- *Corresponding Author:
- B. Li
Department of Occupational Health, 1Central Laboratory, Jinshan Hospital, Fudan University, No. 1508, Longhang Road, Jinshan district, Shanghai, 201508, China
E-mail: libingbm@163.com
- Y.L. Zhou
Department of Occupational Health, 1Central Laboratory, Jinshan Hospital, Fudan University, No. 1508, Longhang Road, Jinshan district, Shanghai, 201508, China
| Date of Submission | 28 May 2016 |
| Date of Revision | 01 September 2016 |
| Date of Acceptance | 11 September 2016 |
| Indian J Pharm Sci 2016;78(5):591-601 |
This is an open access article distributed under terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Acrylonitrile is a potent mutagen and carcinogen for hepatocytes. A role of toll-like receptor 4 in the immune toxicity of acrylonitrile has been suggested in many studies. The following study was designed to assess the quantitative alteration of toll-like receptor 4 under the influence of sodium thiosulfate which was used to antagonize acrylonitrile-induced immune toxicity in rats. 70 Sprague Dawley rats were divided into 7 groups. Rats of control group were gavaged with saline for 4 weeks. Rats of acrylonitrile groups were daily gavaged by acrylonitrile of different concentrations for 4 weeks, whereas all rats of sodium thiosulfate groups received gavage of acrylonitrile 10 mg/kg/d for 4 weeks and for the 4th w, meanwhile daily intraperitoneal injection of sodium thiosulfate of different concentrations was administered to detoxify acrylonitrile. All rats were anesthetized and serum levels of aspartate aminotransferase and alanine aminotransferase were measured to evaluate liver damage. Spleens of rats from different groups were dissected for histopathologic examination and immunohistochemistry of toll-like receptor 4 protein in spleen. Concentrations of interleukin-1β and tumor necrosis facor-α in blood samples were also analyzed. Acrylonitrile decreased body and spleen weight of the rats and increased the levels of serum aspartate aminotransferase and alanine aminotransferase. There were significant visible changes in spleen microstructure in rats treated with acrylonitrile alone when compared with control group. Moreover, it was proved in our study that acrylonitrile of 10 mg/kg could cause the most significant increase in the quantity of toll-like receptor 4 protein, when compared with both acrylonitrile (L) and acrylonitrile (H) group. The expression of toll-like receptor 4 protein of all acetonitrile groups was stronger than that of all sodium thoisulfate groups, which was in accordance with the inflammation caused by acrylonitrile with or without the influence from sodium thiosulfate. Among all sodium thiosulfate groups, toll-like receptor 4 expression of sodium thoisulfate (L) group was stronger than that of sodium thoisulfate (M) and sodium thoisulfate (H) group. Administration of sodium thiosulfate on the fourth week after first 3 w treatment with acrylonitrile alleviated acrylonitrile-induced morphological alterations in spleen, hepatic enzymatic indexes including serum aspartate aminotransferase and alanine aminotransferase, as well as the expression of toll-like receptor 4. Therefore, we suggested that sodium thiosulfate could antagonize the immune toxicity induced by acrylonitrile, which was reflected by quantitative change in expression of toll-like receptor 4 protein.
Keywords
Acrylonitrile, TLR4 protein, spleen, sodium thiosulfate
Acrylonitrile (ACN) is a widely used chemical in the production of plastics, resins, acrylic fibers and synthetic rubber. ACN production and demand have been increasing since 1979, particularly in Asia-Pacific region [1]. ACN production in Shanghai accounts for nearly 50% of the national output. Therefore, thousands of Shanghai locals are possibly exposed to ACN during its production and usage, which highlight the importance of our study on detoxification by sodium thiosulfate (Na2S2O3, SCN) against ACN. ACN is known to produce several toxic effects on experimental animals as well as human beings by inhalation and cutaneous contact. Former epidemiological research shows that long-term exposure to ACN causes damages to components of immune system such as lymphocytes and spleen leading to the decrease in the counts of CD4 and CD8 cells and so on [2-4]. Although the mechanisms of the toxicity of ACN have not been completely elucidated, the effects of cyanide released from ACN and the ACN molecules themselves are both considered to play some role in ACN toxicity and animal studies have shown that ACN inhibits esterase and cytochrome C oxidase in T lymphocytes, which are the critical components of mitochondrial respiratory enzymes system in immune cells, which can directly influence the efficiency of the immune responses elicited by the involved immune cells [5].
We have previously carried out research on ACN toxicity on endocrine, nervous and reproductive organs, on its effects on aging and so on. Some other epidemiological investigations on ACN immunotoxicity in animal studies have confirmed that ACN can affect lymphocytes and spleen whereas the mechanism of the immunotoxicity caused by ACN remains unclear, which is worth a further in depth study. In recent years, it has been discovered that toll receptors (Toll like Receptor, TLR) are important transmembrane receptors involved in signal transduction related to innate immune. They are mainly expressed on the surface of antigen presenting cells (APC) and other cells associated with innate immunity, such as neutrophils, mast cells, basophils and eosinophils. They initiate signal transduction, lead to secretion of inflammatory mediators and play important roles in natural immune defenses [6-8]. The emerging concept of TLRs as key molecules shaping the effectiveness of the immune responses against microbes is further supported by the experiments in which the mice lacking MyD88 are incapable of developing antigen-specific Th1 responses [9].
TLR4 is a subtype of the TLR family, which is expressed mainly on the surface of antigen-presenting cells and the cell surface of neutrophils, mast cells, basophils, etc. Studies on signal transduction mediated by T cell receptor have revealed that TLR4 protein on lymphocytes present in spleen and some cytokines were all likely to play critical roles in signal transduction pathways controlling and modulating immune responses. Correspondingly, neutralization of several immune mediators such as interferon (IFN) or tumor necrosis factor (TNF), inhibition of inducible nitric-oxide synthase (iNOS), or T cell depletion, leads to outbreaks of infections [10]. Therefore, a continuous network of actions, most likely in concert with activated T cells, may be required to generate active immunological responses. Based on former research achievements and knowledge on TLR mentioned above, we hypothesize that activation of pathogen pattern recognition receptors (PRRs), such as TLR and other receptors can trigger a cascade of cell activities including release of some proinflammatory factors so that the quantity of TLRs is related to the extent of damages on organs and the degree of the ensuing inflammation as exposed to toxic materials like ACN.
Materials and Methods
The study was conducted according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and performed with every effort to minimize the number of the animals involved and their sufferings. Male Sprague Dawley rats (12 w old) were purchased from the Shanghai Silaike Company of China and group-housed (4 or 5 per cage) in temperature (20±1°) and humidity (55±5%) controlled room, under a reversed 12 h day/night cycle.
ACN was provided by Shanghai SSS Reagent Co., Ltd. (Shanghai, China). TLR4/β-actin monoclonal antibody was supplied by Santa Cruz (USA). Secondary antibodies to horse radish peroxidase and prestained protein markers were offered by Millipore (Billerica, MA, USA). Trizol reagent was supplied by Invitrogen Corporation, USA. SYBR fluorescent dye was purchased from Roche (Germany).
Experimental design
The experimental flow chart was shown in the diagram attached below (fig. 1). Seventy rats were randomly divided into seven groups: Con, ACN-L, ACN-M, ACN-H, SCN-L, SCN-M and SCN-H (n=10 for each group; L, M, H representing low, mediate and high concentration of ACN or SCN). Rats of ACN groups (including ACN-L, ACN-M and ACN-H group) were daily gavaged with ACN for 4 weeks. Rats of Con group were given saline by gavage instead of ACN for 4 w whereas rats of SCN groups (including SCN-L, SCN-M and SCN-H group) were firstly gavaged with ACN 10 mg/kg/d (i.e. mediate concentration of ACN applied in our study) for 3 w and later from the 4th w these rats were daily intraperitoneally injected with SCN of different concentrations in addition to daily gavage of ACN (10 mg/kg/d ).
Fig. 1: Scheme to show the experimental procedures for different groups.
All rats involved in the experiment were randomly divided into seven groups: Con, ACN-L, ACN-M, ACN-H, SCN-L, SCN-M and
SCN-H (n=10 for each group). Rats in ACN groups (including ACN-L, ACN-M and ACN-H group) were daily gavaged with ACN
of different concentrations corresponding to 5, 10 and 20 mg/kg/d concentration, respectively for 4 weeks. Rats in Con group were
given only saline by daily gavage for 4 weeks whereas rats in SCN groups (including SCN-L, SCN-M and SCN-H group) were given
daily intraperitoneal injection (i.p.) of SCN of 0.05, 0.1 and 0.2 g/kg/d concentration, respectively, only for the 4th week, in addition
to daily gavage of ACN of 10 mg/kg/d from the first to the fourth week.
Spleen histology and immunohistochemistry
Rats were anesthetized with 10% chloral hydrate and weighed. Spleens were separated from the rats by dissecting tools and weighed. Sections of 5 μm thickness from spleens were made by a rotary microtome and stained with haematoxylin and eosin. They were examined and photographed under a light microscope. Spleen specimens were fixed in 10% neutral formalin, dehydrated, cut into thinner sections of 4 μm and embedded in paraffin. After these sections were dewaxed by benzene and ethanol of gradient concentrations, they were placed in 3% hydrogen peroxide/methanol solution for 30 min and then placed in hot citric acid for another 15 min. Hydrogen peroxide solution (3%) was used to inactivate endogenous peroxidase. Normal goat serum (closed) was added into antibody diluted to 1:100 at 4° overnight. Then biotin-labeled secondary antibody was applied and sections were incubated at room temperature for 15 min. Then peroxidase solution was added and 3,3'-diaminobenzidine (DAB) was used to color. Afterwards they were dehydrated, made transparent and mounted on slides. The positive signal was brown; 5 randomly selected slices were studied at high magnification (400X) and analyzed by using Image-Pro Plus analysis software to determine the mean absorbance value (A value) of the positive components.
Spleen specimens were prepared in ice cold lysis buffer (50 mM TrisHCl, pH 7.4, 50 mM NaCl, NP-40 1%, Triton X-100 1%, 0.5 mM EDTA, sodium deoxycholate 1%) which contained protease inhibitors. Total proteins were separated by electrophoresis in 8% denaturing SDS/polyacrylamide gel and then transferred to a Hybond ECL nitrocellulose membrane (GE Healthcare Europe, Milan, Italy). After saturation of nonspecific binding sites with 5% nonfat milk in Tris-buffered saline (TBS) 1×Tween 20 (0.05%), the membrane was immunoblotted overnight at 4° with the primary antibody against TLR4 (1:500) and subsequently probed with an anti-goat secondary antibody (1:1000, Santa Cruz Biotechnology Inc.) overnight at 4°. The membrane was stripped (Restore Western Blot Stripping buffer, Pierce Biotechnology, Rockford, IL, USA) and re-immunoblotted with antiactin primary antibody (1:7500), and then with anti-rabbit secondary antibodies (1:5000) (Santa Cruz Biotechnology Inc.). The immunoreactive bands were visualized through enhanced chemiluminescence by using an ECL-plus kit (GE Healthcare Europe) following the manufacturer protocol.
Plasma inflammatory cytokines concentration
Every rat was given 10% chloral hydrate (0. 4 ml/100 g/body weight) for anesthesia. Blood samples were collected from the rat hearts and centrifuged at 2000 rpm for 15 min. The supernatant was collected and stored at –80° for subsequent analysis. The plasma proinflammatory cytokines, i.e. TNF-α, IL-1β levels were measured with a radioimmunoassay (RIA) kit and the obtained values were expressed as pg/ml.
Statistical analysis
Quantitative data are shown as the mean±SEM. Data were analyzed by using SPSS software version 15.0 for Windows (SPSS Inc., Chicago, IL). Statistical analysis was performed by using the Student’s t-test or analysis of variance which is appropriate. P-value<0.05 was considered to be statistically significant.
Results and Discussion
The effects of ACN on body and spleen weight of rats were presented in table 1. Daily administration of ACN for 4 weeks significantly reduced the body and spleen weight of rats. There was significant decline in both body and spleen weights of rats in ACN-M and ACN-H group when compared with control group (P<0.05). But when compared ACN-M with ACN-H groups, it showed no difference (P>0.05, Table 1). On the other hand, after treatment with SCN, the body and spleen weight of rats increased, there was significant difference between ACN-M and any SCN-M group ( P<0.05). There was no significant difference between any SCN group (including SCN-L, SCN-M and SCN-H group) and control group (P>0.05).
| Group | n | Body weight (g) | Spleen weight (g) |
|---|---|---|---|
| Control | 10 | 268±7.31 | 0.87±0.05 |
| ACN-L | 10 | 235±5.36 | 0.66±0.03 |
| ACN-M | 10 | 221±6.31* | 0.65±0.05* |
| ACN-H | 10 | 218±6.52* | 0.62±0.06* |
| SCN-L | 10 | 258±7.31# | 0.83±0.05# |
| SCN-M | 10 | 246±5.31# | 1.03±0.02# |
| SCN-H | 10 | 235±4.33# | 1.13±0.03# |
After four-week exposure to ACN, the body and spleen weight of the rats both decreased. There was significant decline in the weight of the body and spleen in ACN-M and ACN-H group compared with control group (both P<0.05) but t test showed no significant difference between ACN-M and ACN-H group. After detoxification by sodium thiosulfate (SCN), there was significant increase in all SCN groups when compared with any ACN-M group (all P<0.05). * represented P<0.05 compared with control group; # represented P<0.05 compared with ACN-M group.
Table 1: Changes in the Body and Spleen Weight of the Rats in Different Groups
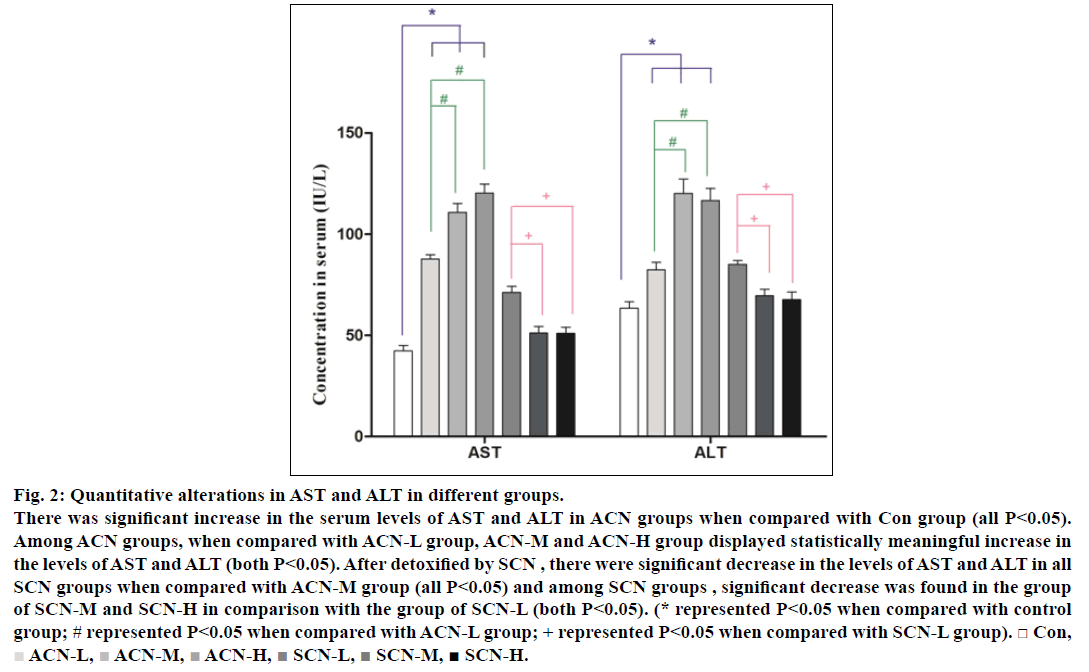
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) and have been reported as the sensitive indicators of liver injury and acrylonitrile poisoning. Quantitative changes in serum AST and ALT for rats of different groups were presented in fig. 2. Therefore, by the end of the 4th w, the rats treated alone with ACN showed a significant increase in the serum levels of AST and ALT (P<0.05) when compared with control group and the rats treated with ACN (10 mg/kg/d) for 4 weeks and i.p. of SCN only for the 4th w displayed less increase in serum AST and ALT as compared with ACN groups. Among all ACN groups, the concentrations of AST and ALT in ACN-M and ACN-H groups were higher than those in ACN-L group (all P<0.05) while among all SCN groups, the levels of AST and ALT in SCN-M and SCN-H group were lower than those in SCN-L group (P<0.05) but still higher than those in control group and there were significant decrease in the levels of AST and ALT in all SCN groups when compared with ACN-M group (P<0.05)
Fig. 2: Quantitative alterations in AST and ALT in different groups.
There was significant increase in the serum levels of AST and ALT in ACN groups when compared with Con group (all P<0.05).
Among ACN groups, when compared with ACN-L group, ACN-M and ACN-H group displayed statistically meaningful increase in
the levels of AST and ALT (both P<0.05). After detoxified by SCN , there were significant decrease in the levels of AST and ALT in all
SCN groups when compared with ACN-M group (all P<0.05) and among SCN groups , significant decrease was found in the group
of SCN-M and SCN-H in comparison with the group of SCN-L (both P<0.05). (* represented P<0.05 when compared with control
group; # represented P<0.05 when compared with ACN-L group; + represented P<0.05 when compared with SCN-L group). □ Con,  ACN-L,
ACN-L,  ACN-M,
ACN-M,  ACN-H,
ACN-H,  SCN-L,
SCN-L,  SCN-M,
SCN-M,  SCN-H.
SCN-H.
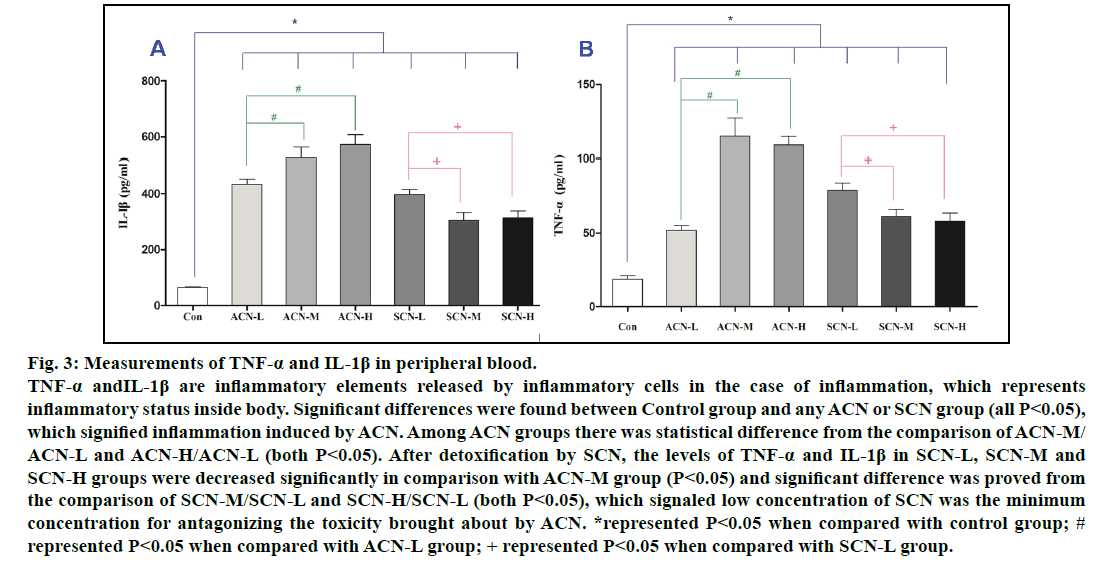
The serum TNF-α, IL-1β levels reflect the magnitude of inflammatory responses and are released in the inflammation caused by ACN. It was confirmed that serum TNF-α and IL-1β of ACN groups were significantly higher than those of control group. Furthermore, the concentrations of TNF-α and IL-1β in ACN-M and ACN-H groups were higher than those in ACN-L group (P<0.05). Among all SCN groups, the levels of TNF-α and IL-1β in SCN-M and SCN-H groups were lower than those in SCN-L (P<0.05) and still significantly higher than those in control group (P<0.05). Nonetheless, the levels of TNF-α and IL-1β in SCN-L, SCN-M and SCN-H groups were decreased significantly in comparison with ACN-M group (P<0.05), which signaled low concentration of SCN was the minimum concentration for antagonizing the toxicity brought about by ACN (fig. 3).
Fig. 3: Measurements of TNF-α and IL-1β in peripheral blood.
TNF-α andIL-1β are inflammatory elements released by inflammatory cells in the case of inflammation, which represents
inflammatory status inside body. Significant differences were found between Control group and any ACN or SCN group (all P<0.05),
which signified inflammation induced by ACN. Among ACN groups there was statistical difference from the comparison of ACN-M/
ACN-L and ACN-H/ACN-L (both P<0.05). After detoxification by SCN, the levels of TNF-α and IL-1β in SCN-L, SCN-M and
SCN-H groups were decreased significantly in comparison with ACN-M group (P<0.05) and significant difference was proved from
the comparison of SCN-M/SCN-L and SCN-H/SCN-L (both P<0.05), which signaled low concentration of SCN was the minimum
concentration for antagonizing the toxicity brought about by ACN. *represented P<0.05 when compared with control group; #
represented P<0.05 when compared with ACN-L group; + represented P<0.05 when compared with SCN-L group.
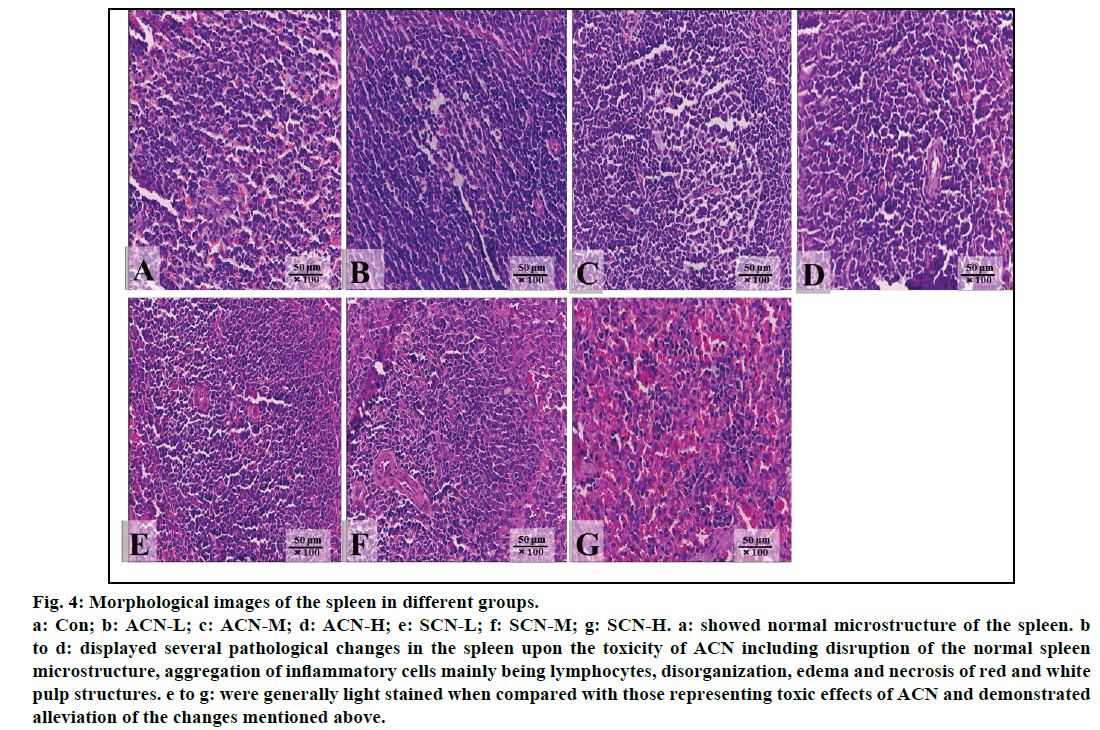
Significant changes were shown in the photographs of the spleen from the rats treated with ACN alone as compared with control group (fig. 4). Normal microstructure of spleen with orderly arranged red pulp and white pulp could be observed in control group (fig. 4a). The slides of the group treated with ACN alone were darkly stained and exhibited several pathological changes, such as disorganized splenic microstructure and indistinct red and white pulp due to infiltration and accumulation of lymphocytes (figs. 4b-4d) whereas in the groups detoxified by SCN, all slides were lightly stained in comparison with those of ACN groups due to alleviated inflammatory changes including less infiltration and accumulation of lymphocytes and partially restored arrangements of red and white pulp. However, mild inflammatory cell infiltration could be still observed (figs. 4e-4g).
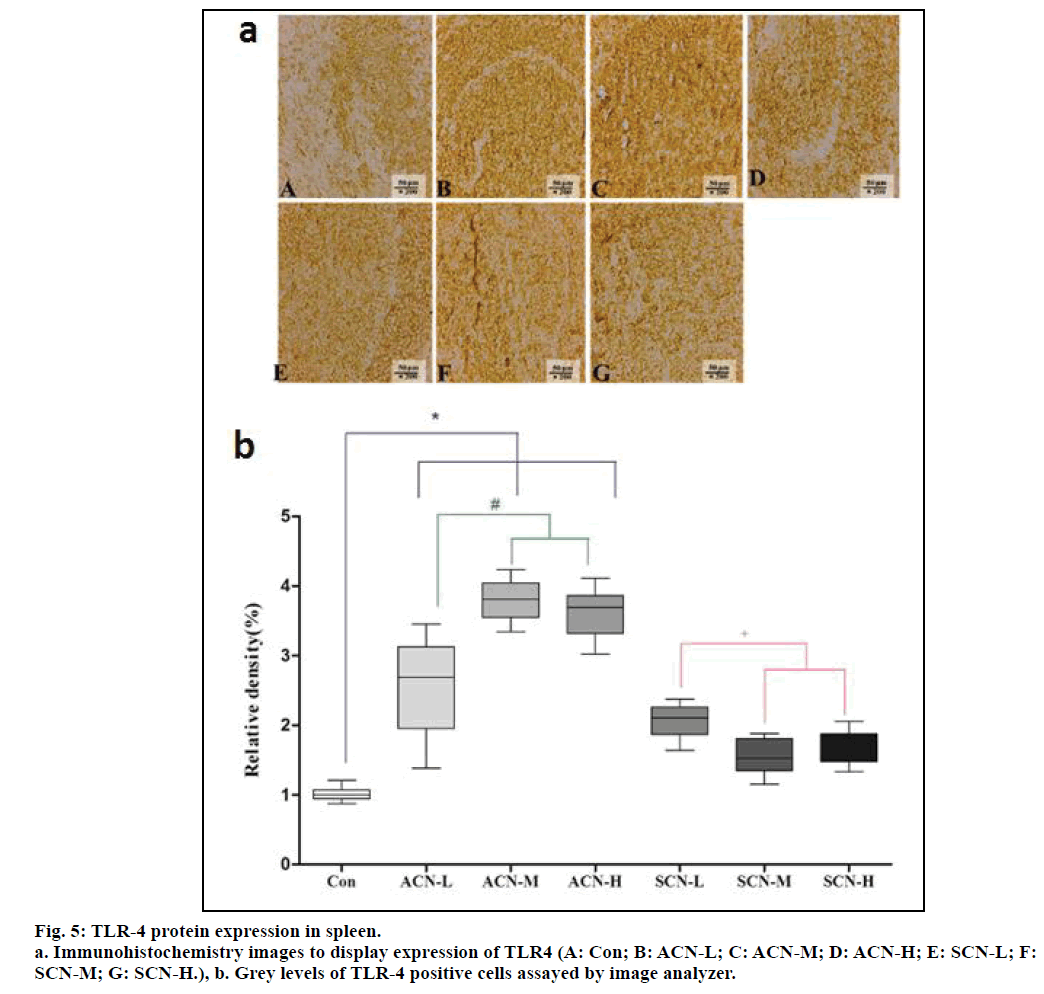
Fig. 4: Morphological images of the spleen in different groups.
a: Con; b: ACN-L; c: ACN-M; d: ACN-H; e: SCN-L; f: SCN-M; g: SCN-H. a: showed normal microstructure of the spleen. b
to d: displayed several pathological changes in the spleen upon the toxicity of ACN including disruption of the normal spleen
microstructure, aggregation of inflammatory cells mainly being lymphocytes, disorganization, edema and necrosis of red and white
pulp structures. e to g: were generally light stained when compared with those representing toxic effects of ACN and demonstrated
alleviation of the changes mentioned above.
From fig. 5a, lighter positive staining was directly visible in the image representing control group, which signaled less positive cells while compared with all the other images. On the other hand, evidently darker positive staining could be noticed from the comparison between any ACN group and any SCN group, which implied more positive cells in ACN groups. The gray levels of different groups display different magnitudes of TLR-4 expression. According to fig. 5b, the relative density of TLR4 positive cells representing con group was significantly less than that of any ACN group. Among ACN groups, the relative density of TLR4 positive cells in ACN-L group was less than that of ACN-M and ACN-H group (P<0.05). Whereas, there was decrease in the relative density of TLR4 of SCN-L, SCN-M and SCN-H group when compared with the group of ACN-M (P<0.05) and the expression of TLR- 4 significantly decreased in the group of SCN-M and SCN-H when compared with the group of SCN-L (P<0.05).
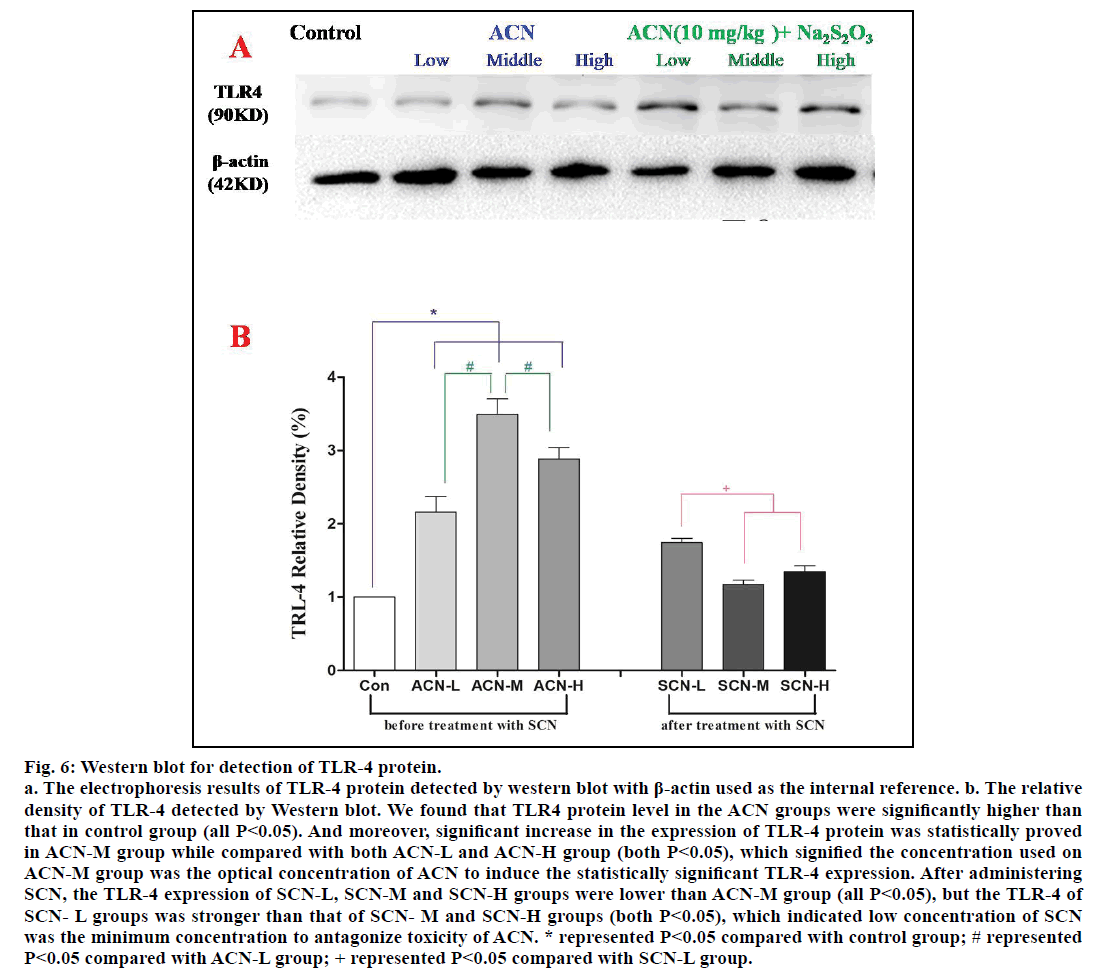
The quantitative level of TLR-4 protein was detected by western blot and β-actin was used as the internal reference. According to fig. 6a, we found out that TLR4 protein level in the groups exposed only to ACN was significantly higher than that in control group (P<0.05); among ACN groups, significant increase in TLR-4 protein in the group of ACN-M was observed when compared with ACN-L and ACN-M group (P<0.05). However, the TLR-4 expression of SCN-L group was stronger than that of SCN-M and SCN-H group (P<0.05), but there was no significant difference between SCN-M and SCN-H groups (P>0.05) and the TLR-4 expression of SCN-L, SCN-M and SCN-H groups were lower than ACN-M group (P<0.05).
Fig. 6: Western blot for detection of TLR-4 protein.
a. The electrophoresis results of TLR-4 protein detected by western blot with β-actin used as the internal reference. b. The relative
density of TLR-4 detected by Western blot. We found that TLR4 protein level in the ACN groups were significantly higher than
that in control group (all P<0.05). And moreover, significant increase in the expression of TLR-4 protein was statistically proved
in ACN-M group while compared with both ACN-L and ACN-H group (both P<0.05), which signified the concentration used on
ACN-M group was the optical concentration of ACN to induce the statistically significant TLR-4 expression. After administering
SCN, the TLR-4 expression of SCN-L, SCN-M and SCN-H groups were lower than ACN-M group (all P<0.05), but the TLR-4 of
SCN- L groups was stronger than that of SCN- M and SCN-H groups (both P<0.05), which indicated low concentration of SCN
was the minimum concentration to antagonize toxicity of ACN. * represented P<0.05 compared with control group; # represented
P<0.05 compared with ACN-L group; + represented P<0.05 compared with SCN-L group.
ACN (CH2=CHCN), a highly reactive compound with active vinyl and cyanide groups, has been widely using in various synthetic chemical industries. Recent studies have proved that ACN could produce chronic toxicity on both experimental animals and human beings. Our last preliminary epidemiologic study on ACN toxicity carried out in Shanghai, China has manifested higher incidence of excessive diseases among the workers exposed to ACN in their occupational environments, which could be possibly related to ACN toxicity. Several researches already pointed out ACN toxicity on reproductive, endocrine, nervous and even immune systems. It is well acknowledged that the toxicity induced by ACN is mainly related to its release of CNgroup inside the body and the strength of the toxicity is determined by the quantity and the release rate of CN- group. Apart from this major mechanism, it is also proved that ACN itself or its metabolites could cause toxicity by binding with DNA or RNA to disturb replication and expression of DNA. On the other hand, detoxification by SCN is attributed to transformation of more toxic group like CN- into much less toxic SCN- salts and the subsequent discharge of SCN salts through urine [11].
As we have all known that damages on organs cause immune responses, or on the contrary, we can say immune responses are the normal responses against inward or foreign injuries from living organisms. So from the aspect of immunity, our attention was drawn to a molecule concerning several immunity responses, PRRs, which are located on immune cells. PRRs are generally recognized as the initiators of all immune responses. Upon ligand binding, PRRs induce activation of inflammatory processes that ultimately leads to pathogen clearance or restoration of tissue homeostasis. In addition, PRRs regulate these processes by controlling activation of a complicated network of transcription factors, which are able to induce appropriate immune responses to a specific ligand. TLRs are the first and best characterized member of PRR family, the essential regulators of innate immune responses [12] and over a long period in the past they were believed to be autonomous proteins able to recognize and initiate all the immune responses to a given stimulus. TLR1, TLR2, TLR4, TLR5 and TLR6 are primarily, but not exclusively, localized to plasma membrane of immune cells and bind lipid or protein elements that are either expressed on the surface of pathogens or released from pathogens into extracellular space. But lately, increasing evidence supports the idea that TLRs do not autonomously orchestrate inflammatory responses against a given ligand. And as a representative to TLRs, TLR4 identifies external antigen by trans membrane structure which causes a consecutive expression of proinflammatory cytokines such as TNF-α and IL-1β [13] and in turn activates dendritic cells to capture a variety of pathogenic microorganisms, induces macrophage activation, directly regulates the functions of T cells, and initiates adaptive immune functions [14,15]. Cytokines and other inflammatory mediators also play important roles in natural immunity [16]. All these knowledge mentioned above favored our idea of measuring TLR4 as the major indicator of the extent of damage caused by ACN and in the meantime using TNF-α and IL-1β as the co-factors to demonstrate inflammation. On the other hand, it was reported that SCN could attenuate acute lung injury caused by ACN in mice [17]. So, in our study we chose SCN as the antidote for ACN to further explore the role of TLRs in ACN toxicity.
All indexes we used in our experiments have different meanings. AST and ALT is the sensitive hepatic enzyme indexes related to liver injury caused by ACN and proinflammatory factors like TNF-α and IL-1β are the indexes reflecting degree of inflammation correlated with organ injuries. Whereas histopathology images of the spleen represented the status of an immune organ upon ACN toxicity or detoxification by SCN, which were the aggregation place for immune cells. And as an important transmembrane receptor, which is involved in activation of immune responses, TLR4 can be found in immune cells in spleen. Hence, the number of TLR4 positive cells or quantitative of TLR4 protein both represent the degree of inflammation occurring in the spleen.
In the beginning, our study showed significant increases in both hepatic enzymes (AST and ALT) and inflammatory response indexes (serum TNF-α and IL-1β), histopathological changes in spleen, as well as an statistic meaningful increase in the level of TLR4 protein in the rats treated with ACN alone, which meant we successfully established animal models of ACN-induced liver damage. And increase in TNF-α and IL-1β signified the inflammation induced by damages caused by ACN. Further findings on the expression of TLR-4 protein by Western blot (fig. 6b) revealed the statistically significant increase in TLR-4 protein caused by ACN of the concentration applied on ACN-M group while compared with either ACN-L or ACN-H group, which proved that 10 mg/ kg was the optimal exposure concentration of ACN to cause the most significant increase in TLR-4 protein, which is targeted by our study. So we chose 10 mg/ kg ACN group to give SCN of different concentrations to establish SCN-L, SCN-M and SCN-H groups to estimate detoxification mechanism of SCN.
With the subsequent administration of SCN after ACN, ACN-induced spleen histopathological changes were significantly relieved which was displayed by the images we enclosed here and the liver injury were greatly alleviated, which was expressed as the striking decrease in AST and ALT along with decrease in serum TNF-α, IL-1β and quantity of TLR4 proved by immunohistochemistry of TLR4 positive cells in spleen and by western blot to quantitatively measure TLR4 protein. So, SCN obviously mitigated spleen damage, liver injuries and TLR4 changes induced by ACN. As for the mechanism of SCN detoxification, it is well known that SCN can biotransform the CN group released from ACN into less toxic SCN group and according to the alterations in quantity of TNF-α and IL-1β observed here in our study we supposed that SCN could also inhibit the release of proinflammatory factors like TNF-α and IL-1β to reduce the inflammation caused by ACN toxicity. Similar findings were reported by Li et al. [18] and Di Gioia et al. [19].
After SCN detoxification, inflammation level had reduced to some extent, but was still higher than that of control group (P<0.05). The comparisons of all the indexes among all SCN groups demonstrated that SCN of the low concentration (i.e. 0.05 g/kg) was the minimum concentration required for detoxification, which could maintain all the indexes representing liver and spleen damage, inflammation (TNF-α and IL-1β; TLR4) to the lower level or degree at the same time.
The decrease in the quantity of TNF-α and IL-1β represents detoxifying effects of SCN. The reason why those factors were still higher in comparison with those of control group is possible that SCN can prevent further toxic effects from ACN but it could not make those done damages undone so that relatively slight inflammations still accompanied the already existing damages.
From comparisons about expression of TLR-4 among different groups and analysis of grey level of TLR4 positive cells, it could be deduced that treatment with ACN evidently induced expression of TLR4, which implied the role of TLR4 in inflammation caused by ACN and significant results about the quantity of TLR4 protein from comparisons of ACN-M/ACN-L and ACN-M/ACN-H proved the concentration applied on ACN-M group was the optimal concentration to establish the liver damage model by using ACN, which caused the most significant increase in TLR-4 protein (fig. 6b). And subsequent treatment with SCN obviously mitigated the expression of TLR4 induced by ACN. But between SCN-M and SCN-H there was no significant difference observed, which meant there was no obvious dose-related antidote function of SCN against ACN. And from the founding that the TLR- 4 expression of SCN-L, SCN-M and SCN-H groups were lower than ACN-M group (P<0.05), it could be inferred that SCN-L was the minimum concentration needed for producing antidote effects.
Therefore, in ACN toxicity there was significant increase in hepatic enzymes (AST and ALT), inflammatory indexes and expression of TLR-4 protein. After detoxification by SCN, there was a statistically meaningful decrease in the indices mentioned above, which proved the reliability of SCN as the antidote against the toxic damages on organs (liver and spleen) and the ensuing inflammation brought about by ACN.
About how TLRs can trigger immune responses there exists several different explanations as follows.
Transmembrane proteins, such as TLRs and Most PRRs, regulate defense mechanisms by activating highly conserved transcriptional signaling pathways, including the TLR4 MyD88-independent and AP-1 pathways. TLR4 MyD88-independent pathway was activated through an IFN autocrine/paracrine loopinduced by TLR. Further research will be required to fully understand the signals that regulate innate immune homeostasis [20]. The regulatory role of IRAK-M on TLR signaling and the requirement of IRAK in response to TLR stimulation, respectively, have been confirmed in gene deficient mice [21,22]. Another important modulator of TLR signaling is the single immunoglobulin IL1Rrelated (SIGIRR) molecule, which is transiently down regulated after TLR-mediated activation and blocks signaling by sequestering IRAK and TRAF-6. TLRs are long believed to have autonomous recognition and transcriptional signaling capacity and TLR-mediated recognition of MAMPs and DAMPs can occur at the plasma membrane or intracellularly [23-27].
It is well known that SCN functions as the antidote for ACN majorly by two different mechanisms. On one hand, it can remove the toxic cyanide ions released from ACN by transforming them into nontoxic sulfocyanide and discharge it. On the other hand, SCN can modulate cellular immunity and have been used to treat allergic reactions. Meanwhile, ACN has multiple toxic effects on organisms and can influence many biochemical processes including inflammation. Over a long period, biotransforming effect of SCN overshadowed its modulation on immunity and antiinflammation effects. The purpose of our study is to highlight SCN effects on immunity by focusing on the quantitative alteration of TLR4, which is the soul of our research.
In this study we have proved that in ACN-group there was significant increase in the level of TLR protein while compared with control group and ACN of the mediate concentration produced the significant increase in the quantity of TLR4, which indicated ACN-M was the optimal concentration fitting for our study, while after administration with SCN, TLR4 decreased in the case that there was no significant difference between SCN-M and SCN-H in addition that SCN-L showed its effect to reduce TLR4 so we assumed that SCN-L concentration was the minimum concentration to detoxify ACN, at least for rats. From the comparison of TLR4 protein between poisoning and detoxification we can suppose that TLRs are correlated to the degree of inflammation induced by ACN and can be used as the reference point for detoxification of ACN, which indirectly proved that anti-inflammation was a possible mechanism of SCN antidote effects. In the future research, we can even measure other signaling proteins which are possibly involved in TLR mediated signal pathways such as IRAK, SIGIRR to further elucidate the signal pathways in depth.
Financial support and sponsorship
The research was supported by the Science Foundation of Shanghai Jinshan District Health Bureau (#JSKJKTQN- 201201), the Shanghai Municipal Health Bureau, China (#2013- 114) and Jinshan Hospital Medical Key Subjects- Department of Occupational Diseases (2014-31).
Conflicts of interest
The author declares no competing interests.
References
- Yuanqing H, Suhua W, Guangwei X, Chunlan R, Hai Q, Wenrong X, et al. Acrylonitrile has distinct hormetic effects on acetyl-cholinesterase activity in mouse brain and blood that are modulated by ethanol. Dose Response 2013;11:49-59.
- Anonymous. Production soared in most chemicals sectors. Chem Eng New 1995;73:38-44.
- Han FA, Yun H, Lu RZ, Jiang FP, Wang SH, Xing WG. Study on health and neurobehavioral effects of workers occupational exposed to low concentration of acrylonitrile. J Environ Occup Med 2008;25:125-29.
- Xia ZL, Jin FS. Investigation of disease spectrum of workers exposed to acrylonitrile. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Zazhi 2000;18:72-4.
- Zabrodskii PF, Kirichuk VF, Germanchuk VG, Belikov VG. Mechanisms of immunotoxic effects of acrylonitrile. Bull Exp Biol Med 2000;129:463-65.
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 2010;11:373-84.
- Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun 2009;388:621-25.
- Zanin-Zhorov A, Cohen IR. Signaling via TLR2 and TLR4 directly down-regulates T cell effector functions: the regulatory face of danger signals. Front Immunol 2013;4:211.
- Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, et al. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res 2010;51:3196-206.
- Botha T, Ryffel B. Reactivation of latent tuberculosis infection in TNF-deficient mice. J Immunol 2003;171:3110-118.
- Farooqui MY, Ahmed AE. In vivo interactions of acrylonitrile with macromolecules in rats. Chem Biol Interact 1983;47:363-71.
- O'Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors - redefining innate immunity. Nat Rev Immunol 2013;13:453-60.
- Yang SS, Tian Q, Zhou HX, Meng L, Zhang ZF, Wang Q, et al. Expression and significance of TLR4 and NF-kB on inflammatory injure after intracerebral hemorrhage in rats. Chongqing Medical 2014;43:584-86.
- Ling GS, Bennett J, Woollard KJ, Szajna M, Fossati-Jimack L, Taylor PR, et al. Integrin CD11b positively regulates TLR4-induced signalling pathways in dendritic cells but not in macrophages. Nat Commun 2014;5:3039.
- Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science 2004;303:1522-26.
- Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol 2003;4:920-27.
- Heldwein KA, Fenton MJ. The role of Toll-like receptors in immunity against mycobacterial infection. Microbes Infect 2002;4:937-44.
- Li C, Huang J, Wang P, Li X, Fan W, Shi J, et al. The effects of acrylonitrile on T lymphocyte subsets and expression of toll-like receptor 4 in rats. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2014;32:481-86.
- Di Gioia M, Zanoni I. Toll-like receptor co-receptors as master regulators of the immune response. Mol Immunol 2015;63:143-52.
- Yang CH, Tian L, Ling GS, Trendell-Smith NJ, Ma L, Lo CK, et al. Immunological mechanisms and clinical implications of regulatory T cell deficiency in a systemic autoimmune disorder: roles of IL-2 versus IL-15. Eur J Immunol 2008;38:1664-76.
- Robbs BK, Lucena PI, Viola JP. The transcription factor NFAT1 induces apoptosis through cooperation with Ras/Raf/MEK/ERK pathway and upregulation of TNF-alpha expression. Biochim Biophys Acta 2013;1833:2016-28.
- So T, Croft M. Regulation of the PKCθ-NF-κB axis in T lymphocytes by the tumor necrosis factor receptor family member OX40. Front Immunol 2012;3:133.
- Larsson S, Löfdahl CG, Linden M. IL-2 and IL-4 counteract budesonide inhibition of GM-CSF and IL-10, but not of IL-8, IL-12 or TNF-α production by human mononuclear blood cells. Br J Pharmacol 1999;127:980-86.
- Lin AC, Dissanayake D, Dhanji S, Elford AR, Ohashi PS. Different toll-like receptor stimuli have a profound impact on cytokines required to break tolerance and induce autoimmunity. PLoS One 2011;6:e23940.
- Luna LG. Manual of histology, staining methods of Armed Forces Institute of Pathology. 3rd ed. New York: McGraw-Hill; 1968. p. 325-33.
- Quesniaux V, Fremond C, Jacobs M, Parida S, Nicolle D, Yeremeev V. Toll-like receptor pathways in the immune responses to mycobacteria. Microbes Infect 2004;6:946-59.
- Sakaguchi M, Marutani E, Shin HS, Chen W, Hanaoka K, Xian M, et al. Sodium thiosulfate attenuates acute lung injury in mice. Anesthesiology 2014;121:1248-57.