- Corresponding Author:
- Suvarna kini
Department of Pharmaceutical Chemistry, Manipal College of Pharmaceutical Sciences, Manipal - 576 104, India. E-mail: suvarna.gk@manipal.edu
| Date of Submission | 29 May 2006 |
| Date of Revision | 4 August 2007 |
| Date of Acceptance | 2 February 2008 |
| Indian J Pharm Sci, 2008, 70 (1): 105-108 |
Abstract
The 1,3,5-trisubstituted-2-pyrazolines were synthesized by refluxing isoniazid with various substituted diarylchalcones in N,N-dimethylformamide at 120-140°. The physical and spectral data such as M.P., R f , elemental analysis, IR, NMR and Mass was obtained for the synthesized compounds and the structures were confirmed. The screening of the synthesized compounds for antimicrobial activity was performed against Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli and Aspergillus niger .
Keywords
Pyrazolines, antibacterial agents, antifungal agents
2-pyrazolines are reported as antibacterial [1], antifungal [2-4], antimicrobial [5], antiviral [6], anti-arthritis [7] and antiinflammatory [7,8] agents. Encouraged by these facts, we selected to work on 1,3,5-trisubstituted-2- pyrazolines with different substitutions on the phenyl ring. The antibacterial activity and antifungal activity was screened by cup-plate agar diffusion method and the zone of inhibition for each microorganism at different concentrations of the compounds was measured.
Melting points of the compounds were determined on a Toshniwal ScientiÞ c melting point apparatus and are uncorrected. Purity of the compounds was checked by Thin Layer Chromatography using precoated Merck Silica gel GF254 micro TLC plates and spots were detected under UV. UV spectra were recorded on UV/ Vis spectrophotometer (Shimadzu), UV-1601 PC, IR spectra were recorded in KBr disc on a FTIR_8300, KBr Press (Shimadzu) spectrophotometer at Manipal College Of Pharmaceutical Sciences, Manipal. 1H NMR spectra (DMSO-d6) were obtained using Varian 300 MHz Mercury NMR spectrometer from Shimadzu Analytical Technique Center, Department of Chemistry, Pune University, Pune. Mass spectra of the synthesized compounds were obtained using Mass Spectrometer, Shimadzu QP5050 from Shimadzu Analytical Technique Center, Department of Chemistry, Pune University, Pune. Chemicals were obtained from S.D. Fine Chemicals Ltd dealers from Mangalore.
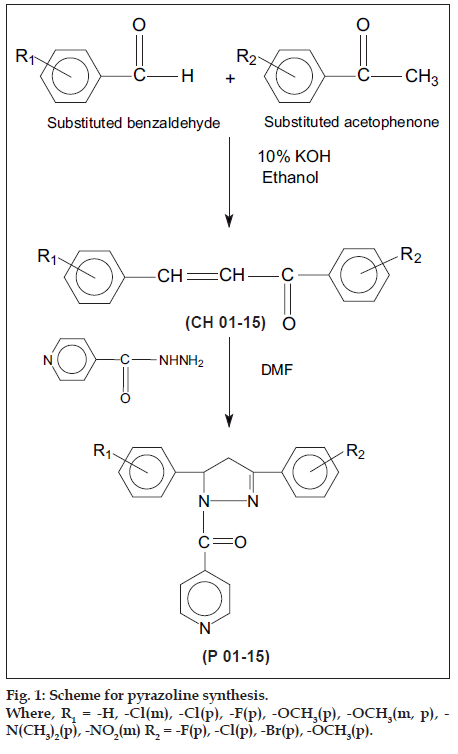
Synthesis of 1,3,5-trisubstituted-2-pyrazolines [compound P13 (5-(4-ß uorophenyl)-3-(4-ß uorophenyl)- 1-isonicotinoyl-pyrazoline)] [9,10] was performed by taking equimolar quantities (0.02 mol) of 4-fluoro benzaldehyde and 4-ß uoro acetophenone in a 250 ml conical flask and dissolving in minimum amount of 95% ethanol. The mixture was then cooled and 8 ml of 10% potassium hydroxide solution was added. The mixture was stirred over a magnetic stirrer for a period of 24 h and left in an ice chest overnight. On the next day, the mixture was poured into a beaker containing ice-cold water. Then the aqueous layer was acidified with conc. HCl. The precipitated chalcone (1-(4-ß uorophenyl)-3-(4-ß uorophenyl)-2-propen-1-one) was Þ ltered at pump and recrystallized from aqueous methanol. IR (KBr)cm-1:1598.9 (C = C), 1660.6 (C = O), 3072.4 (C-H). (1-(4-ß uorophenyl)-3-(4-ß uorophenyl)- 2-propen-1-one) (0.05 mol) was added to isoniazid (0.1 mol) in a 100 ml R.B.F. containing 30 ml of N, N-dimethylformamide. The mixture was refluxed at 120° to 140° for a period of 8-10 h. After cooling, the reaction mixture was poured into a beaker containing ice-cold water and the product separated was scooped off and dried over a clean Þ lter paper. The pyrazoline obtained was recrystallized using benzene-petroleum ether. Single spot on TLC established the purity of the compound. Solvent system used was Hexane: ethyl acetate in 4:1 ratio. IR (KBr)cm-1: 1641.3 (N-C = O), 1595.0 (C = N), 1215.1 (C-N), 2914.2 (C-H,CH2),1H NMR (d values) ppm: aromatic hydrogens 6.7-8.6 d (m, 12H,Ar-H), CH2 methylene 3.4 d (s, 2H, CH2), CH methine 5.6 d (d, 1H,CH-Ar). The other derivatives were prepared in a similar way with different substituted chalcones according to the scheme of synthesis given in fig.1. The physical data of the synthesized compounds is given in Table 1.
| Pyrazoline | R1 | R2 | Rf Value | m.p. (°) | % Yield | ||
|---|---|---|---|---|---|---|---|
| P01 | -F (p) | -Cl (p) | 0.73 | 132-133 | 51 | ||
| P02 | -Cl (m) | -Cl (p) | 0.64 | 123-125 | 55 | ||
| P03 | -OCH3 (p) | -Cl (p) | 0.77 | 172-174 | 58 | ||
| P04 | -N(CH3)2 (p) | -Cl (p) | 0.11 | 163-165 | 49 | ||
| P05 | -Cl (p) | -Cl (p) | 0.17 | 86-88 | 34 | ||
| P06 | -OCH3 (m,p) | -Cl (p) | 0.11 | 166-167 | 50 | ||
| P07 | -N(CH3)2 (p) | -OCH3 | (p) | 0.17 | 188-190 | 52 | |
| P08 | -Cl (p) | -OCH3 | (p) | 0.16 | 214-215 | 59 | |
| P09 | -NO2 | (m) | -OCH3 | (p) | 0.31 | 170-172 | 36 |
| P10 | -OCH3 | (m,p) | -OCH3 | (p) | 0.30 | 188-189 | 47 |
| P11 | -F (p) | -Br (p) | 0.15 | 111-113 | 53 | ||
| P12 | -N(CH3)2 (p) | -Br (p) | 0.14 | 96-98 | 32 | ||
| P13 | -F (p) | -F (p) | 0.19 | 112-114 | 44 | ||
| P14 | -Cl (p) | -F (p) | 0.15 | 162-163 | 65 | ||
| P15 | -OCH3 (p) | -F (p) | 0.12 | 157-158 | 58 | ||
All the compounds gave satisfactory spectral and elemental data
Table 1: Physical data of the synthesized pyrazolines
All the synthesized compounds were screened in vitro for antibacterial activity against Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli at the concentrations 200, 300, 400 and 500 mg/ml and for antifungal activity against Aspergillus niger at 100, 200, 300, 400 µg/ml by cup-plate agar diffusion method [11]. The concentrations used in screening were chosen after determining the MICs of each compound. The solvent used was dimethylsulfoxide (DMSO) further diluted with water. Muller Hinton agar was used as the growth medium for the bacterial species and Sabouraud′s agar was the growth medium for the fungal species. DMSO was used as a control for all the type of microorganisms. The control showed no activity against the strains of microorganisms used. Antimicrobial activity and antifungal activity was measured as a function of diameter of zone of inhibition (mm). The results were compared with standard drugs ciproß oxacin for antibacterial activity and ß uconazole for antifungal activity by measuring the zone of inhibition in mm at 200 and 100 µg/ml respectively. At 200 µg/ml, compounds P14 and P15 were found most effective of the synthesized compounds against S. aureus, (zone of inhibition 15 mm) and compound P14 is the most effective among the synthesized compounds at 200 µg/ml against B. subtilis, (zone of inhibition 16 mm). Compounds P02, P08, P09, P10, P11 were the most effective among the synthesized compounds against E. coli, (zone of inhibition 15 mm) and compound P05 was the most effective at 200 µg/ml among the synthesized compounds against P. aeruginosa, ( zone of inhibition 21 mm). At 100 µg/ml the maximum diameter of zone of inhibition (20 mm) against Aspergillus niger was observed for compound P08; indicating that, it is the most effective among the synthesized compounds against Aspergillus niger. The results of the antibacterial and antifungal activity are given in Table 2.
| Code | S. aureus | B. subtilis | E. coli | P. aeruginosa | A. niger | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | C | D | E | B | C | D | E | B | C | D | E | B | C | D | E | A | B | C | D | ||
| P01 | 12 | 13 | 15 | 16 | 13 | 14 | 16 | 17 | 12 | 13 | 14 | 16 | - | - | - | - | 12 | 13 | 14 | 16 | |
| P02 | 14 | 16 | 18 | 22 | 13 | 14 | 15 | 17 | 15 | 17 | 19 | 21 | 15 | 16 | 18 | 20 | - | - | - | - | |
| P03 | 13 | 14 | 15 | 17 | 14 | 16 | 17 | 19 | 12 | 14 | 16 | 17 | - | - | - | - | 12 | 13 | 15 | 16 | |
| P04 | 13 | 14 | 15 | 17 | 12 | 13 | 15 | 16 | 12 | 13 | 14 | 16 | 18 | 20 | 22 | 26 | 14 | 15 | 16 | 18 | |
| P05 | 12 | 14 | 16 | 17 | - | - | 16 | 19 | 13 | 14 | 16 | 17 | 21 | 24 | 26 | 28 | - | - | - | - | |
| P06 | 12 | 13 | 14 | 16 | 12 | 13 | 14 | 15 | 12 | 13 | 14 | 15 | 20 | 22 | 23 | 25 | - | - | - | - | |
| P07 | - | - | - | 13 | 12 | 13 | 14 | 16 | 12 | 13 | 14 | 16 | 15 | 19 | 22 | 25 | 19 | 21 | 23 | 24 | |
| P08 | 12 | 13 | 15 | 16 | 12 | 14 | 15 | 17 | 15 | 18 | 21 | 23 | - | - | - | - | 20 | 22 | 24 | 26 | |
| P09 | 12 | 14 | 16 | 18 | - | - | - | - | 15 | 16 | 17 | 19 | 12 | 16 | 20 | 23 | 16 | 18 | 20 | 22 | |
| P10 | 13 | 15 | 17 | 20 | - | 12 | 14 | 16 | 15 | 17 | 19 | 21 | 18 | 21 | 24 | 26 | 15 | 17 | 19 | 20 | |
| P11 | 12 | 13 | 14 | 16 | 12 | 13 | 15 | 16 | 15 | 17 | 21 | 23 | 17 | 19 | 22 | 24 | 15 | 16 | 18 | 19 | |
| P12 | 12 | 14 | 15 | 17 | - | - | 12 | 14 | 12 | 14 | 15 | 17 | 17 | 20 | 23 | 25 | 16 | 18 | 20 | 22 | |
| P13 | 12 | 13 | 14 | 15 | 12 | 14 | 16 | 19 | 12 | 13 | 16 | 18 | 13 | 15 | 17 | 20 | - | - | - | - | |
| P14 | 15 | 17 | 19 | 21 | 16 | 18 | 20 | 22 | 14 | 16 | 18 | 20 | 16 | 18 | 20 | 23 | - | - | - | - | |
| P15 | 15 | 17 | 18 | 20 | 14 | 15 | 16 | 18 | 12 | 13 | 14 | 16 | - | - | - | - | - | - | - | - | |
A, B, C, D, E indicates concentration of compounds at 100 µg/ml, 200 µg/ml, 300 µg/ml, 400 µg/ml and 500 µg/ml, respectively. Ciproß oxacin (200 µg/ml) had 26 mm zone of inhibition for S. aureus, 25 mm for B. subtilis, 26 mm for E. coli, 30 mm for P. aeruginosa microorganisms respectively and ß uconazole (100 µg/ml ) had 28 mm zone of inhibition for A. niger organism. Figures indicate diameter of the zone of inhibition in mm including the diameter of cup (i.e. 10 mm). ‘-’ indicates resistance.
Table 2: Results of in vitro antibacterial and antifungal activity
Acknowledgments
The authors are grateful to Shimadzu Analytical Technique Center, Dept. of Chemistry, Pune University, Pune for providing the spectral details in time.
References
- Fahmy AM, Hassa KM, Khalaf AA, Ahmed, RA. Synthesis of some new beta-lactams, 4-thiazolidinones and pyrazolines. Indian J Chem 1987;26:884-7.
- Das NB, Mittra AS. Fungicides derived from 2-pyrazolin-5-ones. Indian J Chem 1978;16:638-40.
- Mittra AS, Rao S. Synthesis and Fungicidal activity of some 2,4-disubstituted thiazoles. Indian J Chem 1977;15:1062-3.
- Rich S, Horsfall JG. Fungitoxicity of heterocyclic nitrogen compounds. ChemAbst 1952;46:11543.
- Shah M, Patel P, Korgaokar S, Parekh H. Synthesis of pyrazolines, isoxazoles and cynopyridines as potential antimicrobial agents. Indian J Chem 1996;35:1282-4.
- Husain MI, Shukla S. Synthesis and Biological activity of 4-(3-Aryl-4-oxo-2-thioxothiazolidin-5-ylimino)-3-methyl-1-(N,N-disubstituted amino-methyl)pyrazolin-5-ones. Indian J Chem 1986;25:983-6.
- Rangari V, Gupta VN, Atal CK. Synthesis, anti-inflammatory and anti-arthritic activity of newer beta-boswellic acid derivatives. Indian J Pharm Sci 1990;52:158-60.
- Nugent AR, Murphy M, Schlachter TS, Dunn CJ, Smith RJ, Staite ND, et al. Pyrazoline Bisphosphonate esters as novel anti-inßammatory andantiarthritic agents. J Med Chem 1993;36:134-8.
- Dhar DN. The Chemistry of Chalcones and Related Compounds. New York: Wiley Interscience; 1981. p. 213.
- Ahmed M, Singh, B, Ranjana Sharma, Talesara GL. Synthesis of 1-(N-Alkoxyphthalimido)-3,5-diaryl-2-pyrazolines. Indian J HeterocyclChem 2004;14:23-5.
- Collee JG, Miles RS, Watt B. Laboratory control of antimicrobial therapy. In: Collee JG, Fraser AG, Marmion BP, Simmons A, editors. Mackie and McCartney Practical Medical Microbiology, 14th ed. New York: Churchill Livingstone; 1996. p. 151-78.