior to experiments. Three hundred milligrams of DS loaded composite beads suspended in 5 ml of dissolution medium was taken in a dialysis bag and sealed at both ends. Dialysis bag was dipped into 500 ml of dissolution medium, stirred at 100 rpm at 37±1º. At time intervals of 30 minutes, 5 ml of the dissolution medium was taken and the DS concentration was determined by UV/Vis spectrophotometer at λmax = 276 nm. These studies were performed in triplicate for each sample and used in data analysis.
Results and Discussion
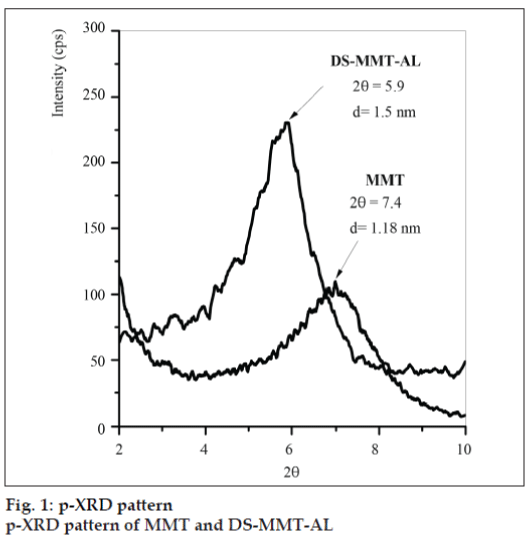
Basal spacing [001] of MMT and DS-MMT-AL was found to be 1.18 nm (2θ=7.4) and 1.5 nm (2θ=5.9), respectively (fig.1). The peak shifting from higher diffraction angle to lower diffraction angle can be attributed to the increase in the basal spacing during the formation of clay-alginate hybrid, indicating successful intercalation of DS and AL into the interlayer of MMT.
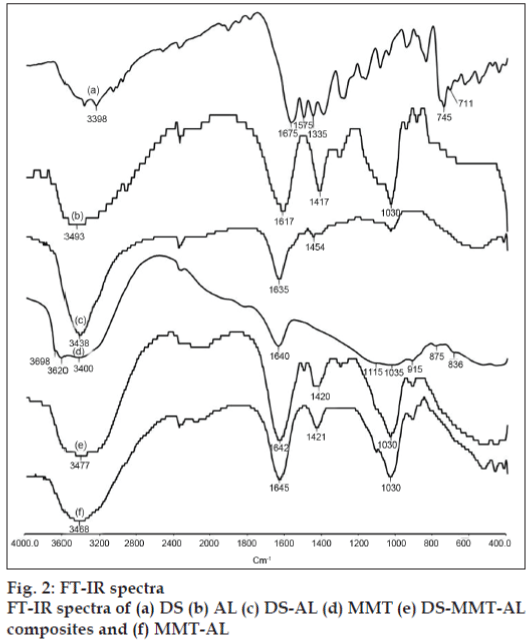
FT-IR spectra of DS, MMT, AL and DS-MMT-AL composite beads are shown in fig. 2. The virgin alginate showed asymmetric and symmetric stretching vibrations at 1617 and 1417 cm-1 due to carboxyl anions, and at 1030 cm-1 due to cyclic ether bridge [6]. MMT shows a broad band centered near 3400 cm–1 due to –OH stretching band for interlayer adsorbed water. The bands at 3620 and 3698 cm–1 are due to –OH band stretch for Al–OH and Si–OH. The shoulders and broadness of the structural –OH band are mainly due to contributions from several structural –OH groups present in MMT. The overlaid absorption peaks in the region of 1640 cm–1 is attributed to –OH bending mode of adsorbed water. The characteristic peak at 1115 cm–1 is due to Si–O stretching (out-of-plane) and the peak at 1035 cm–1 is attributed to Si–O stretching (in-plane) vibration for MMT. The IR peaks at 915, 875 and 836 cm–1 are attributed to AlAlOH, AlFeOH and AlMgOH bending vibrations, respectively [12]. FT-IR spectra of DS showed N-H, C=O, C-Cl stretching vibration at 3398, 1675 and 1335 cm–1, respectively. In the FTIR spectra of DS-AL beads, the asymmetrical band of carboxylate ion was shifted from 1617 cm–1 to 1635 cm–1 whereas carboxyl group of DS was shifted from 1575 cm–1 to 1454 cm–1. The hydroxyl band of sodium AL was shifted from 3493 cm–1 to 3438 cm–1 due the interaction between DS and AL.
Pongjanyakul and Puttipipatkhachorn explained the shift in the IR band of COO¯ while AL interacted with MMT. Incorporation of MMT into AL caused a shift to a higher wave number and decreased the intensity of COO¯ stretching peaks of AL. The OH stretching peak of the silanol group (SiOH) at 3698 cm–1 disappeared in the spectra of MMT-AL composites, and the OH stretching peak of AL shifted to a higher wave number. This may be due to the intermolecular hydrogen bonding between silanol groups on the surface of MMT and the hydroxyl or carboxyl groups of AL. With these results, the bond formation of AL and MMT in the dry state could be used to predict the matrix structure of MMT-AL composites. Intermolecular hydrogen bonding and electrostatic forces between AL and MMT were confirmed by FTIR studies [16].
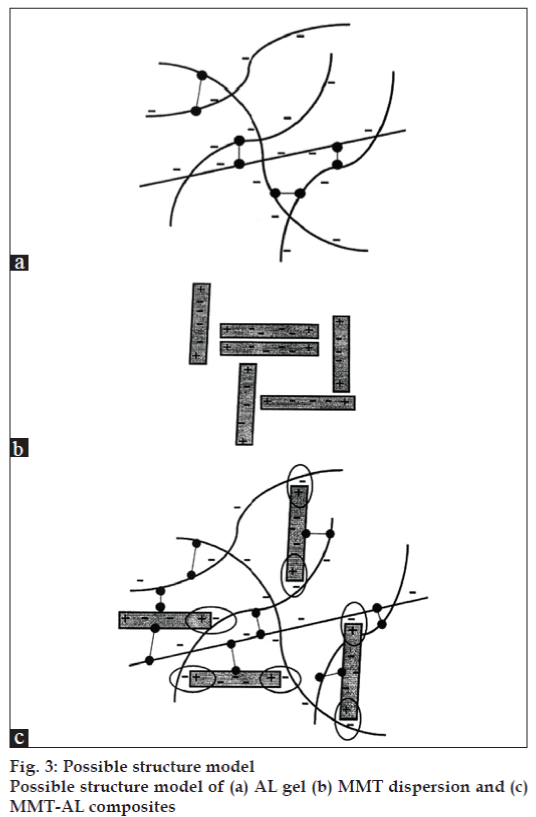
The possible structural model of the MMT-AL composites is shown in fig. 3. The AL molecules possessed a negative charge due to ionization of the carboxyl groups and could form intermolecular hydrogen bonding (fig.3a). The structural model of the MMT-AL composites is presented in fig.3c. It can be seen that AL and MMT formed electrostatic and intermolecular hydrogen bonds, which brought about numerous points of contact to create a three dimensional network. AL chains could also serve as a bridge between neighboring silicate layers when a higher content of MMT was incorporated.
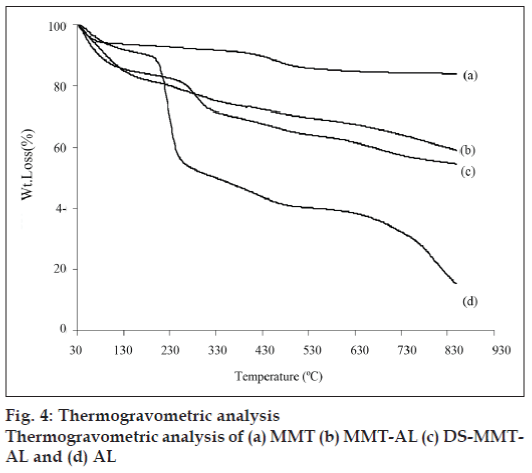
The thermogravimetric analysis (fig. 4) of MMT shows two distinct steps, the first one is due to the loss of free water at <150° and the second weight loss at 550–700° due to the loss of structural OH group from MMT. AL, MMT-AL and the degradation of DS from DS-MMT-AL was observed over a range of 200-500° while AL moiety was degraded 550–700°. The degradation of alginate in MMT-AL was not prominent, may be due to the reinforcement of cross linked Ca-alginate by MMT, and therein increases the thermal stability of MMT-AL composite.
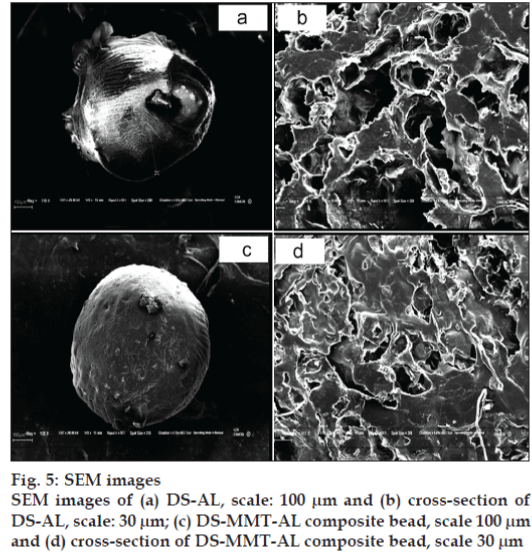
The surface and cross-sectional morphology of DS-AL beads and DS-MMT-AL composite beads are depicted in SEM images (fig. 5). DS-AL beads displayed an unstable structure while DS-MMT-AL composite beads are stable. DS-MMT-AL composite beads demonstrated a more rigid and flat surface as compared to DS-AL beads, whose surface was convoluted. Moreover the AL-DS beads were quite fragile. The rigidity imparted in DS-MMT-AL composite beads may be due to the reinforcement effect of MMT in the AL matrix. The SEM image of DS-AL and DS-MMT-AL composite beads revealed their porous structure (fig. 5 b, d).
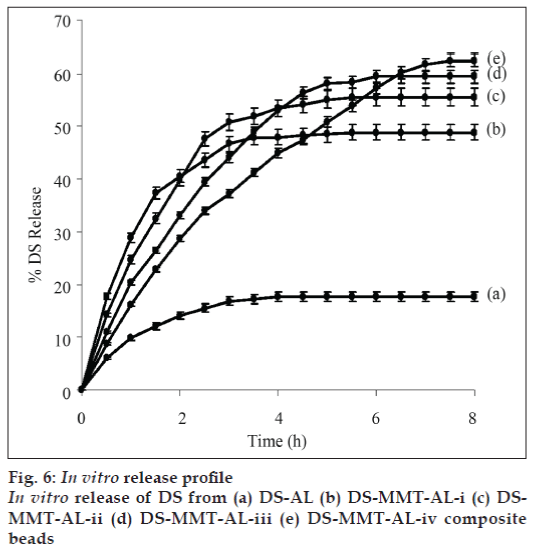
In vitro release profiles of DS from DS-alginate (Formulation ADS-AL) and DS-MMT-alginate composite beads (DS-MMT-AL-i to iv) in simulated gastric fluid and simulated intestinal fluid was done at 37±1º. Only 1-2% DS release was observer in simulated gastric fluid (pH 1.2) after 8 h. The release profile in simulated intestinal fluid showed (fig. 6) 9.8% and 17.7% DS released from DS-AL composite in 1 h, and 4 h, respectively, thereafter the release of DS remained constant up to 8 h. While, in the case of DS-MMT-AL-i to DS-MMT-AL-iv formulation, the average release of DS was ~63% within 5-8 h depending upon the content of MMT in different formulations. The study reveals that the plateau for the release of DS was reached more rapidly in DS-AL as compared to the DS-MMT-AL-i to DS-MMT-ALiv. There was an increase in the release rate of DS from DS-MMT-AL composite beads with an increase in the MMT loading. It may be due to the weak interaction between the DS and MMT as both DS and MMT are negatively charged. The DS encapsulation efficiency was lower in the case of virgin AL beads as compare to DS-MMT-AL composite beads. The DS encapsulation efficiency increases with the increase in the MMT content in DS-MMT-AL composite beads (Table 1). A possible explanation to this behavior is the presence of more hydroxyl groups on the surface of MMT facilitating hydrogen bonding with DS. A release of DS from DS-AL and DS-MMT-AL beads in 8 h was found to be 17.7% (DS-AL) and 48.80%, 55.52%, 59.41% and 62.49% from DS-MMT-AL-i to DS-MMT-AL-iv, respectively.
DS-MMT-AL composite beads were successfully synthesized by gelation methodology. p-XRD study reveals formation of intercalated composites. Reinforcement of AL by MMT resulted into rigid and micro-porous composite beads. The amount of DS released from DS-AL and DS-MMT-AL composite bead is 17.7% and 62.49%, respectively in simulated intestinal fluid. MMT content in DSMMT- AL composite beads regulate the release rate of DS. This approach can be effectively use for the oral administration of DS to enhance its therapeutic efficacy.
Acknowledgements
We are thankful to Council of Scientific and Industrial Research (CSIR) for funding under Network Project: NWP 0010; to Dr. P. Bhatt (p-XRD), Mr. V. Agarwal (FTIR), Mr. Chandrakant (SEM), and Mrs. Sheetal Patel (TGA) from the analytical section of the institute.
References
- Ambrogi V, Fardella G, Grandolini G, Perioli1 L, Cristina MT. Intercalation Compounds of Hydrotalcite-like Anionic Clays with Anti- inflammatory Agents II: Uptake of Diclofenac for a Controlled Release Formulation. AAPS PharmSciTech 2002;3:1-6.
- Khan AI, Lei L, Norquist AJ, O’Hare D. Intercalation and controlled release of pharmaceutically active compounds from a layered double hydroxide. Chem Commun 2001;22:2342-3.
- Noriko S, Yasuaki N, Yoshiteru W, Yasushi K. Intercalation Compounds of Layered Materials for Drug Delivery Use. II. Diclofenac Sodium. Chem Pharm Bull 2001;49:964-8.
- Park JK, Choy BY, Oh J, Kim JY, Hwang SJ, Choy JH. Controlled release of donepezil intercalated in smectite clays. Int J Pharm 2008;359:198-204.
- Patel HA, Somani RS, Bajaj HC, Jasra RV. Nanoclays for polymer composites, paints, inks, greases and cosmetics formulations, drug delivery vehicle and waste water treatment. Bull Mater Sci 2006;29:133-45.
- Daia YN, Lia P, Zhangc JP, Wangc AQ, Weia Q. A Novel pH Sensitive N-Succinyl Chitosan-AL Hydrogel Bead for Nifedipine Delivery. Biopharm Drug Dispos 2008;29:173-84.
- Shilpa A, Agrawal SS, Ray AR. Controlled Delivery of Drugs from Alginate Matrix. J Macromol Sci Polym Rev 2003;C43:187-221.
- Tonnesen HH, Karlsen J. Alginate in drug delivery systems. Drug Dev Ind Pharm 2002;28:621-30.
- Griffin MR, Mphab MD. Epidemiology of Nonsteroidal Anti- inflammatory Drug–Associated Gastrointestinal Injury. Am J Med 1998;104:23S-29S.
- Dong Y, Feng S. Poly (D,L-lactide-co-glycolide)-montmorillonite nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005;26:6068-76.
- Bergaya F, Theng BK, Lagaly G. Handbook of clay science, 1st ed.Amsterdam: Elsevier Publication; 2006. p. 104-13.
- Patel HA, Somani RS, Bajaj HC, Jasra RV. Synthesis and characterization of organic bentonite using Gujarat and Rajasthan clays. Curr Sci 2007;92:1004-9.
- Mirghani A, Idkaidek NM, Salem MS, Najib NM. Formulation and Release Behavior of Diclofenac Sodium in Compritol 888 Matrix Beads Encapsulated in alginate. Drug Develop Ind Pharm 2000;26:791-5.
- Das MK, Senapati PC. Furosemide loaded alginate microsperes prepared by ionic cross linking technique: Morphology and release characteristics. Indian J Pharm Sci 2008;70:77-84.
- Marchal-Heussler I, Maincent P, Hoffman M, Spittler J, Couvreur P. Antiglaucomatous activity of betaxolol chlorhydrate sorbed onto different isobutyl cyanoacrylate nanoparticle preparations. Int J Pharm 1990;58:115-22.
- Pongjanyakul P, Puttipipatkhachorn S. Sodium alginate–Magnesium Aluminum Silicate Composite Gels: Characterization of Flow Behavior, Microviscosity, and Drug Diffusivity. AAPS PharmSciTech 2007;8:E1-E8.