- *Corresponding Author:
- R. Ankam
Glenmark Pharmaceuticals Ltd, R&D Centre, Plot No. C-152, MIDC, Malegaon, Sinnar-422 113, India
E-mail: ankam_1999@yahoo.com
| Date of Submission | 05 September 2008 |
| Date of Revision | 11 June 2009 |
| Date of Acceptance | 10 September 2009 |
| Indian J Pharm Sci 2009, 71 (5): 547-551 |
Abstract
A fast, specific, accurate and precise reverse phase high performance liquid chromatographic method was developed for the simultaneous determination of butenafine hydrochloride and betamethasone in cream formulation. The determination was carried out on licrocart licrosphere RP-select B (250Χ4.6 mm, 5 Î…) column in isocratic mode, the mobile phase consisting of 50 mM ammonium acetate buffer and acetonitrile in the ratio of 60:40, adjusted to pH 4.5 ± 0.1 with glacial acetic acid. The flow rate was 2.0 ml/min and eluent was monitored at 254 nm. The retention times of butenafine hydrochloride and betamethasone were 4.70 min and 7.76 min, respectively, and the resolution factor was greater than 4.0. Linearity of butenafine hydrochloride and betamethasone were in the range of 100-300 μg/ml and 5-15 μg/ml, respectively. The proposed method is also found to be precise and robust for the simultaneous determination of butenafine hydrochloride and betamethasone in cream formulation.
Keywords
RP-HPLC method development and validation, simultaneous determination, butenafi ne hydrochloride, betamethasone
Earlier days the practices of curing the skin diseases with single drug molecule for prolong period, this general practice leads to develop drug resistance for bacterial and fungal strains. Now the practice has been changed and combination of drug molecules implies for simultaneous mechanism of action, this leads to application of drugs for short period, which will exhibit fast curing and minimum development of drug resistance to the microbial strains. There is a simultaneous requirement of fast, potential analytical methods to determine the drugs in the given formulation.
The past decade has witnessed a significant increase in the prevalence of resistance to antibacterial and antifungal agents. Resistance to antimicrobial agents has important implications for morbidity, mortality and health care. Antifungal can be grouped into three classes based on their site of action [1]. Azoles, which inhibit the synthesis of ergosterol (the main fungal sterol); polyenes, which interact with fungal membrane sterols physic chemically and 5-ß uorocytosine, which inhibits macromolecular synthesis. Many different types of mechanisms contribute to the development of resistance to antifungal agents. These mechanisms include alteration in drug target, alteration in sterol biosynthesis, reduction in the intercellular concentration of target enzyme, and over expression of the antifungal drug target.
Butenafine hydrochloride (n-4-ter-butyl-benzyl-Nmethyl- 1-naphthaline methylamine hydrochloride) is a new benzylamine derivative with a chemical structure and mode of action similar to allylamine antifungal. It has the empirical formula C23H27N.HCl and a molecular weight of 353.93. It inhibits squalene epoxidase, an enzyme which converts squalene to lanosterol and leads to accumulation of squalene and is primarily fungicidal against dermatophytes. The azoles antifungal have been extensively used to treat dermatophytosis since the 1970s. The most commonly used topical agents are clotrimazole and miconazole. The azoles act as fungi-static agents by inhibiting the cytochrome P450-dependent enzyme lanosterol 14- demethylase, which is important for the biosynthesis of ergosterol, a component of fungal cell membrane. It is freely soluble in methanol, ethanol and chloroform and slightly soluble in water.
Betamethasone dipropionate is designated chemically as 9-ß uoro-11b-hydroxy-16b-methyl-3,20 dioxopregna-1,4-diene-17,21-diyl dipropionate. It has the empirical formula C28H37FO7 and a molecular weight of 504.6. Betamethasone dipropionate is a synthetic adrenocorticosteroid, for dermatologic use. Betamethasone, an analog of prednisolone has high corticosteroid activity and slight mineralocorticoid activity. Betamethasone dipropionate is a white or almost white, crystalline powder, practically insoluble in water, freely soluble in acetone and in methylene chloride, sparingly soluble in alcohol.
The developed cream formulation contained 1% butenafine hydrochloride synthetic antifungal agent and 0.05% synthetic corticosteroid betamethasone as betamethasone dipropionate is member of the class of steroids. A survey of literature revealed that few chromatographic and spectrophotometric methods are reported for determination of butenafine hydrochloride and betamethasone individually [2-7]. However there is no HPLC method reported for simultaneous determination of butenafine hydrochloride and betamethasone from combine dosage form. The present investigation describes a fast, precise and accurate reverse phase HPLC method for simultaneous estimation of butenafine hydrochloride and betamethasone in the cream formulation.
The drug sample, butenafine hydrochloride was procured from Hetero Drugs Limited, Hyderabad, India, betamethasone dipropionate and cream sample were used in-house. HPLC grade acetonitrile, ammonium acetate and glacial acetic acid were purchased from Merck India Limited, Mumbai, India.
An isocratic high performance liquid chromatography (Jasco system) with intelligent sampler (AS-2057), quaternary gradient pump (PU-2080), intelligent UV/ Vis detector (UV-2075), intelligent column oven (CO- 2065) was used. The chromatography column used was licrocart licrosphere RP-select B (250×4.6 mm i.d., particle size 5 µ) column.
A mixture of 50 mM ammonium acetate buffer (adjusted pH to 4.5 with ten percent solution of acetic acid) and acetonitrile in the ratio 60:40 v/v was filtered through 0.45 µ membrane filter and used as mobile phase. The ß ow rate of mobile phase was maintained at 2.0 ml/min. For calibration, standard butenafine hydrochloride and betamethasone solutions were prepared at concentration range of 100-300 µg/ml for butenafine hydrochloride and 5-15 µg/ml for betamethasone in mobile phase. These standard solutions were injected in duplicate and the average detector response measured at 254 nm.
In-house research and development samples (Lot-1, 2 and 3) two tubes each 5 g cream sample was taken in 100 ml beaker and mixed properly. An accurately weighed quantity of cream equivalent to 20 mg of butenafine hydrochloride and 1 mg of betamethasone was taken in 100 ml volumetric ß ask and dissolved in mobile phase. Volume was made up to mark with mobile phase. Whole solution was transferred in 250 ml volumetric ß ask and chilled it in ice bath for 10 min. The solution was filtered through Whatman filter paper No. 40. The aliquot portion of the filtrate was further diluted to get final concentration and detection was done at 254 nm. The results obtained in the experiment were tabulated in Table 1.
| Formulation | Label Content % w/w |
Amount found % w/w |
% Drug found | Standard Deviation |
|---|---|---|---|---|
| Butenafine HCl | ||||
| Lot-1 | 1.0 | 10.004 | 100.40 | 0.59 |
| Lot-2 | 1.0 | 0.999 | 99.93 | 0.55 |
| Lot-3 | 1.0 | 0.989 | 98.93 | 0.67 |
| Betamethasone | ||||
| Lot-1 | 0.05 | 0.050665 | 101.33 | 0.60 |
| Lot-2 | 0.05 | 0.049665 | 99.33 | 0.59 |
| Lot-3 | 0.05 | 0.49165 | 98.33 | 0.69 |
Table 1: Analysis Of Cream Containing Butenafine Hcl And Betamethasone
The method was validated for accuracy, precision, specificity and robustness (Table 2). The accuracy of the method was determined by calculating recoveries of butenafine hydrochloride and betamethasone by placebo spiked recovery. Known amounts of butenafine hydrochloride (100, 200 and 300 µg/ml) and betamethasone (5, 10 and 20 µg/ml) were added to placebo preparation and the amount of butenafine hydrochloride and betamethasone were estimated by measuring the peak areas.
| Performance parameters | Butenafine HCl | Betamethasone |
|---|---|---|
| Linearity and range | 100-300 µg/ml | 5-15 µg/ml |
| Regression coefficient (r2) | 0.9998 | 0.9998 |
| Regression equation (y=mx+c)* | ||
| Accuracy | 1.94 | 1.99 |
| Precision (RSD, n=6) | 1.17 | 1.69 |
| Specificity | Interference not observed | Interference not observed |
| Stability analytical solution | 0, 6, 12, 18, 24h. (n=5) | 0, 6, 12, 18, 24h. (n=5) |
| (normal conditions) | %RSD 1.10 | %RSD 1.89 |
| Stability of analytical solution | 0, 6, 12, 18, 24h. (n=5) | 0, 6, 12, 18, 24h. (n=5) |
| (In dark refrigerator) | %RSD 1.13 | %RSD 1.9 |
| Intermediate precision (RSD) | 0.81 | 0.66 |
Table 2: Results Of Method Validation Experiments Of Butenafine Hcl And Betamethasone
Linearity experiment was performed by using definite concentrations of butenafine hydrochloride (100, 150, 200, 250 and 300 µg/ml) and betamethasone (5, 7.5, 10, 12.5 and 15 µg/ml) solutions were injected and peak areas were recorded. A linear graph was plotted by using the peak areas against concentration in µg/ ml.
The instrument precision was evaluated by injecting the butenafine hydrochloride (200 µg/ml) and betamethasone (10 µg/ml) solution six times repeatedly and peak areas were measured. The results are reported in terms of relative standard deviation. The intra-day and inter-day precision study of butenafine hydrochloride and betamethasone was carried out by estimating the corresponding responses six times on the same day and six times on the second day. The results obtained were reported in terms of relative standard deviation (RSD). The specificity was estimated by spiking known quantity of drug in to placebo preparation. The chromatography was performed by using appropriate dilutions and quantities of drugs were estimated. Robustness of the method was studied by deliberately changing the experimental conditions like ß ow rate, percentage of organic phase. Stability of the standard and sample solutions were observed at 25+2° for 24 h. The sample solution was assayed at every 6 h intervals up to 24 h.
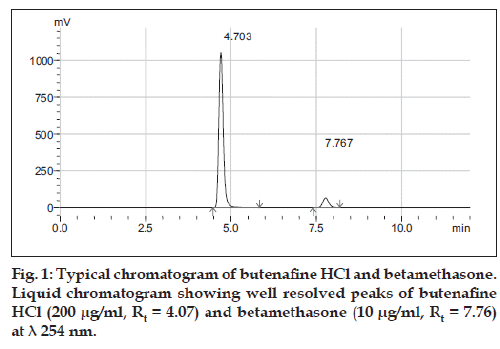
Optimization of mobile phase was performed based on resolution, total runtime, asymmetric factor and theoretical plates obtained for butenafine hydrochloride and betamethasone. Mobile phase consisting of 50 mM ammonium acetate buffer and acetonitrile in the ratio of 60:40, adjusted pH 4.5±0.1 with glacial acetic acid was selected which gave sharp, well resolved peaks for butenafine hydrochloride and betamethasone (fig. 1). The retention time for butenafine hydrochloride and betamethasone were 4.7 and 7.7 min, respectively. The asymmetry factor for butenafine hydrochloride and betamethasone were 1.3, 1.1, respectively. UV spectra of butenafine hydrochloride and betamethasone showed that both the drugs absorbed appreciably at 254 nm, so the same was selected as the detection wave length during the studies. The linearity curve was found to be linear over the range of 100-300 µg/ ml for butenafine hydrochloride and 5-15 µg/ml for betamethasone. The data of regression analysis of the calibration curve are shown in Tables 3 and 4.
| Linearity Level | Conc. (ppm) | Experimental area (a) | Predicted area (y) y=mx+c |
Residuals (b) b=a-y |
|---|---|---|---|---|
| Level-50% | 101.87 | 6118269 | 6094915.2 | 23353.8 |
| Level-75% | 152.80 | 9141247 | 9064783.1 | 76463.9 |
| Level-100% | 203.74 | 11855406 | 12034651 | -179245 |
| Level-125% | 254.67 | 15040202 | 15004518.9 | 35683.1 |
| Level-150% | 305.60 | 18018131 | 17974386.8 | 43744.2 |
Table 3: Results For Regression Analysis Data Of Butenafine Hcl
| Linearity Level | Conc. (ppm) | Experimental area (a) | Predicted area (y) y=mx+c |
Residuals (b) b= a-y |
|---|---|---|---|---|
| Level-50% | 5.01 | 929154 | 936553 | -7399 |
| Level-75% | 7.52 | 1415671 | 1396485.3 | 19185.7 |
| Level-100% | 10.02 | 1836002 | 1856417.6 | -20415.6 |
| Level-125% | 12.53 | 2329220 | 2316349.9 | 12870.1 |
| Level-150% | 15.04 | 2772041 | 2776282.2 | -4241.2 |
Table 4: Results For Regression Analysis Data Of Betamethasone
The accuracy of the method was determined by calculating recoveries of butenafine hydrochloride and betamethasone by placebo spiked method. The recoveries obtained were 99.26-101.98 % for butenafine hydrochloride and 98.02-101.88 % for betamethasone. The high values indicate that the method is accurate. Instrument precision was determined by performing injection repeatability test for standard solution, and the RSD values for butenafine hydrochloride and betamethasone were found to be 1.94 and 1.99, respectively.
For inter-day study RSD values were found to be 0.81, 0.66 for butenafine hydrochloride and betamethasone respectively. The low RSD values indicate that the method is precise. Robustness of the method was studied by changing the ß ow rate of the mobile phase from 2.0 ml/min to 1.8 ml/min and 2.2 ml/min. Using 1.8 ml/min ß ow rate, retention time for butenafine hydrochloride and betamethasone were observed to be 5.2 and 8.5 min, respectively and with 2.2 ml/min flow rate retention time for butenafine hydrochloride and betamethasone were observed to be 4.2 and 6.9 min, respectively without affecting the resolution of the drugs. When mobile phase composition was changed to increase in 2% organic phase, the retention time for butenafine hydrochloride and betamethasone were observed to be 3.9 and 5.9 min, respectively and decrease in 2% organic phase, the retention time for butenafine hydrochloride and betamethasone were observed to be 5.8 and 8.2 min, respectively. The solution stability study revealed that butenafine hydrochloride and betamethasone solutions were stable for 24 h without detectable degradation and the percentage recovery of both the drugs were found to be more than 98.02%.
References
- Mahmoud AG, Louis BR. Antifungal agents mode of action mechanism of resistance and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 1999;12:501-17.
- Yan-Yan LB, Huai-min ZY. Determination of butenafine cream by HPLC. Chinese Hosp Pharm J 2000;06:333-4.
- The United States Pharmacopoeia-30/National formulary-25, Asian edition. Rockville MD: US Pharmacopoeial Convention, Inc.; 2007. p. 1515.
- The United States Pharmacopoeia-30/National formulary-25, Asian edition, Rockville MD: US Pharmacopoeial Convention, Inc.; 2007. p. 1517.
- British Pharmacopoeia Vol. 1. London: The Stationery Office Publications; 2007. p. 259.
- Kedor-Hackmann ER, Gianotto EA, Santoro MI. Determination of betamethasone dipropionate and salicylic acid in pharmaceutical preparations by high performance liquid chromatography. Drug Develop Ind Pharm 1998;24:553-5.
- Dyderski S, Grzeskowiak E, Szalek E, Mrzyglod A. Pharmaceutical availability of betamethasone dipropionate and gentamicin sulfate from cream and ointment. Acta Pol Pharm 2002;59:99-103.