- *Corresponding Author:
- S. S. Agrawal
Delhi Institute of Pharmaceutical Sciences and Research (DIPSAR), Pushp Vihar, MB Road, New Delhi - 110 017, India.
E-mail: agrawal-shyam@indiatimes.com
| Date of Submission | 20 April 2005 |
| Date of Revision | 26 May 2007 |
| Date of Acceptance | 18 July 2007 |
| Indian J. Pharm. Sci., 2007, 69 (4): 535-539 |
Abstract
Since oral bioavailability of atenolol and metoprolol tartrate is poor, different matrix -type transdermal patches incorporating atenolol and metoprolol tartrate were formulated with an objective to study the effect of polymers on transdermal release of the drugs. The polymers selected were polyvinylpyrrolidone, cellulose acetate phthalate, hydroxypropylmethylcellulose phthalate and ethyl cellulose. The patches were formulated using combination of polymers and propylene glycol and 1,8-cineole as plasticizer and penetration enhancer, respectively. The physico-chemical evaluation of the polymer matrices was performed for suitability. The interaction among various components of the matrices was studied by performing Differential Scanning Calorimetry and Scanning Electron Micrography of the formulated patches. In vitro permeation studies were performed using rat abdominal skin as the permeating membrane in Keshary-Chien cell. The results indicated that maximum release was obtained at 48 h (85% and 44% of atenolol and metoprolol tartrate, respectively). The drug permeation studies across cadaver skin showed about 27% of reduction in the amount of drug release as that compared to rat abdominal skin was used.
Transdermal delivery constitutes one of the most important routes for new drug delivery system (NDDS). Transdermal delivery of drugs offers several advantages over conventional delivery methods including oral and injection methods. Transdermal delivery, that traditionally uses a patch containing drug substance pressed onto the skin, is non-invasive, convenient and painless, and can avoid gastrointestinal toxicity (e.g. peptic ulcer disease) and the hepatic first pass metabolism.
Systemic hypertension represents a significant risk factor for the development of atherosclerotic coronary artery disease and myocardial infarction, cerebrovascular accidents and congestive heart failure [1]. A major barrier to the management of hypertension is the extent to which patients comply with the treatment regimen. Transdermally delivered antihypertensives provides the patient a unique and convenient dosing schedule while providing nearly constant serum levels of medication over a prolonged period.
In view of the above-mentioned factors, we aimed to develop the transdermal delivery of certain β1-blockers i.e. atenolol and metoprolol tartrate (both the drugs are incompletely absorbed from GIT and have half–lives of about 6-7 h, respectively). Hence, these drugs were chosen for delivery via transdermal route. Various formulations of these drugs were prepared in three different rate-controlling polymer matrices. These formulations were subjected to physico-chemical evaluation and in vitro permeation studies performed across rat and cadaver skin.
Materials and Methods
Atenolol IP and metoprolol tartrate were obtained as gift sample from Dabur India Ltd. and Astra-Zeneca Pharma India Ltd, respectively. Polyvinylpyrrolidone (PVP) was obtained from USP technologies and hydroxypropylmethylcellulose acetate phthalate (HPMCP) from Shasun Drugs and Chemicals, Cuddalore. Cellulose acetate phthalate (CAP), ethyl cellulose (EC) and propylene glycol were purchased from CDH (P) Ltd. and 1,8-cineole from Sigma Chemical Company. The cadaver skin was procured from Department of forensic medicine, AIIMS, New Delhi 110049. The drug samples were characterized by means of UV, IR methods along with determination of solubility and pH for their authentication.
Preparation of drug containing polymer matrices
Films composed of different polymers (CAP, EC AND HPMCP) with PVP were prepared in methanol/acetone mixture (Table 1). Propylene glycol (40%w/w of dry weight of polymers, used as a plasticizer) and 1,8-cineole (penetration enhancer) were added with continuous stirring using Teflon-coated magnetic bead placed in magnetic stirrer. Drug (33% w/w) was added to the polymer solutions and stirred continuously for an hour and the solutions were casted on the backing mambrane (aluminum foil) and dried in a dessiccator at room temperature. Backing membrane was prepared by wrapping aluminum foil over the Teflon mold. The films were then packed in aluminum foil and stored in a dessiccator until further evaluation.
| Formulations | Atenolol | Metoprololtartarte | ||||
|---|---|---|---|---|---|---|
| Al | A2 | A3 | MI | M2 | M3 | |
| Drug (%w/w) | 33 | 33 | 33 | 33 | 33 | 33 |
| Polymers (%w/w) | ||||||
| PVP | 88 | 88 | 88 | 88 | 88 | 88 |
| EC | 12 | - | - | 12 | - | - |
| HPMCP | - | 12 | - | - | 12 | - |
| CAP | - | - | 12 | - | - | 12 |
| Plasticizer (%w/w) propylene glycol |
40 | 40 | 40 | 40 | 40 | 40 |
| Enhancer (%w/w) | ||||||
| 1,8-cineole | 20 | 20 | 20 | 20 | 20 | 20 |
| Solvent ratio acetone: methanol |
9:1 | 8:1 | 8:1 | 9:1 | 4:1 | 4:1 |
Table 1: Composition of various formulations of atenolol (a) and metoprolol tartrate (m)
Evaluation of polymer matrices
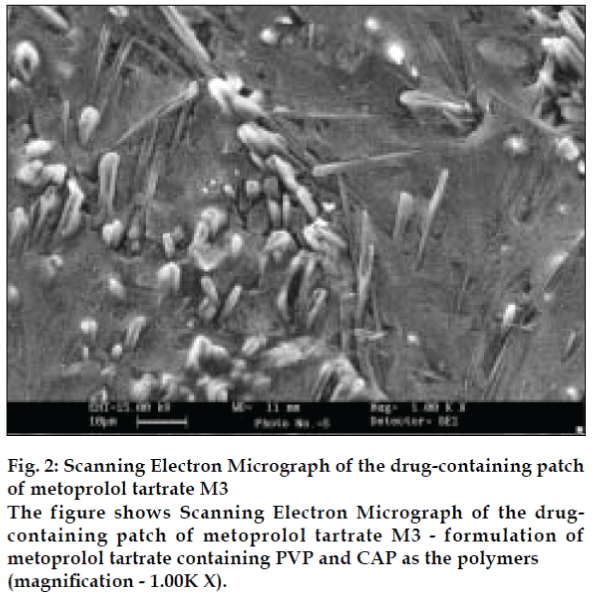
The polymer matrices were evaluated for physical appearance, thickness, uniformity of weight, drug content and effect of aging. The thickness of films was measured at different places using a micrometer and the average was taken. Weight variation test was done by cutting and weighing polymeric films of 1 cm2 size. The average weight was then calculated. The drug content per cm2 was determined by dissolving 1 cm2 of the drug containing polymeric matrices in methanol, diluting with methanol and analyzing for drug content. The effect of aging on physical appearance was studied by packing the polymeric films in properly sealed aluminum foil and then stored in a dessiccator at ambient conditions for 30 days. The scanning electron micrographs (SEM) of formulations A2 and M3 were taken to determine whether the drug is uniformly distributed throughout the polymeric matrix or not, the SEM shows the surface characteristic of the transdermal patch (figs. 1 and 2).
In vitro skin permeation studies with polymeric matrices
The transdermal patches were subjected to in vitro evaluation across rat dorsal skin. After removal of epidermal hair, skin was cleaned and any adhering subcutaneous tissue and blood vessels were removed. The skin was mounted overnight (12 h) on receptor phase to remove any water-soluble (UV absorbing) material [2].
The in vitro skin permeation of atenolol and metoprolol tartrate from various transdermal patches was studied using locally fabricated Keshary-Chien type of diffusion cell [3]. The diffusion cell consists of two parts; the upper part i.e. the donor compartment and contains the active ingredient and the carrier adhesive/patch; the bottom part contains the receptor solution, the water jacket for temperature control, and the sampling port. The effective permeation area of the diffusion cell and receptor cell volume was 1.0 cm2 and 17.5 ml, respectively. The temperature was maintained at 37±2°. the receptor compartment contained 17.5 ml of phosphate buffer saline (PBS) IP (pH 7.4) stirred by magnetic stirrer. The permeability studies were carried out across both rat and cadaver skin. Samples (3.0 ml) were withdrawn and replaced with the same volume of fresh receptor solution, through the sampling port of the diffusion cell at predetermined time intervals till 48 h. the absorbance of withdrawn samples were measured at 274 nm for atenolol and 273 nm for metoprolol tartrate [4]. The experiments were done in triplicates, simultaneously blanks were also run and the average values reported.
The matrices showing promising results from rat skin were evaluated across cadaver skin to determine actual permeation across human skin. Full thickness human chest skin samples were obtained after autopsy. Underlying fatty tissue was removed. Epidermal membranes were prepared using heat separation method. In this method, the full thickness skin samples were immersed in water at 60° for 45 seconds [5]
Evaluation of skin irritation potential of polymeric matrices
The primary skin irritation studies were done using modified Draize test [6]. The hair of rabbits were removed by shaving from the dorsal area on both sides 24 h before test, one side of the back of each rabbit i.e. untreated skin area serves as the control for the test. Medicated patch was secured on experimental side using adhesive tape and the non-medicated patch was adhered on the control side of six rabbits. These patches were covered with occlusive covering to approximate the condition of use. The medicated patches were changed after 48 h. and the fresh patches were secured at the same site. However the patches on the control side were not changed. The patches were secured on the back for seven days. After removal of patch after a week each of the areas were examined for any sign of erythema or oedema.
Results and Discussion
The results of physico-chemical evaluation of different polymeric films showed uniform drug content and minimum batch variation (Tables 2 and 3). The thickness of the patches varied from 552 to 600 µm. The results also showed uniformity in weight per cm2 of area. The physical appearance of the patches and the effect on ageing indicated that the patches need to be stored in properly sealed air tight packing to keep them protected from extremes of moisture that may alter their appearance, thus, the properties were found to be within limits and satisfactory.
| Physical property | Formulations | ||
|---|---|---|---|
| Al | A2 | A3 | |
| Drug content (mg/cm2±SD) | 14.34±1.55 | 14.64±1.33 | 14.89±1.12 |
| Physical appearance | Translucent | Transparent | Transparent |
| Dry | Moist | Dry | |
| Non-sticky | Sticky | Non-sticky | |
| Flexible | Flexible | Flexible | |
| Weight variation (%±SD) | 97.09±1.08 | 101.87±0.99 | 95.03±1.13 |
| Thickness (µ1m±SD) | 570±1.1 | 565±0.93 | 552±0.99 |
| Effect of aging on appearance | |||
| If left open in a dessicator | Translucent | Opaque | Transparent |
| Dry | Dry | Dry | |
| Flexible | Brittle | Brittle | |
| If stored in properly sealed bags | Translucent | Opaque | Transparent |
| Dry | Moist | Dry | |
| Non-sticky | Sticky | Non-sticky | |
| Flexible | Flexible | Flexible | |
Table 2: Result of physico-chemical evaluation of formulated patches of atenolol.
| Physical property | Formulations | ||
|---|---|---|---|
| M1 | M2 | M3 | |
| Drug content (mg/cm2=5D) | 14.32±2.38 | 14.12±2.13 | 14.37±1.38 |
| Physical appearance | Translucent | Transparent | Transparent |
| Dry | Moist | Dry | |
| Flexible | Flexible | Flexible | |
| Weight variation (%-±5D) | 95.61±0.86 | 98.35±1.13 | 94.45±0.86 |
| Thickness (.tm=SD) | 600±1.13 | 593±0.95 | 589±0.65 |
| Effect of aging on appearance | |||
| If left open in dessicator | Translucent | Opaque | Transparent |
| Dry | Dry | Dry | |
| Flexible | Brittle | Flexible | |
| If stored in properly | Translucent | Opaque | Transparent |
| Moist | Moist | Moist | |
| Flexible | Flexible | Flexible | |
Table 3: Physico-chemical evaluation of formulated patches of metoprolol tartrate.
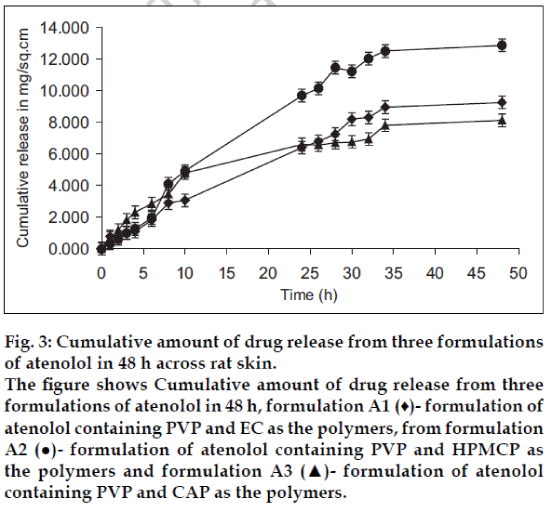
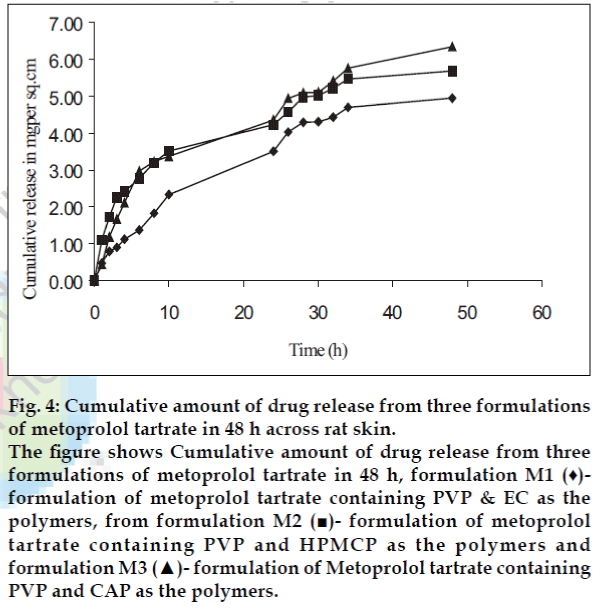
The in vitro permeation data across rat skin showed apparent zero-order kinetics. The cumulative amount of atenolol released from three polymeric films in 48 h was found to be between 8.13 to 12.87 mg and the flux values were found to be 223.2, 328.8 and 174.2 ug/cm2/h, respectively (fig. 3). Maximum amount of drug released from formulation A2 was found to be 85 .0% . The cumulative amount of metoprolol tartarate released from three polymeric films in 48 h was found to be between 4.94 to 6.34 mg and the flux found to be 112.0, 104.9 and 124.6 ug/cm2/h, respectively (fig. 4). Maximum amount of drug released was found to be 44% for formulation M3.
Fig. 3: Cumulative amount of drug release from three formulations of atenolol in 48 h across rat skin. The figure shows Cumulative amount of drug release from three formulations of atenolol in 48 h, formulation A1 (♦)- formulation of atenolol containing PVP and EC as the polymers, from formulation A2 (●)- formulation of atenolol containing PVP and HPMCP as the polymers and formulation A3 (▲)- formulation of atenolol containing PVP and CAP as the polymers.
Fig. 4: Cumulative amount of drug release from three formulations of metoprolol tartrate in 48 h across rat skin. The figure shows Cumulative amount of drug release from three formulations of metoprolol tartrate in 48 h, formulation M1 (♦)- formulation of metoprolol tartrate containing PVP & EC as the polymers, from formulation M2 (■)- formulation of metoprolol tartrate containing PVP and HPMCP as the polymers and formulation M3 (▲)- formulation of Metoprolol tartrate containing PVP and CAP as the polymers.
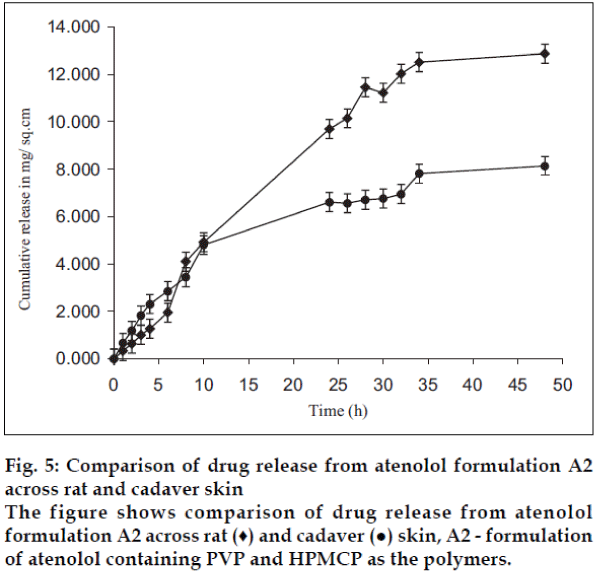
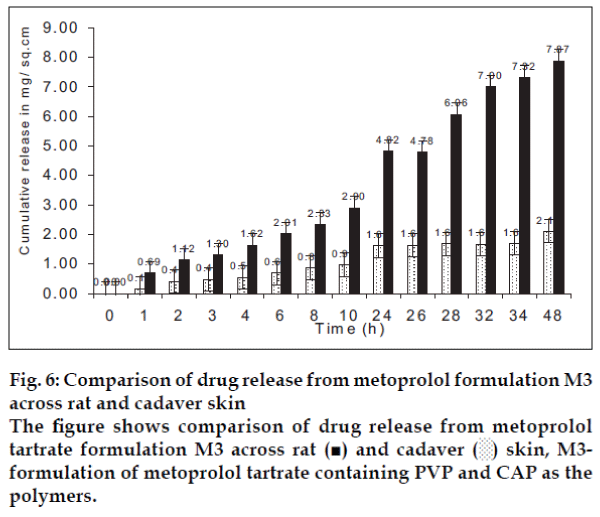
The permeation data of formulations A2 and M3 across cadaver skin showed that cumulative amount of drug released from the patch in 48 h. was found to decrease to 4.22 and 2.10 mg for atenolol and metoprolol, respectively (figs. 5 and 6). This confirmed that rat skin is more permeable for drugs than human skin. These results are in accordance with the findings that indicated that hairless mouse skin and rat skin were about three to four times permeable than cadaver skin.
The primary skin irritation studies of formulation A2 and M3 showed that formulation A2 causes slight irritation after 7 days of application (modified Draize test). Irritation subsided within few hours after removal of patch. Formulation M3 was not found irritant in primary skin irritation studies.
Acknowledgements
The authors are thankful to Astra-Zeneca for providing gift sample of metoprolol tartrate, Dabur India for providing atenolol and Prof. T. D. Dogra, All India Institute of Medical Sciences, New Delhi for providing cadaver skin.
References
- Sclar DA, Skaer TL, Chin A, Okamoto MP, Gill MA. Utility of a transdermal delivery system for antihypertensive therapy.Part 1. Am J Med 1991;91:S50-6.
- Waranis RP, Sloan KB. Effect of vehicles and prodrug properties and their interactions on the delivery of 6-mercaptopurine through skin: Bisacycloxymethyl-6-mercaptopurine prodrugs. J Pharm Sci 1987;76:587-95.
- Keshary PR, ChienYW. Mechanisms of transdermal controlled nitroglycerin administration (I): Development of a finite dosing skin permeation. Drug Develop Ind Pharm 1984;10:883-913
- Moffat AC, editor. Clark's isolation and identification of drugs, 2 nd ed. Pharmaceutical Press: London; 1986. p. 362, 779.
- Kligman AM, Christopher E. Preparation of isolated sheet of human stratum corneum. Arch Dermatol 1963;88:702.
- Draize JH, Woodward GS, Calvery HO. Method for the study of irritation and toxicity of substances applied topically to the skin and mucus membrane. J PharmacolExpTher 1994;82:377-90.