- *Corresponding Author:
- U. Shah
Ramanbhai Patel College of Pharmacy, Charotar University of Science and Technology, CHARUSAT Campus, Changa, Anand-388 421, India
E-mail:umangshah.ph@gmail.com
| Date of Submission | 02 March 2018 |
| Date of Revision | 08 September 2018 |
| Date of Acceptance | 06 February 2019 |
| Indian J Pharm Sci 2019;81(2):273-281 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Stability-indicating reversed-phase high-performance liquid chromatography method was developed and validated for the simultaneous estimation of ezetimibe and glimepiride. Chromatographic separation was achieved with a Shimadzu’s high performance liquid chromatograph and PhenomenexL1 analytical C18 column with a mobile phase of acetonitrile:ammonium acetate buffer (30 mM):methanol (50:45:5 %, v/v/v). The flow rate was set at 1.5 ml/min and the detection wavelength was 232 nm. Quality by design approach was employed for optimization of method parameters like proportion of mobile phase, concentration of buffer and a model highlighting the design space was generated. This developed chromatographic method gave well resolved symmetric peaks. Ezetimibe and glimepiride were eluted at 6.7 and 4.4 min, respectively. This method was validated according to ICH Q2(R1) guideline. The method was linear in range of 50-400 µg/ml for ezetimibe and 5-40 µg/ml for glimepiride with r2=0.9999 and 0.9996, respectively. The sample recoveries were in good agreement with the respective label claim, which suggested non-interference from formulation additives in the estimation. Forced degradation studies were carried out and the stressed samples were analysed using the developed method.
Keywords
Quality by design, stability-indicating, ezetimibe, glimepiride, RP-HPLC
Quality by design (QbD) approach has been introduced by the Food and Drug Administration for the pharmaceutical development to ensure predefined quality attributes of the product. Simultaneously, the application of the QbD concept to the analytical method development leads to a more robust method. As per the International Conference on Harmonisation (ICH) Q8(R2) guidelines, QbD is defined as “a systematic approach to development that begins with predefined objectives and emphasizes product and process control, based on sound science and quality risk management”. Thus in this approach, the variables that overall contribute to the quality of the method are identified, their interactions are studied and finally a method with optimum values of the variables is developed [1]. Stability studies are an integral part of the drug development program. The need for the stability studies on a drug candidate arises from the fact that the chemical integrity of the drug substance should be maintained until the compound is delivered to the intended site of action. Any form of chemical instability may affect the bioavailability and can further lead to toxic effects [2,3].
Ezetimibe (EZE; figure1a), chemically (3R,4S)-1- (4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3- hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one, is a plasma cholesterol lowering agent that acts by decreasing cholesterol absorption in the small intestine. It is soluble in methanol [4,5]. EZE is official in Indian Pharmacopoeia [6].
Glimepiride (GLM; figure1b), chemically 3-ethyl-4- methyl-N-{2-[4-({[(4-methylcyclohexyl) carbamoyl] amino}sulfonyl)phenyl]ethyl}-2-Oxo-2,5-dihydro- 1H-pyrrole-1-carboxamide, is a sulfonylurea antidiabetic agent that works by causing the pancreas to release insulin, which helps to lower blood sugar. It is soluble in methanol [7,8] and is official in Indian Pharmacopoeia [9].
Literature survey revealed that several analytical methods have been reported, such as ultraviolet spectrophotometry [10], high-performance liquid chromatography (HPLC) [11,12], liquid chromatographymass spectrometry (LC-MS) [13] and stability study [14] for the estimation of GLM either individually and in combination with other drugs and similarly for EZE [15-17]. Few HPLC methods were reported for the simultaneous estimation of EZE and GLM in literature. Pavani et al. reported the separation of EZE and GLM from bulk and tablet dosage form by an HPLC method using 0.01 N of potassium di hydrogen ortho phosphate buffer solution and acetonitrile as a mobile phase in the ratio of 30/70 (v/v) [18]. Sudheer et al. developed and validated an HPLC method for simultaneous estimation of EZE and GLM using phosphate buffer (pH 3.6) and acetonitrile in the ratio of 45:55 (v/v) as a mobile phase [19].
All reported liquid chromatographic methods for the simultaneous estimation of the EZE and GLM have used phosphate buffer as one of the components of the mobile phase, which can adversely affect the lifespan of the column. The phosphate buffer at an intermediate and high pH values complexes with the silica surface and weakens the surface silica-siloxane bonds. Thus, a need for a method arises, for estimating the drugs in combination that comprises a mobile phase, which has such solvents and buffers that do not interfere with silica of the chromatographic column as well as contain volatile components that can be used with MS detectors. The ICH Q1A (R2) [20] guideline entitled “Stability testing of new drug substances and products” requires stress testing to be carried out to elucidate the inherent stability characteristics of the active substance. An ideal stability-indicating method is the one that resolves the drug and its degradation products efficiently. So the aim of the present work was to develop and validate [21] a stability-indicating reversed-phase (RP)-HPLC method for simultaneous estimation of EZE and GLM in a combined dosage form within the QbD framework.
Materials and Methods
The GLM reference standard was procured from Baroque Pharma, Sokhda, Khambhat and EZE reference standard was procured from Ranbaxy Pharmaceuticals Ltd., Gurgaon, India. The commercial formulation Eziwa tablets containing 10 mg of EZE and 1 mg of GLM was procured from a local pharmacy. HPLC grade methanol, acetonitrile, and ammonium acetate were procured from Loba Chemicals, India.
The HPLC system (LC-2010C HT, Shimadzu Corporation, Kyoto, Japan) equipped with a SPD M20A photodiode array detector and LC solutions software was used for chromatographic separation. Separation was carried out on a Phenomenex L1 HPLC analytical C18 100 Aº (250×4.6 mm, 5 μ) column. Isocratic condition with mobile phase of acetonitrile:ammonium acetate buffer (30 mM):methanol (50:45:5 %, v/v/v) and 1.5 ml/min flow rate was used for analysis. Ammonium acetate buffer was filtered through 0.45 μ filter. Detection wavelength selected for the estimation of the two drugs was 232 nm.
Preparation of standard solution:
About 100 mg of EZE and 10 mg of GLM were accurately weighed and transferred into a 100 ml clean volumetric flask and diluted up to the mark with methanol. From the above stock solution, 2 ml was pipetted out into a 10 ml volumetric flask and the volume was made with methanol to make concentration 200 μg/ml EZE and 20 μg/ml GLM, respectively.
Method development:
Optimal chromatographic conditions were determined after studying various parameters affecting the chromatographic separation of a mixture including the column, mobile phase ratio, buffer concentration, column temperature and flow rate to achieve maximal separation of the drugs and better peak shape. Various combinations of organic solvents in different ratios were tried to obtain a well-resolved chromatogram of EZE and GLM. The concentration and proportion of ammonium acetate buffer was varied in the mobile phase to obtain good peak shape. The QbD approach was applied to get better resolution between the two drugs and optimization of such robust method to get good peak shape.
Method validation, linearity:
Standard solutions containing 50-400 μg/ml of EZE and 5-40 μg/ml of GLM were prepared. Peak areas for the two drugs were measured at 232 nm. A calibration curve was plotted for peak areas vs. concentration. Regression equation, correlation coefficient, slope and intercept were calculated.
Precision:
Six sample solutions of the same concentration (300 μg/ml EZE and 30 μg/ml GLM) were prepared and injected into the HPLC system as per test procedure. The peak areas for both the drugs in all the sample solutions were determined and percent relative standard deviation (% RSD) was calculated. The intraday and interday precision of the proposed method was determined by analysing the corresponding responses three times on the same day and on three different days of the three standard solution mixtures of 100, 300, and 400 μg/ml of EZE and 10, 30, and 40 μg/ml GLM, respectively. The result was reported in terms of % RSD.
Accuracy:
Accuracy was determined by calculating % recovery by standard addition method. Known amount of standard solution of (50, 100 and 150 μg/ml of EZE and 5, 10 and 15 μg/ml of GLM) were added in preanalysed sample solution having EZE (100 μg/ml) and GLM (10 μg/ml). Peak area of each solution at 232 nm was taken in triplicates and recovery was calculated by using the regression equation.
The detection limit of an individual analytical procedure is the lowest amount of analyte in a sample that can be detected but not necessarily quantitated as an exact value. The quantitation limit of an individual analytical procedure is the lowest amount of analyte in a sample that can be quantitatively determined with suitable precision and accuracy. The quantitation limit is a parameter of quantitative assays for low levels of compounds in sample matrices, and is used particularly for the determination of impurities and/or degradation products. The limit of detection (LOD) and limit of quantitation (LOQ) were separately determined at a signal to noise ratio (S/N) of 3 and 10.
Specificity is the ability to assess unequivocally the analyte in the presence of components, which may be expected to be present. Typically these might include impurities, degradants, matrix. Thus solution of the formulation of EZE and GLM was injected and peak purity was determined for both the peaks. Further stress studies were performed for EZE and GLM to provide an indication of the stability indicating property and specificity of the proposed method.
To assess system suitability of the method, the retention time, peak areas, tailing factor, theoretical plates, and resolution of six replicate injections of standard solution of EZE and GLM having the concentration of 300 and 30 μg/ml respectively, was used and the % RSD values were calculated in each case.
Robustness study was performed to establish the ability of method to remain unaffected for slight variations in the method conditions like mobile phase ratio, flow rate and detection wavelength. No substantial effect was observed on system suitability parameters.
Solution stability:
The solution stability of EZE and GLM was carried out leaving the standard solution in tightly capped volumetric flask for 48 h at room temperature. The peak areas for both the drugs were measured initially, after 24 and 48 h.
Forced degradation studies:
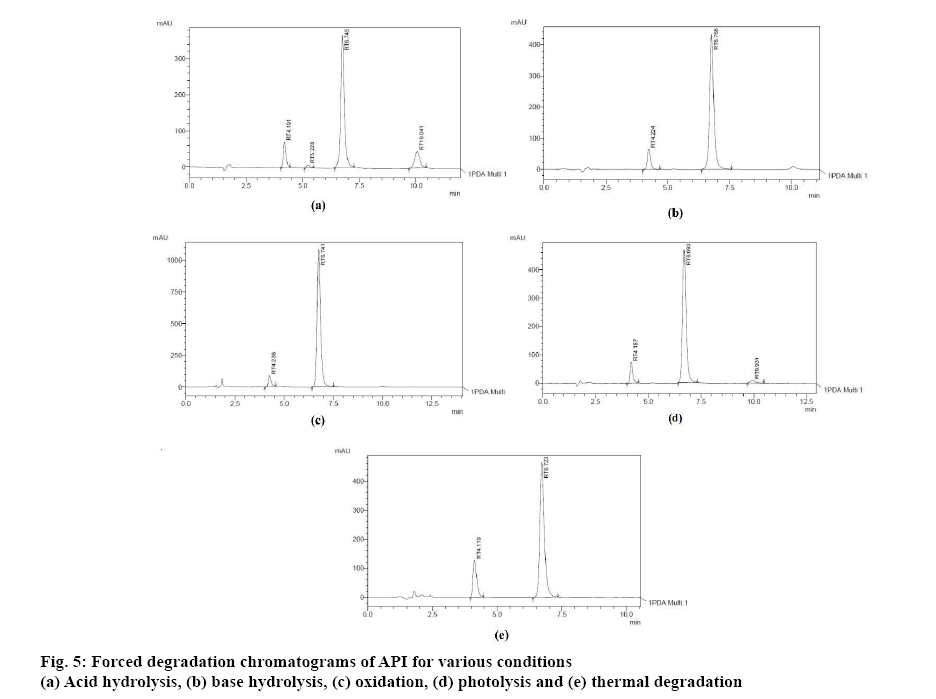
The studies were carried out for API as well as formulation. Stress studies were performed to provide an indication of the stability indicating property of the proposed method. Forced degradation conditions applied to the drug mixture and formulation was acid hydrolysis (0.1 M HCl/50°/15 min), base hydrolysis (0.1 M NaOH/50°/15 min), oxidation (3 % H2O2/50°/15min), photolysis (sunlight/ 1 h) and thermal degradation (60°/1 h). The stressed samples were further diluted in methanol and further studied to evaluate the ability of the proposed method to separate EZE and GLM from their degradation products. Peak purity test was carried out of EZE and GLM by using PDA detector. Assay studies were carried out of stress samples.
Applicability of developed method:
Twenty tablets were weighed accurately and the average weight was determined. The tablets were further crushed; powder equivalent to 100 mg of EZE and 10 mg of GLM was transferred to a 100 ml volumetric flask and dissolved in methanol. An aliquot of 2 ml was withdrawn from the above solution into 10 ml volumetric flask and the final solution was filtered by using 0.45 μ membrane filter to get concentration of 200 and 20 μg/ml for EZE and GLM, respectively.
Results and Discussion
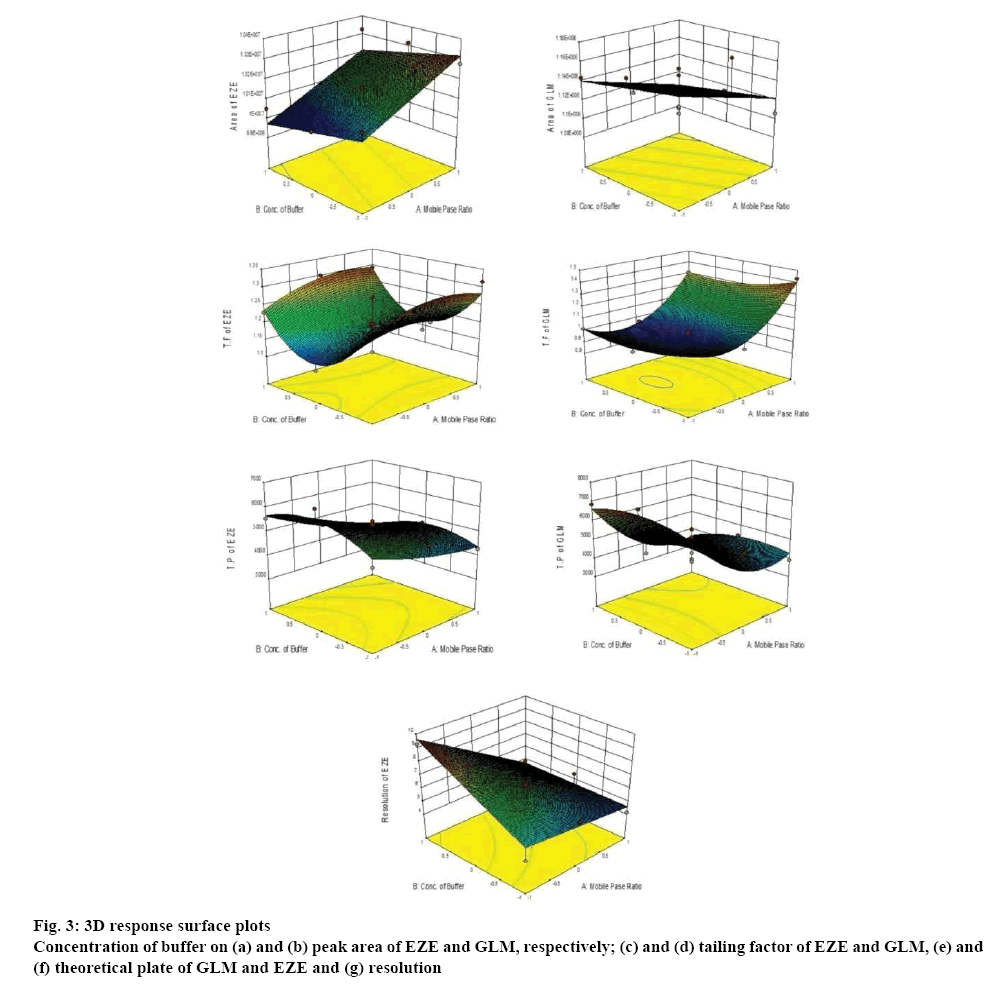
The factors and levels considered in the experimental design are shown in Table 1. Evaluation of the selected critical quality attributes (CQAs) was carried out by ANOVA statistics for experimental design (Table 2). Various proportions of acetonitrile and methanol in the mobile phase were explored for the chromatographic separation of EZE and GLM. The pKa of EZE is 9.73 [22] and that of GLM is 6.2 [23].
| Critical factors | ||
|---|---|---|
| Factors | Levels | Value |
| Mobile phase ratio | -1 0 1 |
45:50:5 50:45:5 55:40:5 |
| Concentration of buffer | -1 0 1 |
25 30 35 |
| Responses | ||
| Peak area Tailing factor Resolution Theoretical plates |
||
Table 1: DoE Summary, Critical Factors and Critical Responses
| Independent Factors | p-value (probability at 95 % confidence interval) | ||||||
|---|---|---|---|---|---|---|---|
| Area EZE | Area GLM | Tailing factor EZE | Tailing factor GLM | Resolution | Theoretical plate EZE | Theoretical plate GLM | |
| X1 | 0.0385 | 0.0940 | 0.3812 | < 0.0001 | 0.0015 | 0.0021 | 0.0048 |
| X2 | 0.0071 | 0.3759 | 0.8845 | 0.0804 | 0.0083 | 0.9107 | 0.6503 |
| X1X2 | 0.4835 | 0.6441 | 0.7628 | 0.5552 | 0.0245 | 0.2853 | 0.6617 |
| X12 | 0.3441 | 0.9203 | 0.3927 | < 0.0001 | 0.1832 | 0.4322 | 0.0463 |
| X22 | 0.1875 | 0.6037 | 0.0078 | 0.0475 | 0.0553 | 0.0228 | 0.4033 |
| X1: mobile phase ratio | *p>0.05 (insignificant) | ||||||
| X2: conc. of buffer | |||||||
| X1X2: interaction | *p<0.05 (significant) | ||||||
Table 2: Anova for 32 Factorial Designs
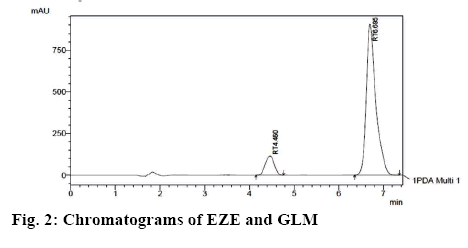
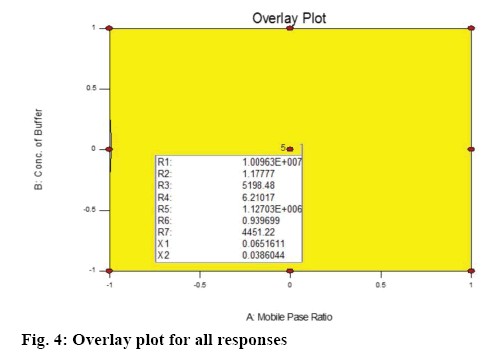
Ammonium acetate buffer was selected to maintain a lower pH of the mobile phase in order to have both the drugs in unionised form for efficient elution through the stationary phase. The organic content of the mobile phase and the concentration of buffer were optimised for good peak shape and resolution of the chromatographic peaks. EZE showed two wavelength maxima at 232 and 250 nm while the wavelength maxima of GLM was 225 nm. The detection wavelength used for the method development for simultaneous estimation of both drugs was 232 nm. The optimized chromatographic condition for the separation and quantification of EZE and GLM was acetonitrile:ammonium acetate buffer (30 mM):methanol in the ratio of 50:45:5 (v/v/v) as the mobile phase with the flow rate of 1.5 ml/min. The optimized chromatogram is shown in figure 2. From the Table 2, the p-values for the studied factors are noted. For the analysis of overall effect of all critical factors, 3D response surface plots were generated that shows simultaneous effect of critical factors on selected responses (figure 3) and an overlay plot of all responses is shown in figure 4. The overlay plot shows that the entire range of the chromatographic conditions having the proportion of acetonitrile from 45 to 55 % v/v and buffer concentration ranging from 25 to 35 mM with the other previously mentioned chromatographic can be applied for the chromatographic separation of EZE and GLM and that any chromatographic condition used within the optimised range will not affect the CQAs. Data for predicted value vs. actual value obtained by cross validation is shown in Table 3.
| Mobile phase ratio | Conc.of buffer | Values | Factors | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | R6 | R7 | |||
| 0.5 | -0.5 | Predicted | 1.01997E+007 | 1.20 | 4862.12 | 5.16 | 1.12418E+006 | 1.09 | 4180.11 |
| Actual | 1.04756E+007 | 1.19 | 5100.10 | 5.31 | 1.18870E+006 | 1.16 | 5005.62 | ||
| -0.5 | -0.5 | Predicted | 1.00927E+007 | 1.19 | 5416.63 | 6.11 | 1.14428E+006 | 0.93 | 5417.56 |
| Actual | 1.06010E+007 | 1.10 | 5277.89 | 7.03 | 1.13235E+006 | 0.88 | 5512.07 | ||
| -0.5 | 0.5 | Predicted | 1.00259E+007 | 1.18 | 5501.23 | 7.70 | 1.13302E+006 | 0.90 | 5358.65 |
| Actual | 1.00533E+007 | 1.09 | 4996.60 | 8.13 | 1.13070E+006 | 0.93 | 5216.47 | ||
| 0.5 | 0.5 | Predicted | 1.01634E +007 | 1.21 | 4743.92 | 5.70 | 1.11826E+006 | 1.05 | 3927.91 |
| Actual | 1.02641E+007 | 1.01 | 4856.9 | 7.90 | 1.16616E+006 | 1.36 | 4284.45 | ||
Where R1 = peak area of EZE, R2 = tailing factor of EZE, R3 = theoretical plates of EZE, R4 = resolution, R5 = peak area of GLM, R6 = tailing factor of GLM, R7 = theoretical plates of GLM
Table 3: Cross Validation Data
Linear correlation was found between peak areas versus concentration of EZE and GLM in the range of 50-400 and 5-40 μg/ml, respectively. The results of method precision, intraday and interday precision (RSD<2 %) proved the method to be precise. The LOD and LOQ for EZE and GLM are shown in Table 4. Accuracy of the method was determined through a recovery study. Results obtained in the range of 100-102 % for EZE and GLM showed good accuracy of the developed method (Table 5). The peak purity index of EZE and GLM peaks obtained from the formulation as well as the stressed samples was found to be more than 0.999. System suitability parameters were found to be within the acceptance criteria (Table 6). Changes in the proportion of mobile phase, flow rate and detection wavelength did not show significant variation in the peak areas of EZE and GLM (Table 7). Stability profile for standard solution mixture of EZE and GLM was studied at 0, 24 and 48 h. The results were expressed as percent drug remaining. The data obtained showed that sample solutions were stable during 48 h when stored at ambient temperature with less than 2 % degradation (Table 8).
| Parameters | EZE | GLM |
|---|---|---|
| Linearity (µg/ml) | 50-400 | 5-40 |
| Correlation coefficient (r2) | 0.9999 | 0.9996 |
| Regression equation | y = 31638x+6095 | y = 35989x+11362 |
| Intraday precision (% RSD) | 0.060–1.36 | 0.29–0.35 |
| Interday precision(% RSD) | 1.135–1.52 | 1.17–1.30 |
| Method precision (% RSD) | 0.60 | 1.055 |
| LOD (µg/ml) | 2.51 | 0.51 |
| LOQ (µg/ml) | 7.61 | 1.56 |
Table 4: Validation Parameters
| Level of recovery(%) | C actual(µg/ml) | C added(µg/ml) | Mean % recovery ±SD | % RSD | ||||
|---|---|---|---|---|---|---|---|---|
| EZE | GLM | EZE | GLM | EZE | GLM | EZE | GLM | |
| 50 | 100 | 10 | 50 | 5 | 101.96±0.32 | 100.57±0.83 | 0.32 | 0.83 |
| 100 | 100 | 10 | 100 | 10 | 100.89±1.69 | 101.59±0.67 | 1.09 | 0.66 |
| 150 | 100 | 10 | 150 | 15 | 101.56±0.76 | 100.25±0.83 | 0.89 | 0.82 |
*n=3
Table 5: Results of Recovery Study
| Parameters | Drug | Mean±SD | % RSD |
|---|---|---|---|
| Retention time (Rt) | EZE | 6.497±0.008 | 0.13 |
| GLM | 4.468±0.001 | 0.03 | |
| Peak area | EZE | 10066729±61316.31 | 0.60 |
| GLM | 1147743±12116.83 | 1.05 | |
| Tailing factor (T) | EZE | 1.503±0.004 | 0.33 |
| GLM | 1.037±0.004 | 0.48 | |
| Theoretical plates (N) | EZE | 4263.388±42.846 | 1.00 |
| GLM | 2826.575±26.032 | 0.92 | |
| Resolution (Rs) | --- | 5.811±0.064 | 1.11 |
*n=6
Table 6: Summary of System Suitability Parameters
| Factor | Peak area | |||
|---|---|---|---|---|
| EZE | GLM | |||
| Mean area±SD | % RSD | Mean area±SD | % RSD | |
| A. Mobile Phase Ratio (ACN:ammonium acetate buffer:methanol) | ||||
| 48:47:5 | 6425960±88467.09 | 1.37 | 679331.3±10219.77 | 1.50 |
| 52:43:5 | 6593183±84290.62 | 1.27 | 675827.7±10943.33 | 1.61 |
| B. Flow rate (ml/min) | ||||
| 1.3 | 6640479±97536.69 | 1.46 | 662859±9856.80 | 1.48 |
| 1.7 | 6466154±103176.8 | 1.59 | 662693±10634.38 | 1.60 |
| C. Wavelength (nm) | ||||
| 230 | 6524847±94394.14 | 1.44 | 665792.7±9026.05 | 1.35 |
| 234 | 6573790±87504.9 | 1.33 | 664376.7±8855.57 | 1.33 |
*n=6
Table 7: Results of Robustness Study
| Time(h) | % Assay | |
|---|---|---|
| EZE | GLM | |
| 0 | 100.00 | 100.00 |
| 24 | 99.80 | 99.86 |
| 48 | 98.48 | 99.19 |
| %RSD | 0.83 | 0.43 |
Table 8: Solution Stability Data
Percent degradation of EZE and GLM observed during the analysis of stressed samples are indicated in Table 9. A similarity in trend for the degradation of the API and formulation was observed in the applied stressed conditions for both the drugs. The extent of degradation of EZE was similar in all the conditions while GLM showed highest degradation in acidic environment and least degradation due to photolysis. The peak purity data of EZE and GLM were evaluated from chromatograms of the degradation samples (figure 5). The assay result for the marketed formulation was found to be well in accordance to the label claim (Table 10).
| Degradation mode | Condition | Time | Temp. | % Degradation of EZE | % Degradation of GLM | ||
|---|---|---|---|---|---|---|---|
| API | Formulation | API | Formulation | ||||
| Acid hydrolysis | 0.1 N HCl | 15 min | 50° | 29.43 | 24.97 | 22.95 | 20.88 |
| Base hydrolysis | 0.1 N NaOH | 15 min | 50° | 20.53 | 28.48 | 18.57 | 16.16 |
| Oxidation | 3 % H2O2 | 15 min | 50° | 16.84 | 22.12 | 14.03 | 12.56 |
| Photolysis | Sunlight | 60 min | --- | 21.80 | 23.82 | 7.21 | 9.605 |
| Thermal | Oven | 60 min | 60° | 25.08 | 22.30 | 17.70 | 16.01 |
Table 9: Results of Forced Degradation Study
| Marketed formulation | EZE | GLM | ||
|---|---|---|---|---|
| Amount found | % Assay | Amount found | % Assay | |
| Eziwa (Label claim:200 mg EZE and 20 mg GLM) |
200.86 | 100.34 | 19.50 | 97.54 |
| 200.01 | 100.007 | 19.63 | 98.19 | |
| 199.15 | 99.57 | 19.81 | 99.07 | |
| 197.16 | 98.58 | 19.66 | 98.32 | |
| 204.15 | 102.07 | 19.62 | 98.12 | |
| 200.56 | 100.28 | 19.57 | 97.88 | |
| Mean | 200.31 | 100.14 | 19.63 | 98.18 |
| SD | 2.30 | 1.14 | 0.10 | 0.51 |
| % RSD | 1.14 | 1.14 | 0.52 | 0.52 |
Table 10: Assay Results
A simple and rapid stability-indicating HPLC method was developed and validated for application in routine quality control tests of EZE and GLM. The method included a simple sample preparation procedure which was a simple extraction with methanol. Based on the understanding of the process, method goals were defined. Experimental design was applied for two factors, mobile phase ratio and concentration of buffer. Mobile phase optimization was given priority to attain complete separation of both drugs. This achieved using the QbD approach. Utilizing the three level factorial design options, an experimental design was created in the design expert software (Stat-Ease Inc., Statistic made easy, Minneapolis, MN, USA, version 9.0.0) for Critical Method Parameters. For the analysis of overall effect of all critical factors, 3D response surface plots were generated which showed simultaneous effect of critical factors on selected responses. The peak purity data confirmed the specificity of the method. The developed method was validated as per the ICH guidelines. The results of method validation showed that the method linear and precise. It was found to be highly robust for the deliberate variations. The samples were exposed to stress conditions, which were optimised so as to cause degradation of the drugs up to 30 %. The peak purity data of EZE and GLM from chromatograms of the degradation samples indicated that drug peak were homogeneous with no co-eluting peaks. This indicated the absence of any interference in the assay of EZE and GLM from the known/unknown impurities which might arise due to degradation of the drug substances.
An innovative QbD approach was applied for developing a stability-indicating RP-HPLC method for the simultaneous estimation of EZE and GLM in the presence of degradants produced during forced degradation. The degradation products generated did not interfere with the drug peaks. The CQAs were studied and a design space (volume in which the method is robust) was defined and visualised. The developed method was validated in compliance with the ICH guidelines. Hence, this developed method could be used for quality control analysis of EZE and GLM in their pure form and their combined dosage forms.
Conflict of interest
The authors declare no conflict of interest.
References
- ICH, Q8(R2), Harmonized Tripartite Guideline, Pharmaceutical Development. Available from: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1/Step4/Q8_R2_Guideline.pdf.
- Shah U, Kavad M, Raval M. Development and Validation of Stability-indicating RP-HPLC Method for Estimation of Pamabrom in Tablets. Indian J Pharma Sci 2014;76(3):198-202.
- Blessy M, Patel RD, Prajapati PN, Agrawal YK. Development of forced degradation and stability indicating studies of drugs-A review. J Pharm Anal 2014;4:159-65.
- Ezetimibe. [cited 2014 Oct 2]. Available from: https://medlineplus.gov/druginfo/meds/a603015.html
- Ezetimibe, Drug Bank. [cited 2014 Oct 2]. Available from: http://www.drugbank.ca/drugs/DB00973.
- Indian Pharmacopoeia. Vol. 2. Ghaziabad, India: The Indian Pharmacopoeia Commission. Ministry of Health and Family Welfare, Govt. of India; 2014. p. 1727-9.
- Glimepiride-oral, Amaryl. [cited 2014 Oct 2]. Available from: http://www.medicinenet.com/glimepiride-oral/article.htm.
- Glimepiride, Drug Bank. [cited 2014 Oct 2]. Available from: http://www.drugbank.ca/drugs/DB00222.
- Indian Pharmacopoeia. New Delhi, India: Controller of Publication, Govt. of India, Ministry of Health and Family Welfare; 2010. p. 1418 -9.
- Altinoz S, Tekeli D. Analysis of glimepiride by using derivative UV spectrophotometric method. J Pharm Biomed Anal 2001;24(3):507-15.
- Balkrishna VM, Babar S, Kulkarni N. Development of UV spectrophotometric method for the simultaneous estimation of simvastatine and ezetimibe in tablet dosage form by simultaneous equation and absorbance ratio method. Int J PharmTech Res 2011;3(3):1459-66.
- Jain D, Jain S, Jain D, Amin M. Simultaneous Estimation of Metformin Hydrochloride, Pioglitazone Hydrochloride, and Glimepiride by RP-HPLC in Tablet Formulation. J Chromatogr Sci 2008;46(6):501-04.
- Yuzuak N, Ozden T, Eren S, Ozilhan S. Determination of Glimepiride in Human Plasma by LC-MS-MS. Chromatographia 2007;66(1):165-68.
- Bonfilio R, Peres C, Salgado HR, De Araujo MB, Tarley CR. Multivariate development and validation of a stability-indicating HPLC method for the determination of glimepiride in tablets. J AOAC Int 2013;96(5):960-67.
- Beludari MI, Prakash MV, Mohan GK. RP-HPLC method for simultaneous estimation of rosuvastatin and ezetimibe from their combination tablet dosage form. Int J Chem Anal Sci 2013;4(4):205-09.
- Sahu S, Narayan UL, Garnaik B, Patro, SK. Stability Indicating RP-HPLC method for determination of Ezetimibe in pure and pharmaceutical formulation. Int J Res Pharm Biomed Sci 2013;4(4):1249-55.
- Yardimci C, Ozaltin N. Simultaneous Determination of Ezetimibe and Simvastatin in Pharmaceutical Preparations by MEKC. J Chromatogr Sci 2008;48(2):95-99.
- Pavani P, Srilekha A, Sreedhar B. Stability Indicating RP-HPLC Method Development and Validation for Simultaneous Estimation of Glimepiride and Ezetimibe in bulk and tablet dosage form. Int J Pharm Sci Res 2015:6(3);1066-77.
- Sudheer N, Shilpa K, Ajitha A, Uma Maheswara Rao V. Method Development and Validation of Simultaneous Estimation of Ezetimibe and Glimepiride by RP-HPLC. Int J Pharm Res Anal 2016;6(1);47-52.
- ICH, Q1A(R2): Stability Testing of New Drug Substance and Drug Product. Available from: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1A_R2/Step4/Q1A_R2__Guideline.pdf.
- ICH, Q2(R1): Validation of Analytical Procedure: Text and Methodology. Available from: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf
- Ezetimibe, LogP. [cited 2018 Sep 5]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Ezetimibe#section=LogP
- Baliarsingh OP, Biswal S, Sahoo J, Murthy PN. Physicochemical Properties of Glimepiride in Solid Dispersion with Polyethylene Glycol 20000. Int J Pharm Sci Nanotechnol 2009;2(2):537-43.