- *Corresponding Author:
- R. Manohar
Ayurvidye Trust, Bangalore-560 085, India
E‑mail: rammanoharp@gmail.com
| Date of Submission | 16 August 2013 |
| Date of Decision | 05 April 2014 |
| Date of Acceptance | 12 April 2014 |
| Indian J Pharm Sci 2014;76(3):240-245 |
Abstract
A study in 2010 reported that administration of 2 g of O. sanctum leaves for 30 days to laboratory male albino rabbits showed adverse effect on sperm count and male hormones. The dose and duration at which this testing was reported was commented upon as being high. It is learnt that basis this publication a few European regulators have imposed restrictions on usage of O. sanctum. Recognizing the need for evaluation, a review has been made of the posological considerations related to decision on dose of a drug in pharmaceuticals (drug development stages) and in Ayurvedic science as part of history of use and current usage. Specifically, we report the dose range as per documented tradition, marketed products containing O. sanctum as an ingredient and current clinical practice. Greater consultation is suggested before deciding the studies on Ayurvedic herbs. Regulatory action of banning use of O. sanctum needs a review and may need to be replaced with an advisory.
Keywords
Ayurveda, male hormones, Ocimum sanctum, posology, Tulasi
An animal study reported administering 2 g per day of Ocimum sanctum leaf juice for 30 days, resulting in significant reduction of sperm count, while testosterone levels were raised. FSH and LH levels were also significantly raised. While the authors have highlighted the potential use of O. sanctum leaves as a contraceptive, these findings raise concerns of safety when using formulations that contain O. sanctum indicated for conditions like cough and fever [1]. One of the authors of this communication, pointed out in a letter, which was published in International Journal of Ayurvedic Research that the dose of O. sanctum leaves is 2-3 g and therefore it appears that the study evaluated the effect of O. sanctum, at very high dose in animals, compared to a human adult dose. The results reported in the above study [1], could unleash a potential scare about the safety of O. sanctum, which is known as Tulasi in Ayurveda and used widely in Ayurvedic practice. The findings of the above study need to be put in proper perspective highlighting the fact that the antifertility effect was observed at a very high doses than seen in actual clinical practice. In case of pharmaceuticals, dose ranges are arrived at through controlled clinical trials after obtaining regulatory permission to test in human subjects.
For example, in the case of fenofibrate, a drug indicated in hypercholesterolemia and hypertriglyceridemia, the recommended dose for adult is an initial dose of 67 mg 2-4 times a day (micronized) or 200 mg/day in divided doses (nonmicronized), while in case of children it is 5 mg/ kg daily [2]. The recommended doses and strength of a number of drugs are laid out in the National Formulary of India [2].
Posological formulae exist to calculate doses for vulnerable populations like pregnant mothers, infants and children based on the human adult dose approved by the regulators and also for doses for use in laboratory animal studies. For example, metformin, an antidiabetic drug to test on rabbits, the dose is computed as below. The dose of metformin in rabbit is 0.07×human dose. So, if metformin human dose (average 60 kg) is 1000 mg/day, rabbit (1500 g wt) dose would be 1000 mg×0.07= 70 mg/day [3]. Ayurveda uses different considerations for deciding and computing the dose for drugs. According to Ayurvedic texts, the dose of herbal paste and powder for a one month old infant is one ratti (125 mg), which is to be increased each month by the same quantity (125 mg). When the child is one year old, it is to be increased by 1 masa (1 g) every year until completion of 16 years. From age 16 onwards the dose will be constant (16 masa=16 g) until 70 years of age, and then above the age of 70 the dose will be tapered gradually until it is once again 125 mg. The dose for decoction is four times this quantity [4].
For Ayurvedic drugs, the first step normally is to refer to the documented texts of Ayurveda to get authentic information on all aspects of drugs. Government of India, Ministry of Health has in the Drugs and Cosmetics Act, which regulates Ayurvedic Medicines amongst recognized 57 authoritative texts in its first Schedule as official books. Recipes and drugs mentioned in these texts get regulatory approval for usage including production at industrial levels and marketing. These books provide information on each drug which includes in case of drugs of botanical origin the name of the herb, part(s) of the plant to be used, process methods, dosage form, duration and anupãna (vehicle). Unlike pharmaceuticals, Ayurveda has laid greater stress on the ability of the patient to tolerate a particular dose of a drug while still getting the desired pharmacological action. The Tulasi (Ayurvedic physicians) are taught develop an expertise to assess the “agni” (loosely interpreted in this context as digestive ability and metabolic capability at cellular level) and “bala” (loosely interpreted in this context as tolerability of the patient to that dose of the drug). In addition, the physician considers the prakr. ti (psycho-somatic constitution). For example, ginger is removed from the formulation s.ad.an.ga (with six herbs as ingredients) when administered to a person or in a disease with predominance of pitta to prevent an adverse reaction [5]. Another alternative is to increase the quantity of sandal in the formulation to pacify pitta. In diarrhea, nut grass is added in double the quantity [6]. Chebulic myrobalan is another example of a drug that should be used with care and is generally contraindicated in a pitta predominant person. Chebulic myrobalan is contraindicated in summer and should be administered in lower doses or given in combination with other substances like jaggery that can neutralize unwanted effects [7]. Unlike posological considerations in pharmaceuticals, where body weight is an important determinant, weight of the patient is not the primary determinant but gets considered as part of the prakr.ti in fixing dose of the drug. It is common practice in Ayurveda to use poly ingredient recipes and single herb based treatment are seldom used. There is greater stress in Ayurveda to use selected dosage forms during treatment and depending on the dosage form, its availability in the body as well as ability of the patient to absorb and metabolize the drug from the product also varies. Hence the dosage form also becomes a determinant factor while deciding the dose of Ayurvedic drugs.
For example, decoctions are typically administered in the acute condition of the disease known in Ayurveda as the Ama stage of disease [8]. Extracts of herbs in ghee are administered in chronic condition known as Nirama stage of the disease after digestion and absorption has improved [8].
Most of the other determinant factors for the dose are same or similar for both pharmaceuticals and Ayurvedics. However, timing of the administration of medicines is based on different parameters. Dosage forms like decoctions are to be taken on empty stomach. In diseases affecting the respiratory system, medicines are to be administered frequently in divided doses. Depending on the type of dos.a involved specific timings in relation to food have been advised for drug administration [9]. Table 1 summarizes different dosage forms of Ayurveda, their doses and considerations for variation in dosage.
| Ayurvedic dosage form | Description | Usual dose | Variation in Dose | Remark |
|---|---|---|---|---|
| Svarasa | Freshly extracted juice of a plant | Infants‑2 ml, bid. | 24 ml (without | Honey, sugar, jaggery, ghee, |
| Children‑5 ml, bid | application of heat)/48ml | oil, can be given as vehicle 6 g | ||
| Adults‑10 ml, tid | (with application of heat) | |||
| Kalka | Soft mass or paste with juice | Infants‑…… Children‑5 g, | 12 g | Jaggery–12 g, honey/ghee/oil/ |
| prepared by pounding the herbs | bid. Adults‑10 g, bid | sugar candy‑24 g, decoction/ | ||
| milk/water‑48ml | ||||
| Cūrn.a | A dry powder filtered through a | Infants‑2.5 g, bid. | 4‑12 g | |
| fine cloth | Children‑5 g, bid. | |||
| Adults‑10 g, bid | ||||
| Kvātha | Preparation obtained by boiling | Infants‑2 ml, bid | 10‑60 ml | Currently used decoctions |
| the herbs with water | Children‑5 ml, bid | are in concentrated form. So | ||
| Adults‑15 ml, qid | consumed after dilution | |||
| Avaleha | Semisolid preparation by | Infants‑2.5 g, oid. | 6‑48 g | |
| solidifying decoction/juice, | Children‑ 5 g, oid. | |||
| jaggery/sugar, powders/pulp, of | Adults‑10 g, oid | |||
| prescribed drugs, ghee/oil/honey | ||||
| Vatī | Solid dosage form prepared by adding fine powder of herbs to liquefied jaggery/sugar/Guggulu/ water/honey |
Infants‑1/2 pill, bid Children‑1 pill, bid Adults‑2 pills, bid |
125 mg‑15 g | |
| Taila(āvarti) | This is prepared by using one part of herbal paste, four parts of oil, 16 parts of decoction this is prepared by using one part of herbal paste, four parts of ghee, 16 parts of decoction |
Infants‑. Children‑5 drops, bid. Adults ‑10 drops, bid Infants‑2 ml, oid Children‑5 ml, bid Adults‑10 ml, bid |
6‑12 ml (internal) | |
| Arista | Fermentative preparations | Infants‑2 ml bid Children‑5 ml, bid Adults‑30 ml, bid |
15‑98 ml |
Table 1: Dosage Forms
In light of the above, inadequate Ayurvedic literature reference, non-consultation with Ayurvedic experts, use of a single point reference to one or two published Ayurvedic books can lead to arbitrary decisions putting limitations on the usefulness of the study data. The level at which a herb known in Ayurveda is taken up for studies either on laboratory animals or on human volunteers, should be decided after thorough study of its Ayurvedic use, especially due to the number of determinants to be considered while deciding the dose of an Ayurvedic drug. Getting adequate information and documenting the same with respect to the lowest and the highest doses to be adopted should be the first step in such studies.
It is common practice to refer to one or two official books of Ayurveda to document the dose of the drug. More often, pharmacologists and clinical personnel refer a secondary source of publication. There is a lacuna in this area due to absence of potential guidelines to review the dose of Ayurvedic drugs on the basis of various determinants. Authors wish to propose a few potential approaches that can strengthen the documentation and hence a better decision on the dose of an Ayurvedic drug.
Taking the case of O. sanctum, a three tier approach is being proposed, which contains, a. documenting the dose and usage levels as enshrined in classical formulations/recipes in which O. sanctum is an ingredient and computing the quantity of raw O. sanctum prescribed (Table 2); b. documenting the usage levels of O. sanctum in licensed Ayurvedic products marketed in India (as proprietary Ayurvedic medicines duly approved by the state licensing authorities under the Drugs and Cosmetics Act (and Rules) and computing the quantity of raw O. sanctum present in the product. (Table 3); and c. documenting the current day usage of O. sanctum as prescribed by practicing Tulasi through an interview.
| Name of the product | Vilvādigulikā | Mānasamitra vat.aka | Nastapuspāntaka rasa | Tribhūvanakirti rasa | |||
|---|---|---|---|---|---|---|---|
| Composition | 43.07 mg in 700 mg of the | 0.74 mg in 165 mg | 2.46 mg in 125 mg of the | 3.90 mg in 125 mg of | |||
| finished product | of finished product | finished product | the finished product | ||||
| Dose per day | 700 mg–1400 mg | 165 mg–660 mg | 250 mg–500 mg | 125 to 250 mg | |||
| Crude Tulasiconsumed/day | 43.07‑86.14mg | 0.74‑2.96mg | 2.46‑9.84 mg | 3.90 to 7.80 mg | |||
| Astān.gahrdaya, Uttarasthāna, | |||||||
| Reference | Sahasrayoga, | Bhaisajyaratnāvalī, | Rasāmrta, 9.80 | ||||
| . . | Gutikāprakarana, 93 | . | . | ||||
| 36.84-85 | Yonivyāpatcikitsā, 51-57 | ||||||
Table 2: Tulasi In Classical Ayurvedic Formulations
| Product name and company | Composition | Dose/day | Total consumption of crude tulasi per day* (mg) |
|---|---|---|---|
| Amylcure DS | Each capsule contains 30 ingredients out | 2 caps b.d./t.d.s | 11538.46 (max dose) |
| capsule | of which tulasi is 20 mg | ||
| Dekofcyn cough | Each 5 ml contains 16 ingredients with | 1‑2 tsf, 3 times a day for | 9230.76 |
| syrup, alarsin | Aqueous ext. derived from tulasi is 200 mg | 1–4 weeks | |
| Feverex syrup, | Each 5 ml contains 13 ingredients with | Adults: 2‑3 tsf, t.i.d, | 6923.07 |
| Dhanwantri | extract of tulasi is 100 mg | ||
| B.cough, | Total number of ingredients is 9 out of | 2 tsf. t.i.d | 2720 |
| Badariya | which tulasi is 10 g | ||
| Koflet syrup, | Total number of ingredients is 21 out of | Adults: 1 to 2 teaspoonfuls | 1538.46 |
| Himalaya | which tulasi is 25 mg | three to four times daily | |
| M‑cof syrup, | Total number of ingredients is 15 out of | 1 tsp thrice daily | 900 |
| Mukthi | which tulasi is 6 g | ||
| Kurex light | Total number of ingredients is 9 out of | Adults‑2 tsf 3 times a day | 300 |
| syrup, Megha | which tulasi is 100 mg | ||
| Respicare | Total number of ingredients is 12 out of | 1 to 2 tabs b.d | 100 |
| tablet, Bacfo | which tulasi is 25 mg | ||
| Tussnil syrup, | Total number of ingredients is 8 out of | 1‑2 tspb.i.d | 26.67 |
| KAPL | which tulasi is 20 mg | ||
| Pneumonorm | Total number of ingredients is 15 out of | 1/2 tsp thrice daily | 11.53 (min dose) |
| syrup, Dr. Paleps | which tulasi is 1 mg |
Table 3: Tulasi in Properiatery Ayurvedic Formulations
Tulasi with predetermined qualification and clinical experience were interviewed either face to face or through teleconference and were asked specific questions about O. sanctum such as, do you prescribe O. sanctum individually, which part of O. sanctum plant do you prescribe normally, how does the patient use it, for what conditions do you prescribe O. sanctum, how much do you prescribe for which age group/population and for how many days, have you observed any side effects, do you avoid use of O. sanctum in any conditions. In case of telephonic interviews, the interview data was documented and signed with date and time by one of the authors who conducted the interview. The results of such interviews of ten Vaidyas were given in Table 4.
| Vaidya | Part of tulasi recommended | Dose according to part used | Caution |
|---|---|---|---|
| Vaidya practicingin Pune | Leaf, seeds soaked in water, whole plant |
0‑25 fresh leaves to make 5ml, taken with pinch of black pepper for 3‑4 days, 15‑20 seeds to make a mucilagenous form for 15‑21 days | Leaves to be plucked before fruiting. Avoid fried food, spicy, hot, pungent food, and pepper |
| Vaidya practicingin Varanasi | Leaf, seed | Juice–1tsf.bd for 2‑4 weeks, Powder 1‑2 g.bd, Decoction 20‑40ml.bd. Seeds for 4‑8 weeks. Half the dose of O. sanctum in children | Not prescribed during pregnancy and surgery |
| Vaidya practicing | Leaf | 5 leaves for 2‑3 weeks | |
| in Trissur | |||
| Vaidya practicing | Juice, rarely seeds, whole | 2‑6 leaves daily or minimum of 1 week, | Leaves should not be |
| in Trissur | plant for decoction | Seeds–½‑1 g.bd for 3 months, Powder–1tsf. | chewed |
| bd. Decoction for 1 week | |||
| Vaidya practicing | Juice, rarely seeds, whole | 2‑8 leaves daily or minimum of 1 week, | Leaves should not be |
| in Delhi | plant for decoction | Seeds–½‑1 g.bd for 3 months, Powder–1tsf. | chewed |
| bd. Decoction for 1 week | |||
| Vaidya practicing | Juice, rarely seeds, whole | 2‑7 leaves daily or minimum of 1 week, | Leaves should not be |
| in Delhi | plant for decoction | Powder–1tsf. bd. Decoction for 1‑2 weeks | chewed |
| Vaidya practicing | Leaf | 2‑3 tsp.bd, Powder 1/2tsp as part of their | Not at this dose |
| in Mumbai | medications for URTI | ||
| Vaidya practicing | Leaf, seeds only in yoga, whole | Juice–1tsp for 1‑2 week. Half the dose in | General |
| in Jamnagar | plant only in combinations | children | |
| Vaidya practicing | Leaf, seed, used to extract oil, | 3‑4 leaves for 7 days, Juice–5‑10ml.td. Seeds | |
| in Bengaluru | whole plant for application | and whole plant for 40 days | |
| Vaidya practicing | Fresh leaf and juice, whole | Juice–15ml for macerating a pill | |
| in Trivandrum | plant |
Table 4: Physicians Feedback
Computation of the dose in the tables 2 to 4 was done to determine the quantity of dry O. sanctum that goes into the formulation and quantity of dry O. sanctum that is recommended per day. In the laboratory, experiment was conducted to actually determine the ratio of fresh O. sanctum leaves to the dry leaves. Based on this data in the tables, we have reported calculated quantity of fresh O. sanctum per day. The calculation for extractive values and back computations were done as follows. E.g. Whyral-22 capsule (Table 3), each capsule contains 50 mg of Tulasi Extract (Ocimum sanctum). Approximately 100 mg of raw O. sanctum yields 13 mg of extract. So this capsule containing 50 mg of extract is equivalent to 384.61 mg of raw O. sanctum. In case of recipes (Table 2) in the authoritative texts of Ayurveda, only the formulations which are meant for oral administration have been considered and rest of the formulations which have O. sanctum but is meant for external use have been omitted.
It was observed that most commonly O. sanctum leaves were used followed by complete aerial parts, flowers and seeds in that order while formulating products with O. sanctum. A study of the indications for which O. sanctum was prescribed in various authoritative books revealed that O. sanctum was prescribed for various indications such as, hiccup, cough, poisoning, dyspnea, pain in flanks, bad odour [10], poisoning by snake, spider, scorpion and rat, artificial poison, fever, cholera, indigestion [11], mental abrasions, increasing intelligence and reasoning [12]. There has been no advisory or care proposed in these books while prescribing O. sanctum nor for its use in children or adults except for the dose to be adjusted for children. However, a deeper study revealed that O. sanctum has been attributed with actions that can increase pitta and body heat. It is mentioned to be a microbicidal agent. Because of its pungent taste and sharp and dry properties, it can increase pitta (one of the three doshas representing heat, transformation, digestion and metabolism) and should not be used in high doses or for a prolonged period in individuals with predominance of pitta or in diseases caused by derangement of pitta and rakta (blood), pungent substances with sharp and dry properties and hot potency are not generally considered to be good for fertility and should be used with care as O. sanctum has pungent taste.
In case of Table 3, as exhaustive as possible, a possible attempts have been made to prepare listing of proprietary Ayurvedic medicines marketed in India. However, this listing is based on identified products listed in a directory of Ayurvedic medicines [13]. An issue of Ayurvedline listed 135 products with O. sanctum as ingredient, out of which 72 products indicated for internal use were taken into consideration. Formulations that did not have adequate data or which were indicated for external use were excluded. Authors consider this book to be fairly comprehensive and hence was selected for the study. However, there could be more products in market containing O. sanctum as an ingredient that may not have been included in our data. This does not adversely affect any of our observations as our listing is covering most commonly marketed products from licensed manufacturers. Interviews of practicing physicians were conducted and data from about 10 physicians have been included representing physicians from territories across India. Table 4 gives an indicative list demonstrating the current day usage of O. sanctum in daily practice by Tulasi.
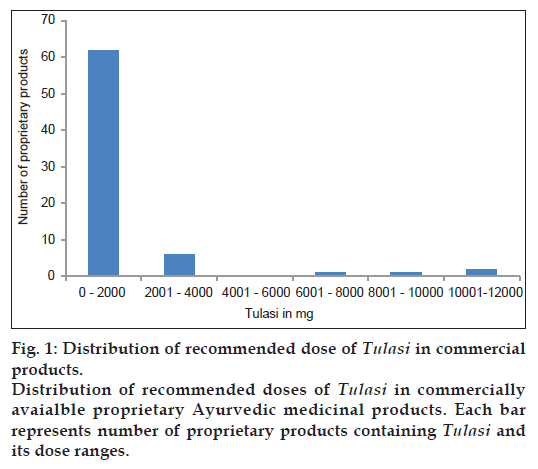
In the classical formulations the quantity of O. sanctum to be consumed per day varied from 0.74 mg to 86.14 mg. In the proprietary products, the daily exposure varied from 11.53 mg to 11538.46 mg. Out of the 72 products, exposure to O. sanctum was less than 4000 mg per day in 68 products. Only in 4 products, it was above 4000 mg (fig. 1). Practicing physicians used different parts of the plant for different purposes. There was some variation in the nature and dosage of use. The daily exposure of O. sanctum ranged from 2 (321 mg) to 25 (2621 mg) fresh leaves to be taken in the form of juice for a period ranging from 3 to 21 days.
From the above analysis, it was found that the recommended daily dosage of O. sanctum for human use in classical Ayurvedic texts, marketed formulations and physician’s prescription was not more than 11.5 g even in exceptional cases. Removing the outliers, the maximum dose was just about 4 g and the minimum dosage across all formulations was found to be 0.00074 g.
As against this, in the earlier quoted study on the antifertility effect of O. sanctum in rabbit (the weight of each rabbit varied from 1.5 kg to 2.5 kg), the daily exposure was 2 g per day. When converted to human dose (considering average weight of a person to be 60 kg), 48 to 80 g of Tulasi leaves would be administered to match the exposure in rabbits. This is an astounding 100000 times greater dose than the lowest dosage used in classical formulations. If compare with proprietary formulations containing exceptionally high dosage of O. sanctum, the doses employed in the rabbit study it would still be seven times greater than the equivalent dose. And if we consider the maximum dose after removing the outliers, it would be twenty times more than the equivalent of the doses used in the study on rabbits. The variations in the dosage of O. sanctum in Ayurvedic formulations can be attributed to the intricacies of dose decision and greater flexibility in fixing doses based on expertise, but there are standard guidelines for posology in Ayurveda.
In the light of the above study, it is quite evident that Ayurveda uses O. sanctum in doses that are significantly lower than the dose at which its antifertility effects were observed in rabbits. Therefore, this animal study and its reporting and results, as a basis for issuing a ban against products containing O. sanctum as an ingredient, needs a review and appears to be not logical, and based on inadequate data. On the other hand, further studies are warranted to explore the dose dependent effects of O. sanctum on fertility. In the present circumstances, an advisory may be issued warning against potential antifertility effects of O. sanctum when consumed in substantially high doses.
References
- Sethi J, Yadav M, Sood S, Dahiya K, Singh V. Effect of tulsi (Ocimum sanctum Linn.) on sperm count and reproductive hormones in male albino rabbits. Int J Ayurveda Res 2010;1:208-10.
- National Formulary of India. Cardiovascular drugs.4th ed. Government of India, Ministry of Health and Family Welfare. Ghaziabad, India: Indian Pharmacopoeia Commission; 2010. p. 309.
- Available from: http://www.naturalhealthresearch.org [Internet]. Bloomingdale: Natural Health Research Institute, 2009. Available from: http://www.naturalhealthresearch.org./nhri/extrapolation-of-animal-dose-to-human [Last accessed on 2013 Oct 03].
- Sarangadhara. SarangadharaSamhita. In: Vidyasagar PS, editor. Aharadigatiadhyaya.Reprint. Varanasi, India: Krishnadas Academy; 1998. p. 71.
- Vagbhata. AshtangaHridaya. In: Paradakar HS, editor. Raktapittacikitsitam. 9th ed. Varanasi, India: ChaukhambhaSurbhartiPrakashan; 2002. p. 577.
- Anonymous. CikitsasarasarvasvamathavaSahasrayogam. In: Krishnan KV, Gopalapillay, editors. KashayayogangalJvarathinu. 19th ed. Mullaykkal, Alapuzha, India: Vidyarambham Publishers; 1990. p.27.
- Bhavaprakash. Bhavaprakashnighantu. In: Dvivedi VS, editor. Haritakyadivarga. 9th ed. Varanasi, India: MotilalBhanarasidas; 1998. p. 5.
- Vagbhata. AshtangaHridaya. In: Paradakar HS, editor. Jvaracikitsitam. 9th ed. Varanasi, India: ChaukhambhaSurbhartiPrakashan; 2002. p. 560.
- Vagbhata. AshtangaHridaya. In: Paradakar HS, editor. Dosopakramaniyam. 9th ed. Varanasi, India: Chaukhambha Surbharti Prakashan; 2002.p. 219.
- Agnivesha. CarakaSamhita. In: Acharya JT, editor. Annapanavidhimadhyaya. 5th ed. Mumbai, India: Munshiram Manoharlal Publishers Pvt. Ltd; 1992.p. 162.
- Vagbhata. AshtangaHridaya. In: Paradakar HS, editor. Sarpavisapratisedha. 9th ed. Varanasi, India: Chaukhambha Surbharti Prakashan; 2002.p. 913.
- Anonymous. Cikitsasarasarvasvam athava Sahasrayogam. In: Krishnan KV, Gopalapillay. editor. Gulikayogangal. 19th ed. Mullaykkal, Alapuzha, India: Vidyarambham Publishers; 1990. p. 139.
- Anonymous. Proprietary medicine index.Ayurvedline-Ayurvedic drug index. 12th ed. Bengaluru, India: Brilliant Printers; 2012.