- *Corresponding Author:
- Y. Inoue
Laboratory of Drug Safety Management, Faculty of Pharmaceutical Sciences, Josai University; 1-1 Keyakidai, Sakado-Shi, Saitama, 3500295, Japan
E-mail: yinoue@josai.ac.jp
| Date of Submission | 04 December 2012 |
| Date of Revision | 23 April 2013 |
| Date of Acceptance | 12 May 2013 |
| Indian J Pharm Sci, 2013;75(4):435-441 |
Abstract
The aim of this study was to prepare an inclusion complex of acetaminophen and β-cyclodextrin (molar ratio of 1:1). A jelly with inclusion complexes formed by kneading was prepared. The formation of inclusion complexes was assessed by powder X-ray diffraction patterns and Fourier transform-infrared spectroscopy. Jellies were prepared with xanthan gum, gelatin, and κ-carrageenan. The concentration of each jelling agent was 0.5, 1.0, and 1.5% w/v. Viscoelasticity and dissolution characteristics were determined and osmometry was performed. PGWater TM , a commercial jelly for fluid replacement, served as a reference for viscoelastic characteristics and dissolution. Powder X-ray diffraction measurement revealed a different diffraction pattern for the kneading than for acetaminophen and β-cyclodextrin. Fourier transform-infrared spectroscopy revealed an absorption peak (at around 1655 cm -1 ) due to the carbonyl group and benzene ring (at around 1610 cm -1 ) of acetaminophen. In contrast, the kneaded mixture (1:1) had a shift in the absorption peak due to the carbonyl group (at around 1650 cm -1 ) in acetaminophen's molecular structure, and the formation of an inclusion complex was noted. The viscosity of xanthan gum-1.0, gelatin-1.5, and carrageenan-0.5 resembled the viscoelasticity of PGWater TM . The acetaminophen in gelatin-1.0 and carrageenan-0.5 had dissolution behavior similar to that of commercial acetaminophen preparations. The osmolality of jellies prepared in different concentrations ranged from about 20-50 mOsm/kg. Results suggested that carrageenan-0.5 could serve as a useful jelly vehicle for acetaminophen.
Keywords
Acetaminophen, β-cyclodextrin, jelly, κ-carrageenan, viscoelasticity
In addition to gastrointestinal symptoms like diarrhea as a complication of liquid feeding [1], patients who have difficulty chewing and swallowing often develop metabolic complications such as dehydration and abnormal blood sugar levels. Semisolid nutrients cause fewer complications than liquid nutrients, and food in the form of jelly is used to prevent complications such as regurgitation. There are various rationales for preparing a drug in jelly form. Calcium polystyrene sulfonate is a treatment for hyperkalemia in patients with chronic renal failure, and a jelly form of this drug reduces graininess. In addition, preparation of a granisetron jelly has improved compliance by inhibiting nausea associated with anticancer agents. In the future, jellies could conceivably be used to facilitate home care. Jellies and jelly vehicles for oral ingestion by patients with dysphagia are being developed and assessed [2]. However, pharmaceutical jelly vehicles for use in feeding tubes and appropriate formulations of those vehicles have not been studied.
Acetaminophen (AAP) is a drug that is widely used as an antipyretic analgesic in clinical practice and its paediatric use is common [3]. On the WHO’s 3-step ladder for cancer pain relief, the first step is the use of nonopioid analgesics such as AAP or nonsteroidal antiinflammatory drugs (NSAIDs) [4,5]. AAP’s usefulness has been recognized in various settings, but the only AAP preparations available are powders, liquids, tablets or suppositories. Thus, drug administration by feeding tube to patients following a gastric resection or patients who have difficulty swallowing requires tinkering with the preparation. In addition, AAP is poorly soluble in water by itself, so its structure has to be tinkered with to improve its solubility.
Cyclodextrins (CD) forms inclusion complexes with various drugs, so it is often used to improve drug solubility [6,7]. CD has been used to stabilize prostaglandin E1 preparations [8] and in a sustainedrelease system to provide resistance to enzymatic degradation of PEGylated insulin [9]. CD has previously been studied in a host of studies and CD is used as a host molecule with molecular characteristics that can be widely altered. As an example, β-cyclodextrins (β-CD) is reported to form an inclusion complex with AAP and improve its water solubility [10].
Thus, we focused as a model drug AAP. Further, AAP inclusion complexes with improved water solubility were prepared in the current study using β-CD as a model drug. This should help facilitate the design of preparations that help improve patient quality of life (QOL). This study also examined AAP in a useful and safe jelly form for the use as a hospital preparation, as reported here.
Materials and Methods
β-CD was donated by MicroBiopharm Japan Co. Ltd., Tokyo, Japan. Xanthan gum (XAN) was purchased from Sigma-Aldrich Corporation, St. Louis, MO, USA; gelatin (GEL) was supplied by Ina Food Industry Co. Ltd., Japan, and κ-carrageenan (CAR) and AAP were of reagent grade and were purchased from Wako Pure Chemical Industries Ltd., Japan. All other chemicals used were of reagent grade. An AAP preparation (Calonal® Tab200) was purchased from Showa Yakuhin Kako Co. Ltd., Japan.
Preparation of physical mixtures
The AAP and β-CD were physically mixed (PM) at a molar ratio of 1:1 in a glass vial for 1 min using a vortex mixer. Kneaded (KD) mixtures were prepared by grinding in a mortar with a pestle for 3 min using a physical mixture at a molar ratio of 1:1 (AAP:β-CD) in 0.5 ml of water with a gross weight of 1000 mg.
Preparation of jellies
Jelly vehicles were prepared using XAN, GEL and CAR. AAP-β-CD inclusion complexes (AAP content: 100 mg) were dissolved by dispersion in 100 ml of distilled water heated to 80º in a water bath. A mixer was used to mix each jelly vehicle with the solution for 3 min to produce an even mixture. The mixture was mixed at 260 rpm to yield a sol. The sol sample was left to stand for 30 min at room temperature and then stored at 4º. XAN, GEL and CAR concentrations in each of the jellies prepared were 0.5, 1.0, and 1.5% w/v (Table 1). PGWaterTM a commercial jelly for fluid replacement from Terumo, served as a reference for viscoelastic characteristics and dissolution.
| Formulation | Percentage of GEL (% w/v) | ||
|---|---|---|---|
| 0.5 | 1.0 | 1.5 | |
| Xanthan gum | XAN-0.5 | XAN-1.0 | XAN-1.5 |
| Gelatin | GEL-0.5 | GEL-1.0 | GEL-1.5 |
| κ-Carrageenan | CAR-0.5 | CAR-1.0 | CAR-1.5 |
Each formulation contained the AAP 1.0 g
Table 1: Concentrations Of Gelling Agents In Jellies
Measurement of powder X-ray diffraction
Powder X-ray diffraction (PXRD) patterns of samples were measured using an X-ray diffractometer (MiniFlex II, Rigaku, Tokyo, Japan) with CuKα radiation, a voltage of 30 KV, a current of 15 mA, a scan range of 2θ=5-40°, and a scan rate of 4°/min.
Fourier transform-infrared spectroscopy
The Fourier transform-infrared (FT-IR) absorption spectra of samples were recorded using a spectrometer (FT/IR-410, Jasco, Tokyo, Japan) based on the KBr disk method The samples were ground with potassium bromide and compressed to obtain disks, and the spectra were recorded at a resolution of 4 cm−1.
Measurement of viscosity and viscoelasticity
Viscosity and viscoelasticity were measured at 25º and 35º using a rheometer (Haake Mars; Thermo Scientific Co., Karlsruhe, Germany) with a 1º×R35 cone rotor. The conditions for viscous measurement were a sample size of 0.2 ml and a gap of 0.051 mm. A flow test was used to determine the relative viscosity of all preparations with the following parameters: a continuous ramp with the shear rate as a controlled variable (0-500 s–1), log mode, and 1 min ramp duration were used for the up curve. The same procedure was used for the downcurve with a reversed shear rate (500-0 s–1) to measure thixotropy and yield stress. The conditions for measurement of viscoelasticity were a sample size of 2 ml and a gap of 1 mm. Stress was gradually increased from 1 to 10 Pa. The viscosity (Eta (mPa•s)) and shear stress (Tau (Pa)) of each sample were measured every 1 s.
Drug release study
Dissolution testing was done with a dissolution tester from Toyama Sangyo Co. Ltd., Osaka, Japan. Sample solutions contained 900 ml of purified water (per the Japanese Pharmacopoeia). Dissolution was measured at a temperature 37±0.5º and paddle speed of 50 rpm in accordance with a dissolution test (the paddle method) in the Japanese Pharmacopoeia, Sixteenth Edition. After addition of the sample, 10 ml of dissolved solution was collected after 5, 10, 15, 30, 45, and 60 min and filtered through a membrane filter with a pore size of 0.45 μm (in dissolution testing of intact AAP, the PM (1:1), and the KD (1:1), samples were measured after 1, 3, 5, 10, and 15 min). Two milliliter of filtrate was accurately measured and accurately diluted to 50 ml with a water/methanol (23/25) mixture to serve as the sample solution. Assays were done with a high-performance liquid chromatograph (HPLC, SPD-20A) from Shimadzu, Kyoto, Japan. Assay conditions were a column of Inertsil ODS-3 (4.6×150 mm, 5 μm), column temperature of 40º, mobile phase of a 0.05 mol/l potassium dihydrogenphosphate:methanol mixture (4:1, pH 4.7), and detection wavelength of 245 nm. AAP retention time was designed to be about 5 min.
Osmometry
The Osmomat 030, a freezing point depression osmometer from Gonotec, Berlin, Germany, was used to measure osmolality. Osmometry conditions were a measurement temperature of 25º, measuring time of about 1 min., measured range of 0-3000 mOsm/kg, and a sample size of 50 μl. Various jelly and physiological saline samples were assembled and their osmolality was measured.
Results and Discussion
Jellies were prepared using complexes with β-CD. The current study used XAN, CAR and GEL, which are commonly used as liquid jelly vehicles and jellies containing AAP were prepared. The CAR-0.5 jelly had viscoelastic characteristics and dissolution like those of commercial jellies and AAP preparations, indicating that it would be a useful formulation for a hospital preparation.
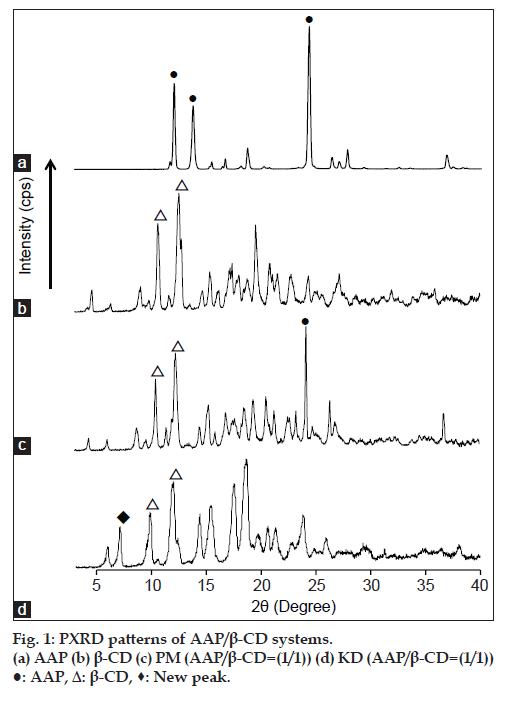
To examine the formation of inclusion complexes by KD, AAP:β-CD molar ratio of 1:1 was prepared. PXRD patterns for the PM and KD of AAP and β-CD are shown in fig. 1. PM was found to have characteristic diffraction peaks due to AAP and β-CD. The KD produced a new peak at 2θ=7.3º that was not produced by AAP or β-CD. Ohmura et al. reported that β-CD inclusion complexes produced powder X-ray diffraction at 2θ of 7.4 and 12.8º; this diffraction pattern is typical of a cylindrical structure [11]. Thus, the KD featured AAP/β-CD complex formation.
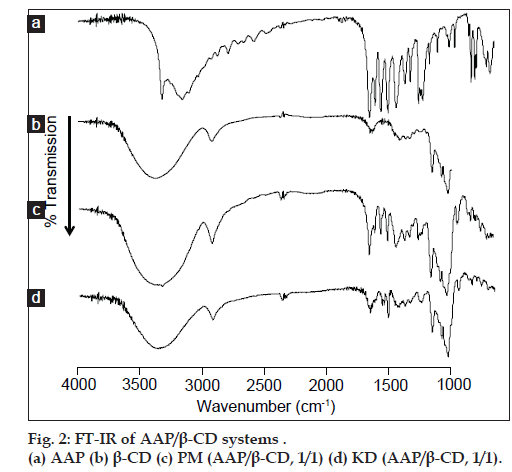
Results of PXRD measurement suggested complex formation by KD. To examine molecular states in complexes, FT-IR absorption spectra were measured. AAP and the PM (1:1) produced absorption peaks due to the carbonyl group (at around 1655 cm–1) and benzene ring (at around 1610 cm–1) in AAP’s molecular structure (fig. 2a and b). KD (1:1) caused a shift in the absorption peak due to the carbonyl group (at around 1650 cm–1) in AAP’s molecular structure (fig. 2d). The shift in the absorption peak due to the carbonyl group is attributed to molecular interaction as a result of the hydrogen bonds of AAP and β-CD. This finding coincides with the results reported by El-Kemary et al. [10]. This molecular interaction is characterized by the hydroxyl groups in the β-CD cavity and the carbonyl group of AAP forming hydrogen bonds and molecular motion being inhibited.
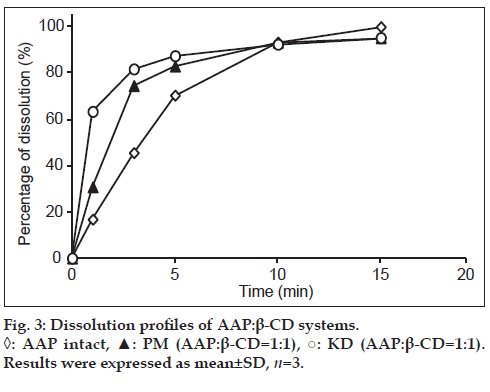
To assess the dissolution of AAP, a dissolution test was conducted with AAP crystals and the PM (1:1) and KD (1:1). Results indicated that the KD (1:1) had the highest rate of dissolution after measurement for 5 min, followed by the PM (1:1) and then AAP crystals, suggesting that the KD (1:1) had improved dissolution (fig. 3). Differences in dissolution were facilitated by the formation of inclusion complexes in the KD, which PXRD measurements confirmed. As a result, in KD, 3 min showed AAP not less than 80%. Therefore, we performed jelly manufacture using the feature of complex. Thus, the readily soluble KD (1:1) can be used to prepare a jelly.
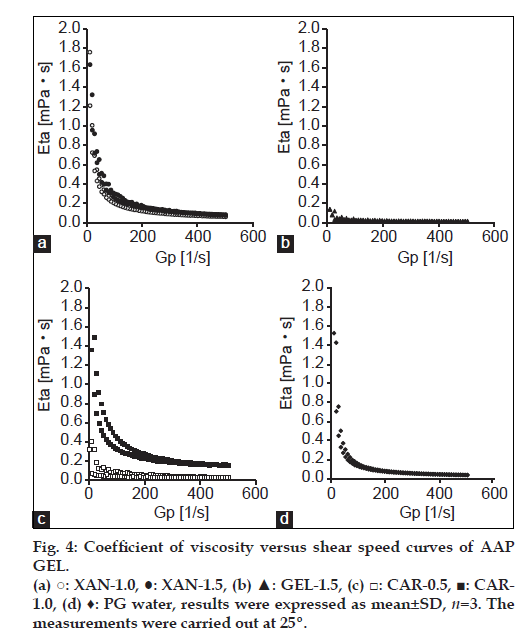
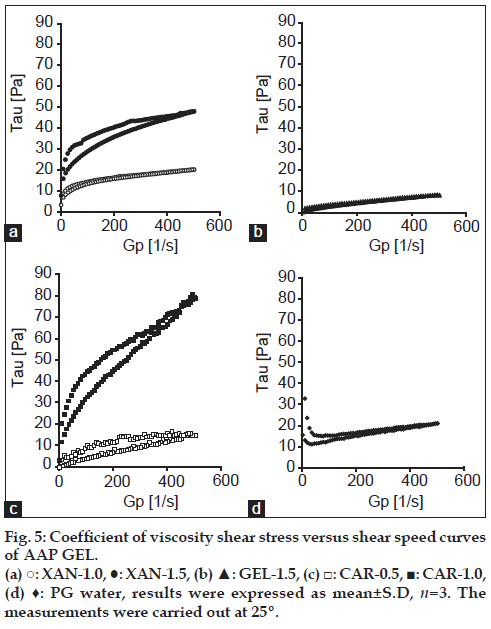
Jelly formulations were prepared using AAP-β-CD inclusion complexes. To compare the viscoelastic characteristics of different jellies at 25°, rheology was determined. In addition, the coefficient of viscosity Eta (mPa•s) and shear stress Tau (Pa) of the commercially available PGWaterTM were measured and compared to the Eta and Tau of different jellies. Results are shown in figs. 4 and 5. PGWaterTM is commonly used to facilitate water intake in forms such as high calorie liquid food supplements. Eta and Tau were measured as clinical indices of viscoelasticity. As shown in fig. 4, the viscosity of XAN, GEL and CAR jellies and PGWaterTM behaved similarly.
The elasticity of XAN-1.5 and CAR-1.0 increased as stress increased (fig. 5). XAN-1.5 and CAR-1.0 had greater elasticity than PGWaterTM. The elasticity of XAN-1.0, GEL-1.5 and CAR-0.5 behaved rather similarly in comparison to PGWaterTM. An advantage of jelly with low viscoelasticity is lessening of the load when pushing a syringe connected to a feeding tube. A smaller load lessens the burden on the caregiver and makes use easier. In addition, ease of use allows food or nutrients to be infused in a short amount of time. This also reduces the need for patients with pressure sores to change position and it reduces the time they must remain in one position. Jelly is not fluid when static; it only flows when subjected to stress. Indicative of a jelly’s dynamic properties, a jelly vehicle’s firmness, and viscosity are correlated, so CAR-1.0 and XAN-1.5 are presumably firm jellies with little plasticity compared to PGWaterTM, and the load presumably increases when the syringe is pushed. In contrast, the elasticity of XAN-1.0, GEL-1.5 and CAR-0.5 behaved similarly in comparison to PGWaterTM, so their structures were flexible and the syringe could be pushed with a smaller load. This makes them easy to use.
In addition, a jelly’s viscosity may affect the ease of chewing and obstruction of feeding tubes [12]. The current results indicated that the viscosity of XAN-1.0, GEL-1.5 and CAR-0.5 resembled the viscoelasticity of PGWaterTM, so XAN-1.0, GEL-1.5 and CAR-0.5 had physical properties akin to those of jellies in clinical use. Thus, there are no concerns about tube obstruction when using a feeding tube and these three jellies can serve as vehicles given their dispersibility and viscosity. At 25º, XAN-1.0, GEL-1.5 and CAR-0.5 resembled a sol. CAR-1.5 solidified, making measurement impossible.
GEL is commonly used as a gelling agent and usually produces highly temperature-sensitive jelly. It has trouble retaining its optimal physical properties until taken and it is known to become a sol at about 30º and melt [13]. In addition, GEL-1.0 had a good rate of dissolution but resembled a sol (data not shown), precluding measurement of its viscoelasticity. Moreover, gelatin reportedly causes allergic reactions. There are also concerns about it having a particular odor. In contrast, stress on CAR at 0.5 resulted in a viscoelasticity like that of PGWaterTM. CAR is known to form a jelly at 40-45º. This property allows a consistent preparation with good dispersibility even at room temperature. In addition, the firmness and elasticity of a CAR jelly can be readily adjusted with minerals or gums, allowing a wide range of uses. This is another reason why CAR-0.5 can readily serve as a jelly vehicle.
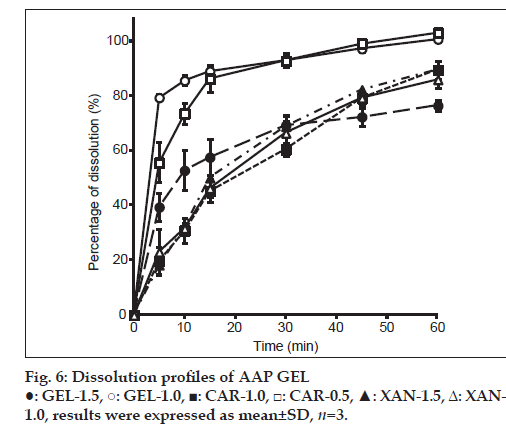
A dissolution test was conducted with different jellies, and results are shown in fig. 6. In GEL-1.0 and 1.5, the difference was observed in the dissolution of AAP by the difference in the concentration of GEL. Although GEL-1.0 showed not less than about 80% of dissolution in 15 min, it was about 60% of dissolution in GEL-1.5. Hence, the difference between the viscosity of GEL and elasticity was considered to have influenced the difference in the dissolution of AAP. Moreover, in CAR-1.0 and 0.5, the difference was similarly observed in the dissolution of AAP by the difference in CAR concentration. This is presumably related to a jelly’s 3-dimensional network structure. CAR is a kind of water-soluble polysaccharide containing sulfate groups which is hydrophilic [14]. With increasing sulfate group, water retaining capacity of polysaccharide increases. In other words, as CAR concentration increases, it is considered that the amount of solvent to keep between the network structure also increases. GEL is a biopolymer with thermo-reversible properties, and at temperatures below 25°, an aqueous gelatin solution solidifies due to the formation of triple helices and a rigid three-dimensional network. When the temperature is raised to approximately 30°, the conformation changes from a helix to a more flexible coil, with a consequent formation of the liquid form of the GEL [13]. Jellies such as gelatin, agar, and carrageenan incorporate vehicles in their structure, and their network structure changes in accordance with the concentration [15]. The low rate of elution of AAP by GEL-1.5 and CAR-1.0 is caused by shrinking of their network structure in accordance with the concentration of the CAR vehicle, and structural characteristics of the substituent presumably limiting elution of AAP from the network structure of the jelly.
XAN-1.0 and 1.5 were both found to have a low rate of dissolution. When the structure of a jelly is disrupted, the jelly’s viscoelasticity decreases and the vehicle dissolves. XAN is pseudoplastic and has a large binding capacity. These characteristics are presumably the reason for its poor rate of dissolution. In addition, XAN is a hydrophilic polymer. Its fast hydration rate causes it to swell with only its surface dissolving. As a result, a thick jelly structure is formed. This can be used as a matrix for controlled release of a drug [16]. The current study similarly found that XAN resulted in the sustained release of AAP.
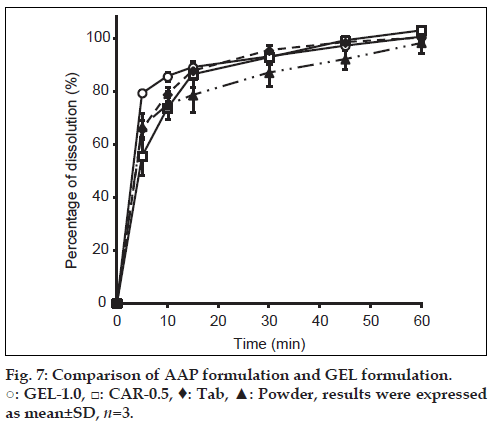
Dissolution by GEL-1.0 and CAR-0.5 was compared using acetaminophen preparations in commercially available forms (Calonal granules and Calonal tablets) (fig. 7). GEL-1.0 and CAR-0.5 both had dissolution behavior similar to that of AAP tablets and granules. This suggests an appropriate level of bioequivalence.
The osmolality of different jellies was measured and compared to the osmolality (mOsm/kg) of physiological saline. Results are shown in Table 2. Osmolality in the human body is typically about 300 mOsm/kg. If a jelly has an osmolality greater than that of a liquid, the rate of water absorption decreases and water pools in the gastrointestinal tract, resulting in clinical manifestations such as diarrhea. The osmolality of different jelly vehicles ranged from 20 to 50 mOsm/kg, which is an acceptable osmolality.
| Formulation | Percentage of GEL (% w/v) | ||
|---|---|---|---|
| 0.5 | 1.0 | 1.5 | |
| Xanthan gum | 30.0(±1.0) | 40.0(±1.0) | 50.0(±1.0) |
| Gelatin | 20.0(±2.0) | 30.0(±1.0) | 20.0(±1.0) |
| κ-Carrageenan | 30.0(±0.0) | 30.0(±1.0) | 30.0(±1.0) |
Results were expressed as mean±SD, n=3
Table 2: Measurement of Osmotic Pressure Of Aap Gel
The results of the current study indicate that CAR-0.5 could serve as a useful and safe jelly vehicle for AAP in a hospital preparation. The design of such preparations can help to improve patient QOL. In addition, a jelly form lessens the burden on the caregiver in terms patients compliance while giving medication. Given the multiple disease incidences in the current society a jelly form may find wider patient complacence in future clinical practice.
References
- Trabal J, Leyes P, Hervás S. Factors associated with nosocomial diarrhea in patients with enteral tube feeding. NutrHosp 2008;23:500-4.
- Miyazaki S, Ishitani M, Takahashi A. Carrageenan gels for oral sustained delivery of acetaminophen to dysphagic patients. Biol Pharm Bull 2011;34:164-6.
- Sullivan JE, Frick GS, Maxwell LG. Fever and antipyretic use in children. Pediatrics 2011;127:580-7.
- McDaid C, Maund E, Rice S. Paracetamol and selective and nonselective nonsteroidalantiinflammatory drugs (NSAIDs) for the reduction of morphine-related side effects after major surgery. Health Technol Assess 2010;14:1-153.
- Kokki H. Nonsteroidalantiinflammatory drugs for postoperative pain: A focus on children. Paediatr Drugs 2003;5:103-23.
- Figueiras A, Carvalho RA, Ribeiro L. Solid-state characterization and dissolution profiles of the inclusion complexes of omeprazole with native and chemically modified b-cyclodextrin. Eur J Pharm Biopharm2007;67:531-9.
- El-Barghouthi MI, Masoud NA, Al-Kafawein JK. Inclusion complexationof Itraconazole with β- and 2-hydroxypropyl-β-cyclodextrins in aqueous solutions. Russ J PhysChem 2006;80:1050-5.
- Kurozumi M, Nambu N, Nagai T. Inclusion compounds of nonsteroidalantiinflammatory and other slightly water soluble drugs with α- and β-cyclodextrins in powdered form. Chem Pharm Bull 1975;23:3062-8.
- Higashi T, Hirayama F, Misumi S. Polypseudorotaxane formation of randomly-pegylated insulin with cyclodextrins: Slow release and resistance to enzymatic degradation. Chem Pharm Bull 2009;57:541-4.
- El-Kemary M, Sobhy S, El-Daly S. Inclusion of Paracetamolinto β-cyclodextrinnanocavities in solution and in the solid state. SpectrochimActa A MolBiomolSpectrosc 2011;79:1904-8.
- Ohmura M, Kawahara Y, Okude K. Electron microscopic observations of inclusion complexes of α-, β-, and γ-cyclodextrins. Polymer 2004;45:6967-75.
- Wendell GD, Lenchner GS, Promisloff RA. Pneumothorax complicating small-bore feeding tube placement. Arch Intern Med 1991;151:599-602.
- Curcio M, Puoci F, Spizzirri UG, Iemma F, Cirillo G, Parisi OI, et al. Negative thermo-responsive microspheres based onhydrolyzed gelatin as drug delivery device. AAPS PharmSciTech 2010;11:652-62.
- Liners F, Helbert W, Van Cutsem P. Production and characterization of a phage-display recombinant antibody against carrageenans: Evidencefor the recognition of a secondary structure of carrageenan chains present in red algae tissues. Glycobiology 2005;15:849-60.
- Sambrook J, Russell D. Molecular Cloning: A Laboratory Manual. 3rd ed. Vol. 1. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2001, p. 5.4-5.13.
- Asghar LF, Chure CB, Chandran S. Colon specific delivery of indomethacin: Effect of incorporating pH sensitive polymers in xanthan gum matrix bases. AAPS Pharm Sci Tech 2009;10:418-29.