- *Corresponding Author:
- A. Chakraborty
Department of Pharmaceutical Sciences and Technology,
Maulana Abul Kalam Azad University of Technology, Kolkata, West Bengal 741249,
India
E-mail: arpan.soleria@gmail.com
| Date of Received | 04 September 2022 |
| Date of Revision | 16 April 2023 |
| Date of Acceptance | 19 March 2024 |
| Indian J Pharm Sci 2024;86(2):509-516 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The condition known as benign prostatic hyperplasia, which causes enlargement in the prostate gland and male pattern hair loss are both treated with a family of drugs known as 5 alpha reductase inhibitors. This study shows that Solanum nigrum L. has 5 alpha reductase inhibitory action, that is helpful in the treatment of androgenic diseases. It was carried out to screen for phytochemicals and to analyze high-performance thin layer chromatography. Using petroleum ether and methanol extract, the 5 alpha reductase inhibitory activity of the plant was compared to the renowned 5 alpha-reductase inhibitor finasteride. The inhibition of extracts to the enzyme was assessed using a biochemical approach to measure the activity of 5 alpha-reductase. Because the substrate nicotinamide adenine dinucleotide phosphate has a particular absorbance at 340 nm, the optical density value of each sample was monitored with an ultraviolet spectrophotometer. Nicotinamide adenine dinucleotide phosphate concentration rises over time in the presence of a 5 alpha-reductase inhibitor because the enzyme 5 alpha-reductase needs nicotinamide adenine dinucleotide phosphate as a substrate. Thus, this technique implies 5 alpha-reductase activity. The method was quite successful in evaluating the plant capacity to block 5 alpha-reductase. It was determined that Solanum nigrum L. petroleum ether extract 125.528±0.634 (μg/ml) and linoleic acid (chemical biomarker of the plant material) 89.672±0.669 (μg/ml) were promising candidates for future investigation into their anti-androgenic activities.
Keywords
5 alpha-reductase, hair loss, nicotinamide adenine dinucleotide phosphate, Solanum nigrum, linoleic acid, high-performance thin layer chromatography
Androgens and the androgen receptors interact to have cellular effects that control important mechanisms involved in the normal growth, structure and operation of the prostate. Additionally, androgen may contribute to the emergence of prostate cancer. Suppression of Dihydrotestosterone (DHT) may prevent the formation of cancer, according to various preclinical and clinical investigations[1]. Testosterone (T) is converted into DHT by the nuclear membrane bound enzyme steroid 5 alpha-Reductase (5α-R), which has two subtypes called as type 1 (5α-R1) and type 2 (5α-R2)[2]. The Nicotinamide Adenine Dinucleotide Phosphate (NADPH)-dependent conversion of T to DHT is catalyzed by 5α-R[3]. Numerous human diseases, including as male pattern baldness in both genders, alopecia, Benign Prostatic Hyperplasia (BPH), prostate cancer, acne and hirsutism, are impacted by the 5α-R and its metabolite DHT[4]. The creation of therapeutic inhibitors of the enzyme was prompted by the knowledge that DHT controls the development of the prostate, androgenic alopecia and that those without the enzyme 5α-R do not produce one[5]. Finasteride and epristeride are two common 5α-R inhibitors on the market; however their use is limited due to a number of side effects. This problem might be solved by using a herbal alternative for 5α-R inhibition. In order to find potential 5α-R inhibitors, we evaluated plants for antiandrogenic activity and their potential to block the 5α-R.
Solanum nigrum (S. nigrum) L. (Solanaceae), also renowned as "black nightshade," is a plant that has been widely used in classical Indian system of medicine as well as other parts of the world to treat conditions, including disease, severe skin problems (such as psoriasis and ringworm), inflammatory disorders, menstrual discomforts, fevers, diarrhoea, eye problems, hydrophobia, and so on. It has been found that S. nigrum L. contains anti-tumor chemicals such as total alkaloids, glycoprotein, steroidal alkaloids and saponins. In Indian traditional medicine, the plant is used as a hepatoprotectant[6]. Considering its status as a weed, some components of the plant may be harmful to humans and animals. However, ripe berries and cooked leaves from edible strains are used as food in some areas, and plant components are also used in traditional medicine. In traditional European medicine, the plant has been used as a sudorific, analgesic, sedative and narcotic[7].
Charak, who was born in 300 BC, was one of the major contributors to the ancient art and science of Ayurveda as a system of medicine. Charak Samhita, Sharangdhar Samhita mentions some plants and their mixture as being traditionally used in the treatment of Khalitya (Alopecia) and Indralupta (Baldness). The berries of S. nigrum L. are one of such herbs that are utilized as an alopecia treatment[8]. To the best of our knowledge, no scientific research has been done on examining the folklore claim that S. nigrum promotes hair development. This is despite the fact that the usage of berries has historically been praised for the alopecia treatment.
Materials and Methods
Collection and identification plant material:
In February, the berries of the S. nigrum plant were collected in Kolkata (West Bengal). Berries were identified and authenticated. The plant material was finely pulverized, air dried and then passed through filter number ten.
Preparation of extracts:
Each of two 1000 ml conical flasks holding 100 g of crushed S. nigrum (berries) contained 500 ml of methanol and 500 ml of petroleum ether. In an airtight container, it was kept in a temperature range of 25°-30° for 3 d. Then it was filtered using ordinary filter paper. The filtrate was stored in a 1000 ml beaker. After filtering, the filtrates were concentrated using a rotary evaporator at a temperatures ranging from 40°-45°.
Drugs and chemicals:
Sisco Research Laboratory (SRL) supplied linoleic acid, NADPH tetrasodium salt. Finasteride, tris- Water (HCl) buffer, and T were purchased from Sigma-Aldrich. Merck in Mumbai supplied sucrose, sodium phosphate, methanol, 95 % ethanol, n-hexane, ethyl acetate, petroleum ether and Ethylenediamine Tetra acetic Acid (EDTA). For all other chemicals in the study, analyticalgrade chemicals were used.
Qualitative evaluation of S. nigrum extracts:
Extracts were submitted to several qualitative analyses to detect plant components such as saponins, phytosterols, alkaloids, glycosides, carbohydrates, flavonoids, proteins, tannins and phenolic compounds by the method of Singh et al.[9].
Standardization of linoleic acid (standard) in S. nigrum extracts berries by High-Performance Thin Layer Chromatography (HPTLC):
Linoleic acid is an omega-6 polyunsaturated fatty acid. It is known to have a variety of physiological properties such as 5α-R inhibitor, anticancer, antifibrinolytic and so on[10]. Linoleic acid is said to be the most prevalent unsaturated fatty acid in S. nigrum oil[11,6]. In a previously published article, linoleic acid was quantified in S. nigrum methanol extract by using HPTLC[12]. Linoleic acid content in S. nigrum extracts in methanol and petroleum ether were measured by using the same HPTLC methodology[12].
The CAMAG, HPTLC system includes the scanning densitometer; LINOMAT V automated sample applicator, automatic development chamber, with WINCATS software. The image documentation tools CAMAG reprostar 3 and CAMAG scanner 3 were utilized. About 10.0 mg of lyophilize methanolic and 2 mg of petroleum ether extract of S. nigrum (berries) was taken in separate 1 ml of eppendorf tube. 1.0 ml of methanol and petroleum ether was added in methanolic and petroleum ether extract of S. nigrum respectively. S. nigrum was applied 8 μl and 10 μl each at the concentration 10 mg/ml and 2 mg/ml of methanol and petroleum ether extract, respectively. In order to serve as a standard marker for quantification, a band of linoleic acid (0.5 mg/ml) dissolved in methanol was applied accordingly in the range of 2-10 μl with 2 μl progressive increment. Application of samples to HPTLC plates was done using a 100 μl syringe (Hamilton, Switzerland). For standardization, an HPTLC plate (silica gel GF254, E. Merck, Germany) with bands for the standard and sample solutions was employed. The mobile phase used for resolving the extracts was n-hexane and ethyl acetate (5:4 v/v). Plate was dried using hand dryer after development. The sulfuric acid-anisaldehyde spraying reagent was used to treat the dry plate. For a short period of time, the plate was kept in a hot air oven at 110°, and the analysis was 540 nm. At 540 nm, colored bands were visible.
Enzyme inhibition assay preparation for 5α-R:
A few changes were made to the approach described by Nahata et al.[13]. Following are the specifics of the process.
Methods of preparation 5α-R solution [13]:
The local hospital in Kolkata provided human prostate (approximately 350 mg), which was minced into small pieces and then mixed with 30 ml of a medium (20 mM sodium phosphate, pH 6.5, containing 0.32 M sucrose and 1 mM EDTA). Then centrifuged the homogenate for 15 min at 4000 rpm (716 g), and the supernatant was utilized as an enzyme source. Using the Bradford technique of protein quantification, the protein content in the supernatant was calculated. Bradford technique of protein quantification was used to estimate the amount of enzyme in the supernatant.
In this method, a stock Bovine Serum Albumin (BSA) solution of 1 mg/ml was produced in deionized water. From stock, two fold serial dilutions with concentration of 0.5, 0.25 and 0.125 mg/ml were made. 5 μl of the produced BSA solution were put to a 96-well microplate at various concentrations. 200 μl of Bradford reagents were added to the BSA solution. At 592 nm, absorbance was observed. A standard curve was created by plotting the standard concentrations against the absorbance at 592 nm. 5 μl of enzyme homogenate solution was incubated for 5 min with 200 μl of Bradford reagent. The protein concentration was then calculated by using the BSA standard curve. The isolated prostate protein content was 0.58 mg/ ml. For the enzyme assay, the solution was further diluted to 100 μg/ml using tissue homogenization media.
Preparation of standard curve of NADPH[13]:
At 340 nm, a standard NADPH curve was created in methanol using concentrations of 1-20 μg/ml. The equation of a straight line revealed a good linear connection between absorbance and concentration, the correlation value (r2), that was equal to 0.9978, and the equation y=0.0244x+0.00056.
Preparation of finasteride solution[13]:
Finasteride stock solution was prepared by dissolving 0.0037 g of powder in 10 ml methanol (1000 μm). Sonication was carried out for 1 h and vortexed for 15 min. Then filtration was carried out by 0.45 μ syringe filter, and filtrate was collected in two separate eppendorf tube. Working solution was prepared by 2 μl of stock finasteride solution diluted with 1998 μl methanol to obtained 1 μm working solutions. Further dilution was done 0.1, 0.2, 0.4, 0.6, 0.8 μm respectively for determine the Half-Maximal Inhibitory Concentration (IC50) value.
Preparation of plant extracts and linoleic acid as the biomarker of plant material:
Each 2 ml eppendorf tube contained 10 mg of the S. nigrum methanolic and petroleum ether extract, which were then combined with the corresponding 2 ml parent solvent. In addition, 2 mg of the plant biomarker linoleic acid was collected in separate 2 ml eppendorf tubes and given 2 ml of methanol. It was mixed in a vortex before being placed in an ultrasonic bath to dissolve the substance entirely. It was preserved for later research after being filtered via 0.45 μ syringe filter. Additionally, dilutions of 25, 50, 75, 100, 150, 200 and 300 μg/ ml were carried out to determine the IC 50 value.
Preparation of test solutions for 5α-R inhibition assay[13]:
T solution (75 μm) in methanol, NADPH solution prepared in methanol (22 μm), extracts solution in parent solvent (1 mg/ml), and 0.5 mole of Tris-HCl buffer in distilled water.
Assay procedure of 5α-R inhibition of S. nigrum and linoleic acid:
Based on the procedure outlined by Nahata et al.[13], 5α-R inhibition experiments were carried out. In a summary, test samples, enzyme homogenate solution, T and NADPH were mix together. Table 1 provides a description of the specific reaction mixtures. All reaction mixtures underwent a 30 min incubation period at 37°. At 340 nm, absorbance was determined spectrophotometrically. From the NADPH standard curve, the test samples corresponding NADPH concentrations were determined. NADPH concentrations that remain in the reaction medium were calculated. The NADPH concentration was calculated as a percentage of NADPH scavenging. To calculate the net absorbance of NADPH, blank absorbance was subtracted from the test samples. For each test substance, the 5α-R inhibition was calculated, showing the test substance’s original efficacy against the enzyme. The percentage of NADPH scavenging potential was used to calculate the percent inhibition of 5α-R. All of the extracts were subjected to a blank test to determine their intrinsic in vitro antioxidant activity or their capacity to convert NADPH to NADP, it would prevent them from initially being able to inhibit the 5α-R present in the reaction medium. The 5α-R inhibition was then calculated for each separate extract to assess the real activity, against the enzyme. Absorbance at 340 nm; Net absorbance of test=(Test absorbanceblank absorbance)
| Sample ID | Methanol (ml) | Tris HCL (ml) | NADPH (ml) | Enzyme (ml) | Finasteride/(ml) | Test sample (ml) | Testosterone (ml) | Total volume (ml) |
|---|---|---|---|---|---|---|---|---|
| Blank control | 4 | 4 | 3 | 1 | 12 | |||
| Negative control | 2 | 4 | 3 | 1 | 2 | 12 | ||
| Finasteride | 4 | 3 | 1 | 2 | 2 | 12 | ||
| Test | 4 | 3 | 1 | 2 | 2 | 12 |
Note: It was considered that 2 ml of 75 µm of T were transformed into DHT by 3 ml of 22 µm NADPH
Table 1: Enzyme, Substrate and Coenzyme Mixture
Calculate the NADPH concentration in each sample from NADPH standard curve prepared previously.
Percentage inhibition=100-((54.78-concentration of NADPH obtained from net absorbance of the test solution)/54.78)×100)
To measure the percentage inhibition of various test sample concentrations required to establish the IC50 value of the test extracts, statistical analysis was performed.
Results and Discussion
The percentage yield of extraction was 3.1 % and 1.54 % w/w in methanol and petroleum ether, respectively. The methanolic extract gives positive test of flavonoids, alkaloids, protein, carbohydrates, tannins, glycosides and saponins.
In petroleum ether extract gives positive test of phytosterols, alkaloids and tannins shown in Table 2.
| Phytochemical screening test | Methanolic extract of S. nigrum | Petroleum ether extract of S. nigrum | |
|---|---|---|---|
| Phytosterols | Libermann-Burchard Test | + | + |
| Liebermann’s reaction | + | + | |
| Glycosides | Keller-Killiani Test | + | - |
| Bontragger’s Test | + | - | |
| Flavonoids | Shinoda test | + | - |
| Ferric chloride test | + | - | |
| Alkaloids | Mayer’s test | + | + |
| Dragendroff's test | + | + | |
| Protein | Millon’s reaction | + | - |
| Xanthoproteic reaction | + | - | |
| Carbohydrates | Molisch’s test | + | - |
| Fehling’s test | + | - | |
| Barfoed reagent test | + | - | |
| Tannins and phenolic compounds | Ferric chloride test | + | + |
| Lead acetate solution | + | + | |
| Saponins | Foam test with water | + | - |
| Foam test with sodium carbonate | + | - | |
Note: (+): Present and (-): Absent
Table 2: phytochemical screening of the extracts of s. Nigrum
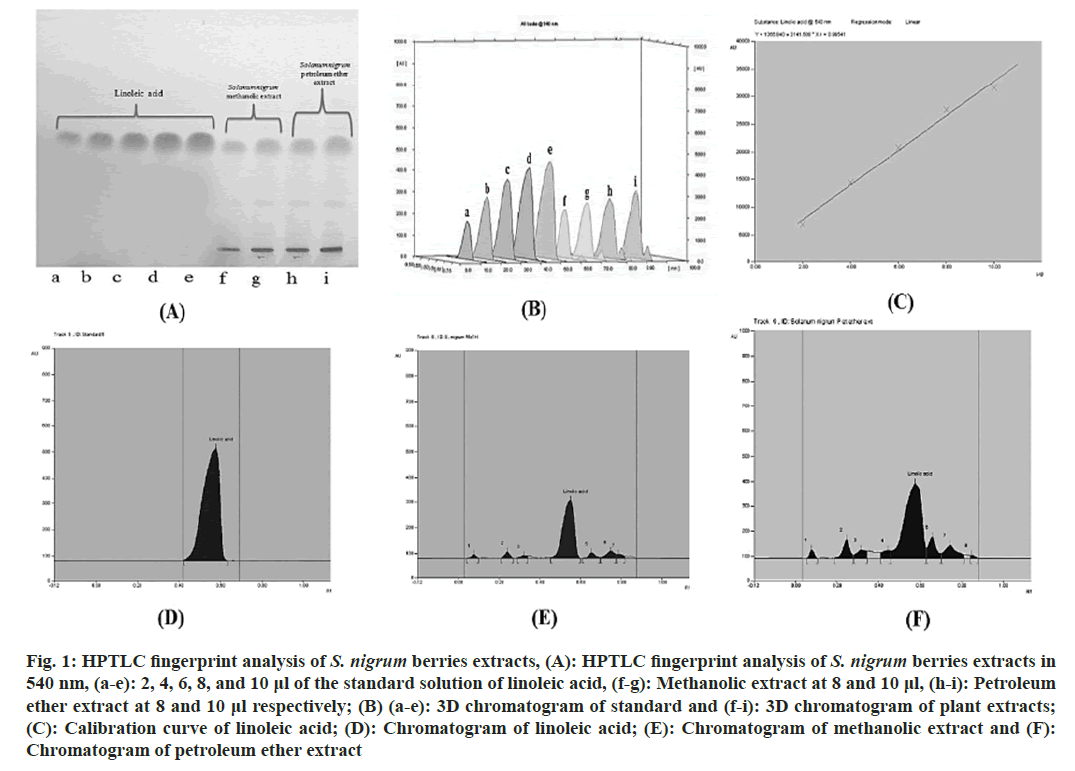
In HPTLC, methanolic as well as petroleum ether extracts of S. nigrum were found to contain 9.65 % and 32.16 %of linoleic acid. A calibration curve with the equation y=3141.508x+1366.840 (correlation coefficient=0.99541) was used to establish this. Standard linoleic acid was shown to have an Rf value of 0.58. In order to verify specificity, the Rf of the standard and sample were compared (fig. 1).
Fig. 1: HPTLC fingerprint analysis of S. nigrum berries extracts, (A): HPTLC fingerprint analysis of S. nigrum berries extracts in 540 nm, (a-e): 2, 4, 6, 8, and 10 μl of the standard solution of linoleic acid, (f-g): Methanolic extract at 8 and 10 μl, (h-i): Petroleum ether extract at 8 and 10 μl respectively; (B) (a-e): 3D chromatogram of standard and (f-i): 3D chromatogram of plant extracts; (C): Calibration curve of linoleic acid; (D): Chromatogram of linoleic acid; (E): Chromatogram of methanolic extract and (F): Chromatogram of petroleum ether extract
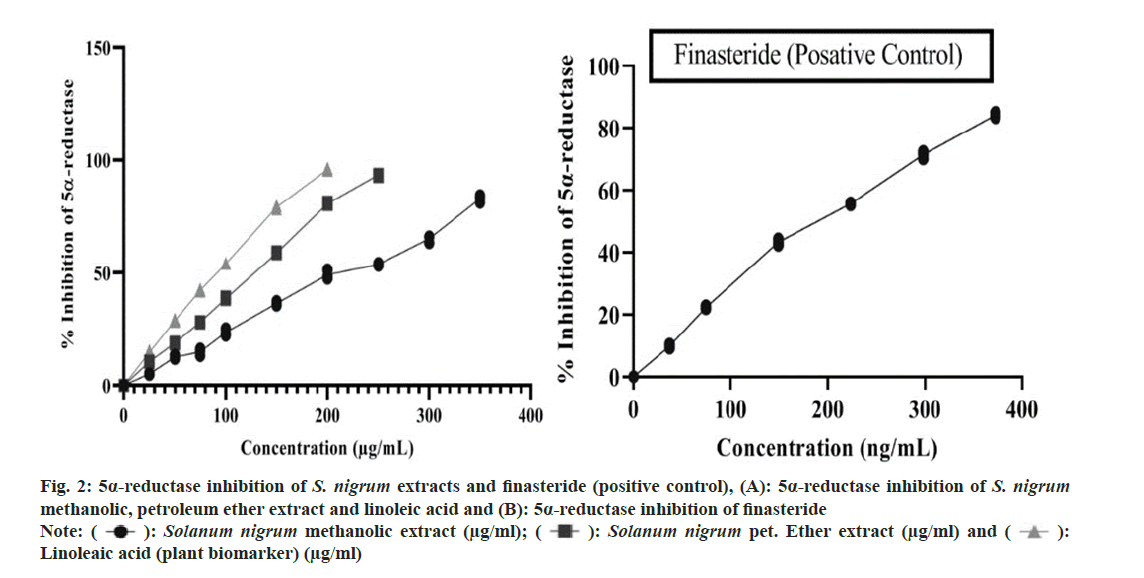
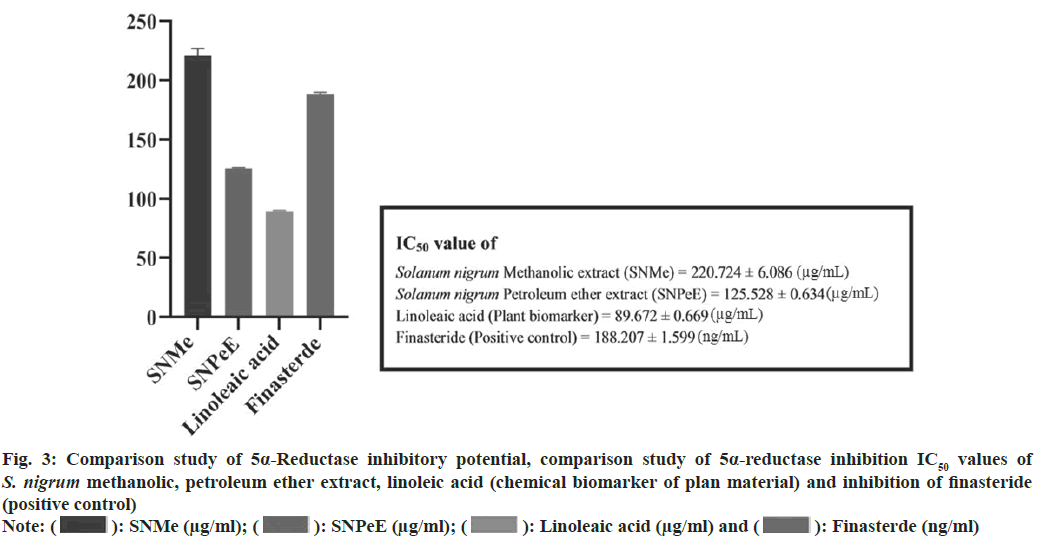
To get IC50 values, statistical analysis was performed. Plotting the curve with the percentage of inhibition vs. the concentrations, several investigations was used to get the IC50 values, which were then represented as mean±standard deviation. The statistical analysis was performed utilizing Graphpad software version 6.0 and one way Analysis of Variance (ANOVA) and the Bonferroni post-hoc test performed. When compared to the reference standard, the p was deemed to have a significant difference of less than 0.05. IC50 value of S. nigrum methanolic and petroleum ether extract (berries) was found 220.724±6.086 (μg/ml) and 125.528±0.634 (μg/ ml). Whereas 5α-R inhibition of linoleic acid and finasteride shown to be 89.672±0.669 (μg/ml) and 188.207±1.599 (ng/ml) respectively. 5α-R inhibition of S. nigrum extract, linoleic acid and finasteride shown in fig. 2 and fig. 3 compares the samples comparative 5α-R inhibitory potential.
Fig. 2: 5α-reductase inhibition of S. nigrum extracts and finasteride (positive control), (A): 5α-reductase inhibition of S. nigrum methanolic, petroleum ether extract and linoleic acid and (B): 5α-reductase inhibition of finasteride
Note: (  ): Solanum nigrum methanolic extract (μg/ml); (
): Solanum nigrum methanolic extract (μg/ml); (  ): Solanum nigrum pet. Ether extract (μg/ml) and (
): Solanum nigrum pet. Ether extract (μg/ml) and (  ):
Linoleaic acid (plant biomarker) (μg/ml)
):
Linoleaic acid (plant biomarker) (μg/ml)
Fig. 3: Comparison study of 5α-Reductase inhibitory potential, comparison study of 5α-reductase inhibition IC50 values of S. nigrum methanolic, petroleum ether extract, linoleic acid (chemical biomarker of plan material) and inhibition of finasteride
(positive control)
Note: ( ): SNMe (μg/ml); (
): SNMe (μg/ml); ( ): SNPeE (μg/ml); (
): SNPeE (μg/ml); ( ): Linoleaic acid (μg/ml) and (
): Linoleaic acid (μg/ml) and ( ): Finasterde (ng/ml)
): Finasterde (ng/ml)
Androgenic alopecia is very prevalent in both men and women[14,15]. DHT is produced when T is converted by the nuclear membrane bound enzyme 5α-R[3]. 5α-R catalyzes the NADPH dependent conversion of T to DHT[16]. The 5α-R and its metabolite DHT have an influence on a variety of human disorders, including male pattern baldness in both sexes, alopecia, benign prostatic hyperplasia etc[4].
Solanaceae family, S. nigrum barries has used in the treatment of alopecia[8]. Linoleic acid is an omega-6 polyunsaturated fatty acid have a variety of physiological properties such as 5α- R, antianaphylactic, etc.,[10]. Methanol as well as petroleum ether extracts of S. nigrum were found to contain 9.65 % and 32.16 % of linoleic acid by HPTLC. Linoleic acid is said to be the most prevalent unsaturated fatty acid in S. nigrum oil[11,6].
To evaluate the therapeutic benefits of several S. nigrum extracts, an in vitro 5α-R inhibitory experiment was conducted. The IC50 value of petroleum ether extract of S. nigrum was more potent than that of methanol, which might be due to nonpolar components contained in petroleum ether extraction medium being more potently active to inhibit enzyme. In the research presented here, a previously described molecule called linoleic acid was identified and quantified in large amounts in the petroleum ether extract of the plant S. nigrum. The linoleic acid standard was found to have the most potent 5α-R inhibitor activity, except for finasteride, which was used as a positive control, as indicated in fig. 2 and fig. 3. Due to the presence of the high concentration of unsaturated fatty acids (linoleic acid, linolenic acid, and so on)[8,9], it may be able to associate the maximum activity of S. nigrum petroleum ether extract with its 5α-R inhibitor activity. It has been demonstrated that inhibition of 5α-R by lipophilic extracts of Sabal serrulata fruits is entirely due to free fatty acid content[17]. Dermal papilla cell proliferation and hair development were induced by the isolation of linoleic acid from Malva verticillata seeds, which triggered Wnt/β-catenin signalling to boost the cell cycle and growth factor release[18]. The spore of Lygodium japonicum is known as the Lygodii Spora, and a 50 % of aqueous ethanol extract showed in vitro 5α-R inhibitory action as well as in vivo anti-androgenic activities. In mice treated with T, it demonstrated hair growth following shaving. Fatty acid such as oleic, linoleic, and palmitic acid were the primary components with anti-5α-R activity[19]. In cultured cells and cellfree systems, certain unsaturated fatty acids can inhibit 5α-R. According to a research, γ-linolenic acid has the most 5α-R inhibitory action, followed by arachidonic acid, α-linolenic acid, linoleic acid, palmitoleic acid, oleic acid, and myristoleic acid in decreasing order of intensity[20]. The seeds of Sesamun indicum, a Chinese plant also used for hair development, contain significant levels of fatty acids; it is probable that this is the mechanism by which Sesamun indicum affects hair growth[13]. Another botanical, Boehmeria nipononivea in acetone extract also inhibits 5α-R and encourages hair growth in mice. Fractionation of the leaves extract revealed six fatty acids including α-linolenic acid, linoleic acid, palmitic acid, elaidic acid, stearic acid and oleic acid[21]. Thus petroleum ether of S. nigrum is most useful for alopecia by inhibiting 5α-R enzyme activity due to rich content of linoleic acid present. S. nigrum can be used for the aforementioned purpose since 5α-R inhibition aids in the treatment of androgenic alopecia. The well-known 5α-R inhibition of free fatty acids found in the saw palmetto extract that include linoleic acid might potentially be considered as a possible action mechanism for S. nigrum[22].
Discussing some of the protocol’s restrictions in our experiment, all reaction mixtures included the same reagents used in the blank along with the examined extracts, allowing us to draw a conclusion. NADPH (22 μm) was added to the blank solution (3 ml) to observed the absorbance that was seen at time zero in the blank control group. Therefore, at this point, a new NADPHonly group without any test samples was preferred for an effective comparison. It would have demonstrated NADPH innate absorbance. So, for a relevant comparison, it is suggested that the experiment in the future add a new group as the NADPH control group. The NADPH amount in the enzyme solution is another element to take into account. This concentration may be tested before to improve accuracy and eliminate any potential NADPH related interference. Since the same amount of enzyme was used for all measurements in this experiment, the results were consistent and the probability that the absorbance would change was almost negligible. This aspect should still be prioritized in future studies to allow for better group comparisons.
Future prospects for this study include a time dependent investigation using various fractions that would have provided more information about the plants. Finding out how precisely the substances affect the enzyme, how well they interact to the androgen receptor that NADPH binds to, and how they block NADPH, whether by competitive inhibition or site-specific inhibition, more research on the potent substances of the study is necessary. It is also unknown if allosteric modulation has a role in the overall interaction of the enzyme activity.
In conclusion, the study found that S. nigrum petroleum ether extract was superior to methanolic extract as a 5α-R inhibitor. The most effective 5α-R inhibitor activity was discovered in linoleic acid (chemical marker of S. nigrum analysis). The petroleum ether extract of S. nigrum contains a significant amount of linoleic acid, which has been proven to be a powerful 5α-R inhibitor. S. nigrum can be used for the aforementioned purpose since 5α-R inhibition aids in the treatment of androgenic alopecia. The ethnomedical usage of plant for treating hair loss is supported by the current investigation. To support the in vitro findings produced by S. nigrum berries 5α-R inhibitory effects, more in vivo research is necessary. The utilization of the petroleum ether extract in a formulation and additional research into S. nigrum berries for the treatment of alopecia may thus be beneficial.
Conflict of interest:
The authors declared no conflict of interests.
References
- Lieberman R. Evolving strategies for prostate cancer chemoprevention trials. World J Urol 2003;21(1):3-8.
[Crossref] [Google Scholar] [PubMed]
- Wright AS, Douglas RC, Thomas LN, Lazier CB, Rittmaster RS. Androgen-induced regrowth in the castrated rat ventral prostate: Role of 5α-reductase. Endocrinology 1999;140(10):4509-15.
[Crossref] [Google Scholar] [PubMed]
- Levy MA, Brandt M, Sheedy KM, Holt DA, Heaslip JI, Trill JJ, et al. Cloning, expression and functional characterization of type 1 and type 2 steroid 5α-reductases from Cynomolgus monkey: Comparisons with human and rat isoenzymes. J Steroid Biochem Mol Biol 1995;52(4):307-19.
[Crossref] [Google Scholar] [PubMed]
- Guarna A, Poletti A, Catrambone F, Danza G, Marrucci A, Serio M, et al. Synthesis of a chemiluminescent probe useful for the purification of steroid 5α-reductase. Bioorgan Med Chem Lett 1996;6(16):1997-2002.
- Russell DW, Wilson JD. Steroid 5α-reductase: Two genes/two enzymes. Ann Rev Biochem 1994;63(1):25-61.
[Crossref] [Google Scholar] [PubMed]
- Saleem TM, Chetty C, Ramkanth S, Alagusundaram M, Gnanaprakash K, Rajan VT, et al. Solanum nigrum Linn: A review. Pharmacogn Rev 2009;3(6):342.
- Kuete V, Karaosmanoglu O, Sivas H. Anticancer activities of African medicinal spices and vegetables. Med Spices Vegetables Afr 2017:271-97.
- Patel S, Sharma V, S Chauhan N, Thakur M, Dixit VK. Hair growth: Focus on herbal therapeutic agent. Curr Drug Discov Technol 2015;12(1):21-42.
[Crossref] [Google Scholar] [PubMed]
- Singh R, Kori ML. Phytochemical studies of Tephrosia purpurea Linn. and Martynia annua Linn. extracts for identification of chemical constituents. Int J Pharm Sci Drug Res 2022;14(2):226-32.
- Duke JA. CRC handbook of medicinal spices. CRC press; 2002.
- Sarma H, Sarma A. Solanum nigrum L., a nutraceutical enriched herb or invasive weed? Int Conference Environ Biosci 2011;21:105-9.
- Chakraborty A, Bhattacharjee A, Dasgupta P, Manna D, Chun WO. Simple method for standardization and quantification of linoleic acid in Solanum nigrum berries by HPTLC. J Chromatogr Sep Tech 2016;7(6):1-4.
- Nahata A, Dixit VK. Evaluation of 5α-reductase inhibitory activity of certain herbs useful as antiandrogens. Andrologia 2014;46(6):592-601.
[Crossref] [Google Scholar] [PubMed]
- Sperling LC. Hair and systematic disease. Dermatol Clin 2001;19(4):711-26.
[Crossref] [Google Scholar] [PubMed]
- Kumar N, Chaiyasut C. Hair growth promoting activity of Carthamus tinctorius florets extract-loaded nanostructured lipid carriers. Int J Pharm Pharm Sci 2015;7(2):252-7.
[Crossref] [Google Scholar] [PubMed]
- Nutbrown M, Hull SP, Randall VA, Cunliffe WJ. Abnormalities in the ultrastructure of melanocytes and the outer root sheath of clinically normal hair follicles from alopecia areata scalps. J Invest Dermatol 1995;995(104):12S-3S.
[Google Scholar] [PubMed]
- Niederprum HJ, Schweikert HU, Zanker KS. Testosterone 5α-reductase inhibition by free fatty acids from Sabal serrulata fruits. Phytomedicine 1994;1(2):127-33.
[Crossref] [Google Scholar] [PubMed]
- Ryu HS, Jeong J, Lee CM, Lee KS, Lee JN, Park SM, et al. Activation of hair cell growth factors by linoleic acid in Malva verticillata seed. Molecules 2021;26(8):2117.
[Crossref] [Google Scholar] [PubMed]
- Paine C, Sharlow E, Liebel F, Eisinger M, Shapiro S, Seiberg M. An alternative approach to depigmentation by soybean extracts via inhibition of the PAR-2 pathway. J Invest Dermatol 2001;116(4):587-95.
[Crossref] [Google Scholar] [PubMed]
- Hakozaki T, Minwalla L, Zhuang J, Chhoa M, Matsubara A, Miyamoto K, et al. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br J Dermatol 2002;147(1):20-31.
[Crossref] [Google Scholar] [PubMed]
- Tung RC, Bergfeld WF, Vidimos AT, Remzi BK. α-Hydroxy acid-based cosmetic procedures: Guidelines for patient management. Am J Clin Dermatol 2000;1(2):81-8.
[Crossref] [Google Scholar] [PubMed]
- Abe M, Ito Y, Oyunzul L, Oki-Fujino T, Yamada S. Pharmacologically relevant receptor binding characteristics and 5α-reductase inhibitory activity of free fatty acids contained in saw palmetto extract. Biol Pharm Bull 2009;32(4):646-50.
[Crossref] [Google Scholar] [PubMed]