- Corresponding Author:
- R. R. Somani
Department of Pharmaceutical Chemistry, VES’s College of Pharmacy, Collector Colony, Chembur, Mumbai–400 614, India
E-mail: rakeshrsomani@gmail.com
| Date of Submission | 04 April 2011 |
| Date of Revision | 30 November 2011 |
| Date of Acceptance | 03 December 2011 |
| Indian J Pharm Sci, 2011, 73 (6): 634-640 |
Abstract
Synthesis of 1,3,4-oxadiazole derivatives of diclofenac and mefenamic acid are described. The target compounds 5-[2-(2,6-dichloroanilino)benzyl]-2-aryl-1,3,4-oxadiazole (3a-3e) and 5-[2-(2,3-dimethylanilino)phenyl]-2-(aryl)- 1,3,4-oxadiazole (6a-6e) were obtained by treating 2 and 5 with various aromatic acids using POCl3 as dehydrating agent. They were purified and characterized by IR, 1H-NMR and elemental analysis. These compounds were further subjected to antiinflammatory, analgesic and acute ulcerogenic activity. Compound 3c and 6d exhibited good antiinflammatory activity and compounds 3c, 3e, 6c, 6d, 6e were found to be non ulcerogenic.
Keywords
Analgesic, antiinflammatory, diclofenac, mefenamic acid, ulcerogenic activity, 1,3,4-oxadiazole
Non steroidal antiinflammatory drugs (NSAIDs) are the first line drugs for the treatment of arthritis, pain and inflammatory disorder [1]. The pharmacological activity of NSAIDs is related to the suppression of prostaglandin biosynthesis from arachidonic acid by inhibiting the enzyme prostaglandin endoperoxidase, popularly known as cyclooxygenase (COX) [2,3]. It was reported that COX exists in two isoforms, COX- 1 and COX-2, which are regulated and expressed differently [4-6]. COX-1 provides cytoprotection in the gastrointestinal tract (GIT), whereas inducible COX-2 selectively mediates inflammatory signals [7-9]. Since most of the currently available NSAIDs in the market show greater selectivity for COX-1 than COX-2 [10], chronic use of NSAIDs, including diclofenac and mefenamic acid, may elicit appreciable GI irritation, bleeding and ulceration [11]. GI damage from NSAID is generally attributed to two factors, local irritation by the direct contact of carboxylic acid (–COOH) moiety of NSAIDs with GI mucosal cells (topical effect) and decreased tissue prostaglandin production in tissues which undermines the physiological role of cytoprotective prostaglandins in maintaining GI health and homoeostasis are the effects shown by the nonselective COX inhibitors [12]. The ulerogenesis due to carboxylate group can be minimized by modifying the acid functionality to ester [13], amide or NO releasing group [14,15]. Also derivatisation of the carboxylic acid group of NSAIDs to various five member heterocycles has been reported to retained antiinflammatory activity with reduced ulcerogenic potential [16-19].
Among the various heterocycles, oxadiazole, especially 2,5-disubstituted-1,3,4-oxadiazole has unique features associated with it. It consists of toxophoric moiety (N-C-O) [20] which enhances its potential as an effective biodynamic molecule, additionally the oxadiazole ring has been reported to be weak acidic in nature and it is an isoster for the ester functionality [21]. The 2,5-disubstituted-1,3,4- oxadiazole is associated with diverse biological activities like anticonvulsant [22], antidepressant [23], non-ulcerogenic antiinflammatory [24], anticancer [25], antimicrobial-antiTB [26] and antiangiogenic [27] etc, which makes it biologically important scaffold.
Thus in our attempt to find new, safer and potent antiinflammatory agent and our continued interest in oxadiazole chemistry [28-33], we built oxadiazole nucleus on the two most popular NSAIDs diclofenac and mefenamic acid and evaluated them for their antiinflammatory, analgesic and ulcerogenic activities.
Materials and Methods
All the chemicals were obtained from commercial suppliers and used without further purification. All the melting points were determined on ‘Veego’ VMP-D apparatus and are uncorrected. Silica gel G plates of 3x8 cm (Sigma-Aldrich) were used for TLC and spots were located by UV or in iodine chamber. The IR spectra were recorded in the 4000-400 cm-1 range using KBr discs on FT-IR 8400 Shimadzu spectrometer. 1H NMR spectra were recorded on Varian Mercury (300 MHz) spectrometer in CDCl3 with TMS as an internal standard and values are expressed in ppm. Elemental analyses were performed for C, H, N and were found within ±0.4% of theoretical values.
Synthesis of methyl {2-[(2,6-dichlorophenyl) amino]phenyl}acetate (1) and methyl 2-[(2,3- dimethylphenyl)amino]benzoate (4)
In a solution of acids (diclofenac and mefenamic acid) (0.033 moles, 10 g) with absolute methanol (50 ml), was added 5 ml of conc. H2SO4 under stirring. It was then refluxed for 6-7 h on water bath, cooled and concentrated under reduced pressure. The residue was poured onto crushed ice and neutralized with 10% NaHCO3 solution to yield solid precipitate. The solid was collected by filtration, washed twice with ice cold water and dried [34].
Synthesis of of 2-{2-[(2,6-dichlorophenyl) amino]phenyl}acetohydrazide (2) and 2-[(2,3- dimethylphenyl)amino]benzohydrazide (5)
To a solution of 1 (0.05 moles, 15.5 g), in absolute methanol (40 ml), 3.75 g of hydrazine hydrate (0.075 moles) was added and the reaction mixture was reflux for 15 h. It was then concentrated, cooled and poured onto ice cold water. The white solid thus separated out was filtered, dried and recrystallised from absolute ethanol [35].
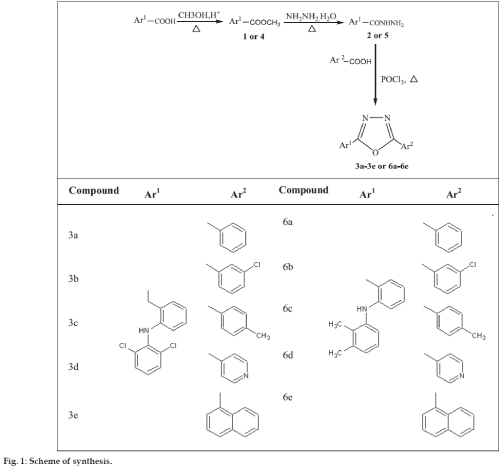
Synthesis of 5-[2-(2,6-dichloroanilino)benzyl]- 2-aryl-1,3,4-oxadiazole (3a-3e) and 5- [2-(2,3- dimethylanilino)phenyl]-2-(aryl)-1,3,4-oxadiazole (6a-6e)
The mixture of 2 or 5 (1 g, 0.01 mole) and various aromatic acid (0.01 mole) was dissolved in 3-5 ml of phosphorus oxychloride (POCl3). The reaction mixture was heated under reflux for 4 h. After completion of the reaction, (monitored by TLC, ethyl acetate:hexane:: 0.5:1.5) mixture was neutralized with ice cold solution of 10% NaHCO3. The precipitate thus obtained was filtered, washed with ice cold water and purified by column chromatography [36] (ethyl acetate:hexane:: 0.5:1.5).
5-[2-(2,6-Dichloroanilino) benzyl]-2-(phenyl)-1,3,4- oxadiazole (3a): Yield 71%, white crystalline solid, m.p. 80-82º, 1H NMR (CDCl3): δ 7.60-7.50 (m, 5H, Ar-H) , 7.42 (d, 2H, Ar-H) , 7.20-7.10 (m, 4H, Ar-H), 6.80-6.70 (t, 1H, Ar-H), 6.60 (s, 1H, NH), 1.55 (s, 2H, CH2). IR (KBr, cm-1): 3254, (NH), 3072 (Ar CH), 1606(C=N), 1101, 1000, 902(C-O-C). Rf; 0.84.
5-{[2-(2,6-Dichloroanilino)benzyl]-2-(3-chlorphenyl)}- 1,3,4-oxadiazole (3b): Yield 72%, whitish yellow solid, m.p. 83-84º, 1H NMR (CDCl3): δ 7.60-7.50 (m, 4H, Ar-H), 7.48-7.40 (m, 3H, Ar-H), 7.30-7.10 (m, 3H, Ar-H), 6.80-6.70 (t, 1H, Ar-H), 6.68 (s, 1H, NH), 1.54 (s, 2H, CH2). IR (KBr, cm-1): 3252, (NH), 2953 (Ar CH), 1604(C=N), 1101, 1096 (C-O-C). Rf; 0.78.
5 - { [ 2 - ( 2 , 6 - D i c h l o r o a n i l i n o ) b e n z y l ] - 2 - ( 4 - methylphenyl)}-1,3,4-oxadiazole (3c): Yield 79%, pinkish white solid, m.p. 79-80º, 1H NMR (CDCl3): δ 7.60-7.50 (m, 4H, Ar-H), 7.40 (d, 2H, Ar-H), 7.20- 7.10 (d, 2H, Ar-H), 7.00 (d, 2H, Ar-H), 6.80-6.70 (t, 1H, Ar-H), 6.68 (s, 1H, NH), 1.50 (s, 2H, CH2), 1.26 (s, 3H, CH3). IR (KBr, cm-1): 3126, (NH), 2923 (Ar CH), 1589(C=N), 1101, 1090 (C-O-C). Rf; 0.81.
5-{[2-(2,6-Dichloroanilino)benzyl]-2-(pyridine-4-yl)}- 1,3,4-oxadiazole (3d): Yield 74 %, white solid, m.p. 82-84º, 1H NMR (CDCl3): δ 7.60-7.50 (d, 2H, Ar-H), 7.52-7.40 (m, 4H, Ar-H), 7.30-7.20 (d, 2H, Ar-H), 7.10 (d, 2H, Ar-H), 6.81-6.70 (d, 2H, Ar-H), 6.68 (s, 1H, NH), 1.50 (s, 2H, CH2). IR (KBr, cm-1): 3326 (NH), 2929 (Ar CH), 1614 (C=N), 1172, 1092, 1022 (C-O-C). Rf; 0.79.
5-{[2-(2,6-Dichloroanilino)benzyl]-2-(1-naphthyl)}- 1,3,4-oxadiazole (3e): Yield 80%, white crystalline solid, m.p. 88-90º, IR (KBr, cm-1): 3128 (NH), 3028 (Ar CH), 1588 (C=N), 1103, 1010, 998 (C-O-C). Anal. Calcd for C25H17Cl2N3O: C, 68.18; H, 3.86; N, 9.54. Found C,68.11; H, 3.82; N, 9.55%. Rf; 0.80.
5-[2-(2,3-Dimethylanilino)phenyl]-2-(phenyl)-1,3,4- oxadiazole (6a): Yield 63%, yellow amorphous solid, m.p. 117-119º, IR (KBr, cm-1): 3276 (NH), 2921 (Ar CH), 1622 (C=N), 1103, 1165, 1010 (C-O-C). Calcd for C22H19N3O: % C, 77.41; H, 5.57; N, 12.31. Found % C, 77.32; H, 5.50; N, 12.01. Rf; 0.80.
5-[2-(2,3-Dimethylanilino)phenyl]-2-(3-chlorophenyl)- 1,3,4-oxadiazole (6b): Yield 70%, yellow amorphous solid, m.p. 117-118º, IR (KBr, cm-1): 3211 (NH), 2921 (Ar CH), 1596 (C=N), 1157, 1089, 977 (C-O-C). Calcd for C22H18ClN3O: % C, 67.64; H, 4.79; N, 11.18. Found %C, 67.43; H, 4.83; N, 11.00.Rf; 0.86.
5-[2-(2,3-Dimethylanilino) phenyl]-2-(4-methylphenyl)- 1,3,4-oxadiazole (6c): Yield 65%, yellow amorphous solid, m.p. 121-123º , 1H NMR (CDCl3): δ 8.30-8.20 (m, 4H, Ar- H), 7.95 (d, 2H, Ar- H), 7.90-7.80 (m, 3H, Ar- H), 7.42 (d, 2H, Ar- H), 7.23 (s,1H, NH), 2.95 (s, 3H, CH3), 2.60 (s, 3H, CH3), 1.56 (s, 3H, CH3). IR (KBr, cm-1): 3326 (NH), 2927 (Ar CH), 1627 (C=N), 1184, 1087, 1049 (C-O-C). Rf; 0.78.
5-[2-(2,3-Dimethylanilino)phenyl]-2-(pyridine-4-yl)- 1,3,4-oxadiazole (6d): Yield 62%, yellow amorphous solid, m.p. 115-116º, 1H NMR (CDCl3): δ 8.42 (d, 2H, Ar- H), 8.30-8.20 (d, 2H, Ar- H), 7.86-7.76 (m, 3H, Ar- H), 7.42 (d, 2H, Ar- H), 7.22 (s,1H, NH), 2.90 (s, 3H, CH3), 2.60 (s, 3H, CH3). IR (KBr, cm-1): 3147 (NH), 2950 (Ar CH), 1608 (C=N), 1186, 1060, 986 (C-O-C). Rf; 0.81.
5-[2-(2,3-Dimethylanilino)phenyl]-2-(1-naphthyl)- 1,3,4-oxadiazole (6e): Yield 74%, yellow solid, m.p. 113-115º, 1H NMR (CDCl3): δ 8.20-8.10 (m 4H, Ar- H), 8.00-7.90 (m, 4H, Ar- H), 7.86-7.76 (m, 3H, Ar- H), 7.60-7.50 (m, 3H, Ar- H), 7.20 (s,1H, NH), 2.90 (s, 3H, CH3), 2.60 (s, 3H, CH3). IR (KBr, cm-1): 3332, (NH), 2929 (Ar CH), 1627 (C=N), 1159, 1089 (C-O-C). Rf; 0.77.
Acute toxicity study
The acute toxicity study was performed as per literature method [37] on female swiss albino mice, weighing 20-25 g. Animals were kept overnight fasting (about 18 h) before administering the doses. Graded doses of compounds (175 mg/kg to 1750 mg/kg) were administered orally. Animals were observed for mortality at least once during the first 30 min, periodically during the first 24 h (with special attention given during the first 4 h) and daily thereafter, for a total of 14 days, except where they needed to be removed from the study and humanely killed for animal welfare reasons or were found dead.
Antiinflammatory activity
This activity was performed as per the literature method [38] on groups of six sprague dawley rats weighing 150-180 g each. A freshly prepared suspension of carrageenan (1.0% w/v, 0.1 ml) was injected in the planter region of right hind paw of each rat one hour after treating them with 10 mg/kg of test compounds. One group was treated as control and the animals of the other groups were pretreated with the test compounds suspended in 1.0% CMC with few drops of tween 80 given orally 1 h before the carrageenan treatment. The paw volume was measured after 1, 3 and 24 h of carrageenan treatment with the help of pleythysmometer. The percent antiinflammatory activity was calculated according to the formula: % Antiinflammatory activity = (Vc – Vt/ Vc) × 100, where, Vt represents the mean increase in paw volume in rats treated with test compounds, Vc represents the mean increase in paw volume in control group of rats.
Analgesic activity
The acetic acid-induced writhing test [39] was performed for measure analgesic activity by an i.p. injection of 1% aqueous acetic acid solution in a volume of 0.1 ml. Each group of six albino mice was kept. Mice were kept individually in the test cage, before acetic acid injection and habituated for 30 min. Analgesic activity was tested after p.o. administration of test drugs at the dose of 10 mg/kg. All compounds were suspended in 1% CMC solution with few drops of tween 80. One group was kept as control and received p.o. administration of 1% CMC. After 1 h of administration of test compounds, 0.10 ml of 1% acetic acid solution was given to mice intraperitoneally. Stretching movements consisting of arching of the back, elongation of body and extension of hind limbs were counted for 5–15 min of acetic acid injection. The analgesic activity was expressed in terms of % pain inhibition. % Analgesic activity = (n–n′/n)×100 Where n = mean number of writhes of control group and n′= mean number of writhes of test group.
Acute ulcerogenesis
Acute ulcerogenesis test was done according to literature method [40]. Albino rats have been divided into different groups consisting of six animals in each group. Ulcerogenic activity was evaluated after p.o. administration of test compounds or standard at the dose of 30 mg/kg. Control rats received p.o. administration of vehicle (suspension of 1% carboxy methyl cellulose). Food but not water was removed 24 h before administration of the test compounds. After the drug treatment, the rats were fed normal diet for 17 h and then sacrificed. The stomach was removed and opened along the greater curvature, washed with distilled water and cleaned gently by dipping in saline. The mucosal damage was examined by means of a magnifying glass. For each stomach, the mucosal damage was assessed according to the following scoring system: 0.0 score was given to normal stomach (no injury, bleeding and latent injury). 0.5 score was to latent injury or widespread bleeding (>2 mm). 1.0 was to slight injury (2–3 dotted lines), 2.0 for severe injury (continuous lined injury or 5–6 dotted injuries). 3.0 to very severe injury (several continuous lined injuries) and 4.0 for widespread lined injury or widened injury. The mean score of each treated group minus the mean score of control group was regarded as severity index of gastric mucosal damage.
Data are expressed as mean±S.E.M; Data analyzed by one way ANOVA followed by Dunnett’s test, significance of the difference between the control group and rats treated with the test compounds. The difference in results was considered significant when P<0.01.
Results and Discussion
The present study was aimed to replace the carboxylate functionality of diclofenac and mefenamic acid with less acidic 1,3,4-oxadiazole nucleus to protect the gastric mucosa from free carboxylate moiety. Five oxadiazole derivatives of diclofenac and mefenamic acid were synthesized as out line in scheme (fig. 1). The titled compounds 3a-3j and 6a-6j were obtained by reacting 2 and 5 respectively with various aromatic acids in the presence of POCl3 as cyclodehydrating agent. Subsequent purification by column chromatography using ethyl acetate: hexane as a mobile phase and silica as a stationary phase yielded final compounds in moderate to higher yields. They were characterized by the IR (KBr) as sharp bands were observed around 3254 cm-1 (NH stretch), 3056 cm-1 (aromatic C-H stretch), 1608 cm-1 (C=N), 1068-1020 cm-1 (C-O-C stretch of oxadiazole ring) and their structures were further confirmed by 1H NMR and elemental analysis..
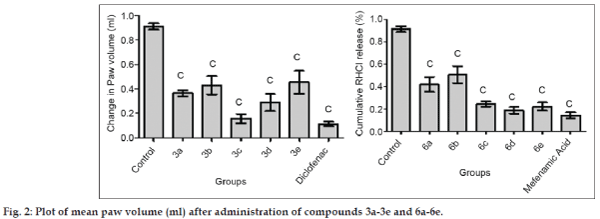
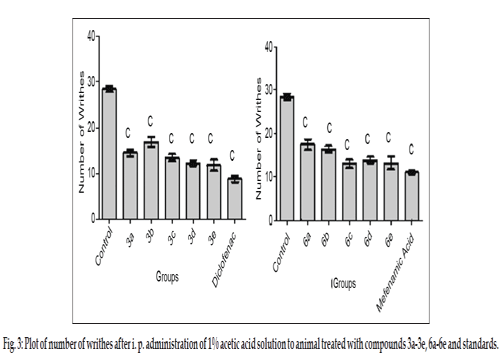
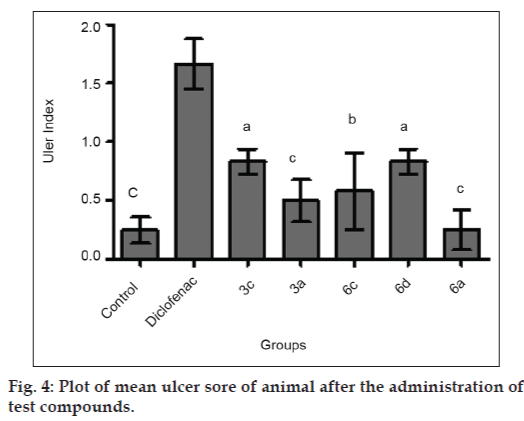
The titled compounds were subjected to pharmacological evaluation which includes acute oral toxicity study, antiinflammatory, analgesic and acute ulcerogenic activities. The toxicity studies revealed that all compounds exhibited LD50 values of 1750 mg/kg which is greater than the LD50 of the diclofenac (95 to 1300 mg/kg)[41] and mefenamic acid (525 mg/kg) [42]. The antiinflammatory activity of the synthesized compounds (3a-3e and 6a-6e) showed that all of these compounds were sufficiently active in inhibiting the inflammation. However compound 3c and 6d exhibited percent inflammation inhibitions of 82.61% and 79.50% (Table 1 and fig. 2). The analgesic activity of the compounds (3a-3e and 6a-6e) was studied by using acetic acid induced writhing test in mice. The results of acetic acid induced writhing test showed that all compounds possess significant analgesic activity as compared with the control group (Table 2 and fig. 3). Ulcerogenic effect of most active oxadiazole derivatives of diclofenac (3c, 3e) and mefenamic acid (6c, 6d, 6e) was evaluated for gastric ulcerogenic potential in rat stress model at 3 times the therapeutic doses. When compared with standard, these compounds showed less ulceration than the standard drugs. The results are shown in (Table 3 and fig. 4).
| Group | 0 h | 3 h | V | %Inhibition |
|---|---|---|---|---|
| (Mean)±SEM | ||||
| Control | 0.93 | 1.86 | 0.93±0.025 | - |
| 3a | 1.30 | 1.66 | 0.36±0.026 | 59.99 |
| 3b | 1.18 | 1.61 | 0.43±0.075 | 52.79 |
| 3c | 1.41 | 1.57 | 0.15±0.037 | 82.61 |
| 3d | 1.34 | 1.63 | 0.29±0.070 | 68.16 |
| 3e | 1.25 | 1.71 | 0.45±0.093 | 50.05 |
| Diclofenac | 1.15 | 1.27 | 0.11±0.017 | 87.37 |
| 6a | 1.21 | 1.63 | 0.41±0.066 | 54.07 |
| 6b | 1.22 | 1.73 | 0.50±0.076 | 44.74 |
| 6c | 1.15 | 1.39 | 0.24±0.021 | 73.28 |
| 6d | 1.15 | 1.34 | 0.18±0.028 | 79.50 |
| 6e | 1.26 | 1.48 | 0.22±0.035 | 75.85 |
| Mefenamic acid | 1.78 | 1.92 | 0.14±0.026 | 84.44 |
Data are expressed as mean±SEM, n=6 animals per group. Data analyzed by one way ANOVA followed by Dunnett’s test, significant when compared with control P<0.05.
Table 1: Mean % Inflammation Inhibition In Rats After Administration Of Compounds 3a-3e, 6a-6e and Standards
| Group | Writhes mean±SEM | %pain inhibition | Group | Writhes mean±SEM | %pain inhibition |
|---|---|---|---|---|---|
| Control | 28±0.666c | - | Control | 28±0.666c | - |
| 3a | 15±0.763c | 46.42 | 6a | 17±1.201c | 39.28 |
| 3b | 17±1.077c | 39.28 | 6b | 16±0.666c | 42.85 |
| 3c | 14±0.763c | 50.00 | 6c | 13±0.966c | 53.57 |
| 3d | 12±0.557c | 57.14 | 6d | 14±0.654c | 50.00 |
| 3e | 12±1.194c | 57.14 | 6e | 13±1.536c | 53.57 |
| Diclofenac | 09±0.703c | 67.85 | Mefenamic Acid | 11±0.365c | 61.17 |
Data are expressed as mean±SEM, n = 6 animals per group. Data analysed by one way ANOVA followed by Dunnett’s test, significant when compared with control ap<0.05,bp<0.01,cp<0.001.
Table 2: Mean % Pain Inhibition After Treatment With Compounds 3a-3e, 6a-6e and Standards
| Group | Ulcer sore, mean±SEm | Ulcer index |
|---|---|---|
| Control | 0.25±0.111c | - |
| 3c | 0.83±0.105c | 0.58 |
| 3e | 0.50±0.182c | 0.25 |
| 6c | 0.58±0.327c | 0.33 |
| 6d | 0.83±0.105c | 0.58 |
| 6e | 0.25±0.170c | 0.00 |
| Diclofenac | 1.66±0.210c | 1.41 |
| Mefenamic acid | 2.00±0.258 ns | 1.75 ns |
Data expressed as mean± SEm, n = 6 animal per group. Data analyzed by one way ANOvA followed by Dunnett’s test, significant when compare with standard (Diclofenac) ns = non significantap<0.05,bp<0.01,cp<0.001.
Table 3: Mean Ulcer Sore And Ulcer Index After Administration Of Compounds
In summary, we disclosed that the effects of replacement of carboxylate functionality of the two most popular NSAIDs, diclofenac and mefenamic acid with 1,3,4-oxadiazole scaffold, with the objective of developing better antiinflammatory profile with minimum ulcerogenic activity and safety profile. The oxadiazole ring being weak acidic in nature and isoster for ester functionality, reduces the ulcerogenicity of the diclofenac and mefenamic acid and retain its antiinflammatory potential. However replacement with oxadiazole scaffold did not improve the analgesic activity of these molecules.
References
- Talley JJ, Brown DL, Carter JS. 4-[5-Methyl-3-phenylisoxazol-4-yl]-benzenesulfonamide, Valdecoxib: A Potent and Selective Inhibitor of COX-2. J Med Chem 2000;43:775-7.
- Smith CJ, Zhang Y, Koboldt CM. Pharmacological analysis of cyclooxygenase-1 in inflammation. ProcNatlAcadSci USA. 1998;95:13313-8.
- Warner TD, Giuliano F, Vaynovie I, Bukasa A, Mitchell JA, Vave JR. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: A full in vitro analysis. ProcNatlAcadSci USA. 1999;96:563-8.
- Marnett LJ, Kalgutkar AS. Cyclooxygenase 2 inhibitors: discovery, selectivity and the future Trends PharmacolSci 1999;20:465-9.
- Dannhardt G, Kiefer W. Cyclooxygenase inhibitors–current status and future prospects. Eur J Med Chem 2001;36:109-26.
- Marnett LJ, Kalgutkar AS. Design of selective inhibitors of cyclooxygenase-2 as nonulcerogenicantiinflammatory agents. CurrOpinChemBiol 1998;2:482-90.
- Parsit P, Reindeau D. Selective Cyclooxygenase-2 Inhibitors. Annu Rep Med Chem 1997;32:211-20.
- Habeeb AG, Rao P, Knaus ED. Design and Synthesis of 4,5-Diphenyl-4-isoxazolines: Novel Inhibitors of Cyclooxygenase-2 with Analgesic and Antiinflammatory Activity. J Med Chem 2001;44:2921-7.
- Almansa C, Alfon J, Cavalcanti FL. Synthesis and Structure− Activity Relationship of a New Series of COX-2 Selective Inhibitors: 1,5-Diarylimidazoles. J Med Chem 2003;46:3463-75.
- Jackson LM, Hawkey CJ. Gastrointestinal effects of COX-2 inhibitors. ExpOpin Invest Drugs 1999;8:963-71.
- Allison MC, Howatson AG, Torrance CJ, Lee FD, Russell IG. Gastrointestinal Damage Associated with the Use of NonsteroidalAntiinflammatory Drugs. N Engl J Med 1992;327:749-54.
- Hawkey C, Laine L, Simon T, Beaulieu AA, Maldonado A. Comparison of the Effect of Rofecoxib (a Cyclooxygenase 2 Inhibitor), Ibuprofen, and Placebo on the Gastroduodenal Mucosa of Patients with Osteoarthritis: A Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Rheum 2000;43:370-7.
- Mohan R, Ramaa CS. Ester prodrugs of flurbiprofen: Synthesis, plasmahydrolysis and gastrointestinal toxicity. Indian J Chem 2007;46B:1164-8.
- Ziakas GN, Rekka EA, Gavalas AM, Eleftheriou PT, Tsiakitzis KC, Kourounakis PN. Nitric oxide releasing derivatives of tolfenamic acid with anti-inflammatory activity and safe gastrointestinal profile Bioorg Med Chem 2005;13:6485-92.
- Galanakis D, Kourounakis A, Doulgkeris C, Rekka E. Synthesis and pharmacological evaluation of amide conjugates of NSAIDs with L-cysteine ethyl ester, combining potent antiinflammatory and antioxidant properties with significantly reduced gastrointestinal toxicity. Bioorg Med ChemLett 2004;14:3639-43.
- Kalgutkar AS, Marnett AB, Crews BC, Remmel RP, Marnett LJ. Ester and Amide Derivatives of the Nonsteroidal Anti-inflammatory Drug, Indomethacin, as Selective Cyclooxygenase-2 Inhibitors. J Med Chem 2000;43:2860-70.
- Duflos M, Nourrisson MR, Brelet J, Courant J, Le BJ, Grimaud GN, et al. N-Pyridinyl-indole-3-(alkyl) carboxamides and derivatives as potential systemic and topical inflammation inhibitors. Eur J Med Chem 2001;36:545-53.
- Kalgutkar AS, Crews BC, Rowlinson SW, Garner C, Seibert K, Marnett LJ. Aspirin-like Molecules that Covalently Inactivate Cyclooxygenase-2. Science 1998;280:1268-70.
- Bhandari SV, Bothara KJ. Design, Synthesis and Evaluation of Antiinflammatory, Analgesic and Ulcerogenicity studies of Novel S-Substituted phenacyl-1,3,4-oxadiazole-2-thiol and Schiff bases of Diclofenac acid as Nonulcerogenic Derivatives. Bioorg Med Chem 2008;16:1822-31.
- Hashimoto M, Ohta H. Studies on Meso-ionic Compounds. XIII). Reaction between Acetic Anhydride and Azoles having Carboxymethylmercapto-group. Bull ChemSoc Japan 1960;93:1394-9.
- Hughes TV, Wetter SK, Connolly PJ. A novel 5-[1,3,4-oxadiazol-2- yl]-N-aryl-4,6-pyrimidine diamine having dual EGFR/HER2 kinase activity: Design, synthesis, and biological activity. Bioorg Med ChemLett 2008;18:896-9.
- .Zrghi A, Faizi M, Shafaghi B, Ahadian A. Synthesis and anticonvulsant activity of new 2-substituted-5-(2-benzyloxyphenyl)-1,3,4-oxadiazoles. Bioorg Med ChemLett 2005;15:1863-5.
- Zarghi A, Faizi M, Shafaghi B, Ahadian A. Design and synthesis of new 2-substituted-5-(2-benzylthiophenyl)-1,3,4-oxadiazolesas benzodiazepine receptor agonists. Bioorg Med ChemLett 2005;15:3126-9.
- Bhandari SV, Parikh JK, Bothara KG. Design, synthesis, and evaluation of anti-inflammatory, analgesic, ulcerogenicity, and nitric oxide releasing studies of novel indomethacin analogs as nonulcerogenic derivatives. J EnzymInhib Med Chem 2010;25:520-30.
- Holla SB, Poojary NK, Bhat SK. Synthesis and anticancer studies on some 2- chloro-1,4-bis-(5-substituted-1,3,4-oxadazol-2ylmetheleneoxy) phenylene derivatives. Indian J Chem 2005;44B:1669-73.
- Suresh Kumar GV, Rajendraprasad Y, Mallikarjuna BP, Chandrashekar SM, Kistayya C. Synthesis of some novel 2-substituted-5-[isopropylthiazole] clubbed 1,2,4-triazole and 1,3,4-oxadiazoles as potential antimicrobial and antitubercular agents. Eur J Med Chem 2010;45:2063-74.
- Kumar A, D’Souza SS, Gaonkar SL, LokanathaRai KM. Antiangiogenic and antiproliferative effects of substituted-1,3,4-oxadiazole derivatives is mediated by down regulation of VEGF and inhibition of translocation of HIF-1α in Ehrlich ascites tumor cells. Cancer ChemotherPharmacol 2009;64:1221-33.
- Somani R, Shirodkar P, Kadam V. Synthesis and Antibacterial Activity of Some New 2,5-Disubstituted-1,3,4-oxadiazole Derivatives. Chin J Chem 2008;26:1727-31.
- Pal T, Somani RR, Shirodkar PY, Bhanushali UV. Quantitative structure activity relationship (QSAR) studies of a series of 2,5-disubstituted- 1,3,4-oxadiazole derivatives as biologically potential compounds. Med Chem Res. 2011;20:1550-5.
- Somani R, Shirodkar P. Synthesis, Antibacterial and Antitubercular Evaluation of Some 1,3,4-xadiazole Analogues. Asian J Chem 2008;20:6189-94.
- Somani RR, Agrawal AG, Bhanushali UV, Kalantri PP, Clercq ED. Synthesis and Biological Evaluation of some 2,5-Disubstituted -1,3,4-Oxadiazole Derivatives. Int J Drug Des Disc2011;2:413-8.
- Somani R, Shirodkar P, Kadam V. Synthesis and Anticancer Evaluation of some Newer 2,5-Disubstituted-1,3,4-Oxadiazole Analogues. Lett Drug Des Disco 2008;5:364-8.
- Somani R, Agrawal A, Kalantri P, Gavarkar P, Clercq ED. Investigation of 1,3,4-Oxadiazole Scaffold as Potentially Active Compounds. Int J Drug Design Disc 2011;2:353-60.
- Sriram D, Yogeeswari P, Devakaram RV. Synthesis, in vitro and in vivo antimycobacterial activities of diclofenac acid hydrazones and amides. Bioorg Med Chem 2006;14:3113-8.
- Amir M, Kumar S. Synthesis and anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation activities of some new 2-[(2,6-dichloroanilino) phenyl]acetic acid derivatives Eur J Med Chem 2004;39:535-45.
- Venkatapuram P, Reddy GS, Mohan A, Konda M. Synthesis of symmetrical and unsymmetrical 1,3,4-oxadiazoles and their interconversion to 1,3,4-thiadiazoles and 1,2,4-triazoles. ARKIVOC 2008;xvii:48-60.
- OECD guideline for testing of chemicals, Acute oral toxicity, environmental Health and Safety Monograph Series on Testing and Adjustment No. 425; 2006. p. 1- 27.
- Schleyerbach R, Weithmann KU, Bartlett RR. Analgesic, anti-inflammatory, and anti-pyretic activity, In, Vogel HG editor, Drug Discovery and Evaluation; Pharmacological Assays, 2nd ed., Berlin; Springer-Verlag: 2002, p 759.
- Schleyerbach R, Weithmann KU, Bartlett RR. Analgesic, anti-inflammatory, and anti-pyretic activity, In, Vogel HG editor, Drug Discovery and Evaluation; Pharmacological Assays, 2nd ed., Berlin; Springer-Verlag: 2002. p. 716.
- Cioli V, Putzolu S, Rossi V, Corradino C. The role of direct tissue contact in the production of gastrointestinal ulcers by antiinflammatory drugs in rats. ToxicolApplPharmacol 1979;50:283-9.
- The European Agency for evaluation of medicinal products (Veterinary Medicines and Inspections) cited in their report, Reference No, EMEA/ MRL/885/03-FINAL, dated, September 2003.
- Material safety data sheet, Spectrum Laboratory Products INC, 14422 S. San Pedro Street Gardena, CA 90248.