- *Corresponding Author:
- V. Vaithilingam
Department of Pharmacy, Karpagam Academy of Higher Education, Coimbatore 641021, Tamil Nadu, India
E-mail: vivek.mph@gmail.com
| Date of Received | 26 October 2021 |
| Date of Revision | 07 September 2022 |
| Date of Acceptance | 02 July 2023 |
| Indian J Pharm Sci 2023;85(4):853-862 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Over several centuries, oral dosage forms have remained incomparably superior to alternative medication delivery methods. For its ease of formulation and administration, its stability, and its excellent rate of patient compliance, the oral route is preferred. Gastric emptying has been a major drawback for drugs that stay for a very short period in the stomach. An innovative method of gastro-intestinal retention allows the dosage form to stay in the gastrointestinal region for a longer period and maximize drug release. Gastro retentive drug delivery system is applied for enhancing bioavailability, local action in the gastric region, maintaining steady drug concentrations and minimising the dosage regimen. This review discusses the approaches used in gastro retentive drug delivery system, which include floating, high density, muco-adhesive, magnetic and various other systems. Floating systems are the most widely used approach under gastro retentive drug delivery system. These are extensively used because of their simplicity. Drug content, hardness, particle size analysis, weight variation and friability are some of the conventional tests for any dosage form. Along with these, buoyancy studies, dissolution studies, and swelling index are also carried out. Techniques like magnetic resonance imaging are widely used in in vivo evaluation. Yet, evaluating entire in vivo efficacy in the case of small animals is a significant drawback. Gastro retentive drug delivery system is regarded as a formulative strategy to improve the activity and bioavailability of the active pharmaceutical ingredient based on its benefits and applications. It is anticipated that numerous more studies will uncover Gastro retentive drug delivery system's full potential.
Keywords
Oral dosage form, gastric emptying, study drug concentration, gastro retentive drug delivery system, mucoadhesive
Oral route is defined as the most convenient route of drug delivery. Oral route is desired for its simplicity in formulation and administration, stability and excellent rate of patient compliance[1]. The oral drug delivery method has a great deal of possibilities for combining medications from different classes and dose ranges. It is one of the earliest dose forms that have evolved in line with more modern developments, such as the use of nanotechnology. The stomach is a major organ in the gastro-intestinal tract. It is located between the oesophagus and the duodenum. The stomach is further divided into four chambers, namely the cardia, fundus, body, and pylorus[2]. Various secretions of the stomach include hydrochloric acid, electrolytes, and glycoproteins like intrinsic factors, mucin, and several other enzymes. Gastric motility and secretion are regulated by neural and humoral mechanisms[3]. Gastric secretions can be facilitated by eating and swallowing (the cephalic phase), the arrival of food in the stomach (the gastric phase), and the entry of food into the intestine (the intestinal phase)[4,5]. Gastric emptying has been a major drawback for drugs that stay for a very short period in the stomach.
An innovative method of allowing the dose form to stay in the gastrointestinal region for a longer amount of time so that maximum release is achieved is gastro-retentive drug administration. The solubility and bioavailability of a substance are likely to increase with such a strategy given the stomach's natural acidity. Pharmacological and physiological approaches can be used to induce gastro retention. By stimulating duodenal or jejunal receptors, some naturally occurring compounds, such as fats and their derivatives, can postpone the emptying of the stomach. Pharmacologically on administering some drugs like propantheline in a dosage form may help delay the gastric emptying[6].
Designing an oral dosage form presents several challenges, including unpredictable gastric emptying, a shorter gastro intestinal transit time, and the existence of an upper small intestine absorption window. A delivery system that can increase the drug's stomach residence time must be developed in order to surmount these challenges[6]. Some medications with short half-lives are eliminated rapidly and call for frequent dosing. Gastro Retentive Drug Delivery System (GRDDS) is used to decrease the number of doses. In order minimize the number of doses, GRDDS is employed. They keep the drug concentration in the plasma stable, allowing for extended release. Additionally, it lessens plasma concentration fluctuations such that it is reproducible[7].
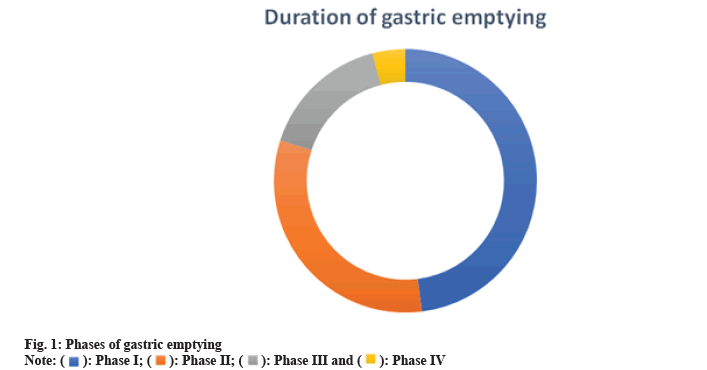
The phases of gastric emptying can be differentiated into four as shown in fig. 1. Phase I, also referred to as the baseline phase, includes irregular contractions and lasts for 30 to 60 min. Phase II is the preburst phase, which begins out as erratic action potentials and contractions and lasts for 20 to 40 min. It gets stronger with time. Phase III, often known as the surge phase, lasts for 10 to 20 min and is characterised by regular, intense contractions. Only 0 to 5 min or 8, 9, are spent in Phase IV[8,9].
Longer stomach retention of the medicine can increase bioavailability, reduce drug waste, and increase the solubility of less soluble pharmaceuticals at high pH. Local drug distribution to the stomach and proximal sections of the small intestine is made possible by this kind of drug delivery mechanism. Formulations based on buoyancy work as a principle that gives a quick and straightforward technique to extend gastric retention duration and also enable sustained release of medications[10].
Physicochemical Factors affecting GRDDS
The physicochemical properties of the gastro-retentive formulations, including shape, size, and density, have a major impact on the efficacy and residence time of the system in the intended location. Dosage form plays an important role in achieving higher stability and maintaining uniform release. In general, round, or spherical shapes are preferred for oral dose forms since they are more effective than other shapes. Tetrahedron and ring-shaped formulations having a flexural modulus of 48 and 22.50 kilo pounds per square inch are reported to have a better gastro-residence time of 24 h compared with other shapes[8]. The formulation's size should make it simple to swallow. Controlling the gastro residence is also crucial. The mean diameter of pyloric antrum is 12.8±7 mm. Designing a formulation greater than this prevents the transition of dosage form from the stomach to intestine, increasing the residence period[11]. According to Vinchurkar et al.[12] a diameter bigger than 7.5 mm can demonstrate improved gastro residence. Gastro retention can be achieved through high density and low-density systems. Systems with lower densities typically float on the surface while systems with higher densities sink. Density should be smaller than that of gastric contents, or 1 g/cm3, in order to achieve gastric floating[13]. To improve residency and encourage sinking at the gastric floor, a high-density system's density should be more than 2.5 g/cm3[14]. Multiple unit formulations show a more expectable release profile and insignificant damaging of performance because of failure of units, allow co-administration of units that have dissimilar release profiles related with single unit dosage forms.
Impact of Food on Gastro Residence
Gastric emptying is significantly influenced by both the fed and unfed states. Low frequency of Migrating Myoelectric Complex (MMC) in the fasting state might cause gastro retention time to increase by up to 400 times, which in turn depends on how the frequency of food intake[15]. In the fed state, the size of the pylorus goes up to 19 mm, resulting in expulsion of food from the stomach, into the intestine[16]. However, presence of food is also found to delay the gastric emptying, as it disrupts the MMC[17]. A meal that is heavy in protein or fat can lengthen the period of gastric retention by 4 to 10 h[18]. Calories only has a minor contribution towards gastric residence. Food viscosity is also reported to delay the gastric emptying rate[19]. The motility pattern of stomach is altered by intake of fatty acids and indigestible polymers; they decrease the gastric emptying.
Patient Oriented Factors affecting Gastric Retention
When an oral dosage form is administered with a large quantity of water, the pH rises to 6-9. In turn, the stomach does not get enough time to release the required acid as the content empties from the stomach. The chances of drug dissolution are higher in a fed state than in fasting conditions. Gastric retention time is more in geriatric patients and less in infants. Gastric retention increases with age. People above 70 exhibit significantly longer residence time[20]. Females tend to display delayed gastric emptying compared to males. This could be justified by the impact of female hormone[21]. Gastro retention time varies with presence of pathological gastric conditions like ulcer, diabetes, thyroid dysfunction, Chron’s disease, etc. Interestingly, emotional state of the patient can also influence the gastric residence. It is seen that mental depression and anxiety can decrease and increase the gastric emptying rate, respectively[17]. The retention of dosage form in the gastric region can vary based on supine and upright positions. Upright positions can favour the low-density systems by retaining the dosage form on the surface of the gastric content, whereas supine position can prolong the residence time for high density systems[18].
Drugs and Gastric Emptying
Several drugs are identified to have effect on gastric emptying rate. This can be due to altered muscle movements or hormonal. Drugs like anticholinergics, antihistamines, tricyclic antidepressants, antihypertensives, antacids can influence gastric emptying. Antacids tend to reduce the acidity of the stomach, increasing the pH, and delay the gastric emptying. Sedatives and anaesthetics are also found to have depressing effect on gastric movements[22].
Mertis and Demerits of GRDDS
Every drug delivery approach comes with its own merits and demerits, so is GRDDS (Table 1)[23-25].
| Merits | Demerits |
|---|---|
| Better bioavailability | Not useful if drugs can cause gastric irritation |
| Minimum number of dosing | Suitable only for drugs that are stable in gastric pH |
| Treating GI issues | Drugs much have greater solubility in gastric fluid |
| Steady drug concentration | May cause problems in adherence to mucus wall |
| Economical | Widely affected by nature of meal and other factors |
| Minimized risk of developing resistance | Swelling based systems may take longer time |
| Appreciable for drugs with short half-lives | |
| Controlled release and minimized fluctuations |
Table 1: Merits and Demerits of GRDDS.
Choice of Drugs for GRDDS
The selection of drug intended for GRDDS is important. The drugs chosen for GRDDS should be stable at the pH of the stomach. For the drugs that are less active or poorly soluble in intestinal pH, opting GRDDS offers better therapeutic efficacy. This approach can also provide benefits in case of local drug delivery to the stomach. Table 2 shows the list of drugs that can be successfully used in GRDDS.
| Property | Drugs | Use |
|---|---|---|
| Local action | Ranitidine | Anti-ulcer |
| Metronidazole, Amoxicillin | Anti-Helicobacter pylori treatment | |
| Control plasma fluctuations | Ciprofloxacin, Clarithromycin | Anti-microbial |
| Unstable in alkaline pH | Captopril | Anti-hypertensive |
| Metronidazole | Anti-protozoal | |
| Metformin HCl | Anti-diabetic | |
| Low solubility at high pH | Diazepam | Anxiolytic |
| Chlordiazepoxide | ||
| Furosemide | Diuretic | |
| Verapamil HCl | Anti-hypertensive | |
| Primary absorption in stomach | Amoxicillin | Anti-microbial |
Table 2: Drugs used in GRDDS.
Likewise, drugs with lesser acidic solubility (phenytoin), less stable in acidic environment (erythromycin), drugs having selective release in colon (corticosteroids) cannot be delivered using this delivery system[25-28].
Approaches for GRDDS
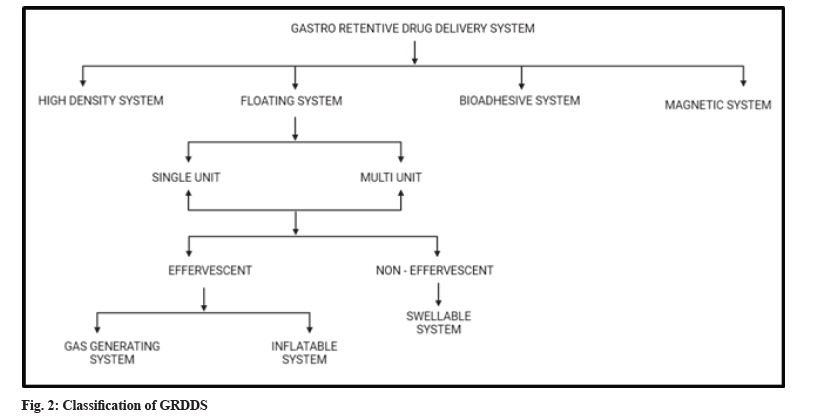
GRDSS has been widely explored in last 4 decades. Various advancements enabled to prolong the duration it stays in stomach like swelling and expanding systems, floating systems, bio adhesive systems, magnetic systems, gas generating systems and high-density systems[8,29]. The classification of GRDDS is shown in fig. 2.
High density GRDDS system:
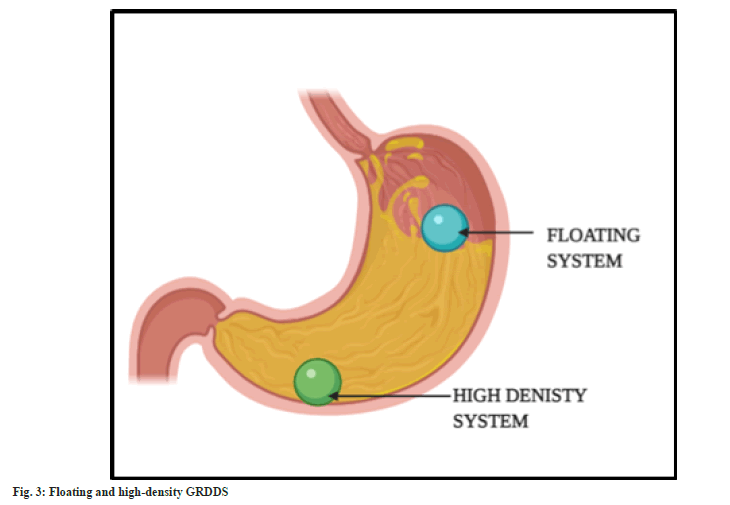
In this technique, the dosage form is made of high-density polymers such that it is denser than the gastric secretions. Therefore, sinks to reach the bottom of the stomach (fig. 3). The prepared dosage form is coated with the polymers. Titanium dioxide, zinc oxide, iron powder, and barium sulphate are among the polymers that are frequently utilised. The density is kept at optimum of 4.9 g/cm3, increasing up to 10.5 g/cm3. The system can tolerate the peristaltic movement of the gastric region and remain settled because of the high density. They slowly sink in the gastric content and reach rugae of the stomach[30,31]. However, dose size is a major obstacle in designing a high-density system. Drugs with large dose size cannot be delivered via GRDDS[32]. They are usually seen in the forms of high-density pellets and tablets[33].
Floating systems:
Under GRDDS, floating systems are the most popular strategy. These are extensively used because of their simplicity. The bulk density of the medication and the type of polymer utilised are the key determinants. The density of the system must be lower than that of the gastric fluid so that it can float on the surface of the gastric contents[34,35]. The system will remain buoyant in the stomach without altering the gastric emptying rate for a longer period of time, as demonstrated in fig. 3. The system is made up of hydrophilic polymers so that it can maintain buoyancy. Most commonly employed polymer is Hydroxypropyl Methylcellulose (HPMC). The polymer hydrates and builds a gelled barrier on the outer surface. On swelling, the drug releases in a determined rate.
Floating system can be further classified as a single unit and multiple unit which contain further sub types as effervescent and non-effervescent system. An effervescent system is made up of swellable polymers along with effervescent materials. For example, sodium bicarbonate. These are also known as gas generating systems. The evolution of carbon dioxide from the system on interaction with the gastric content, results in propulsion which causes floating. Inflatable systems contain a chamber filled with volatile material, which evaporate at the body temperature, inflating the chamber. This promotes the system to float on the gastric contents.
Non effervescent systems, on swelling, tend to restrict their exit through pylorus. It is made up of swellable cellulose type of hydrocolloids, polysaccharides, and matrix-forming polymers like polycarbonate, polyacrylate, polymeth-acrylate, and polystyrene[36-38].
Bioadhesive system:
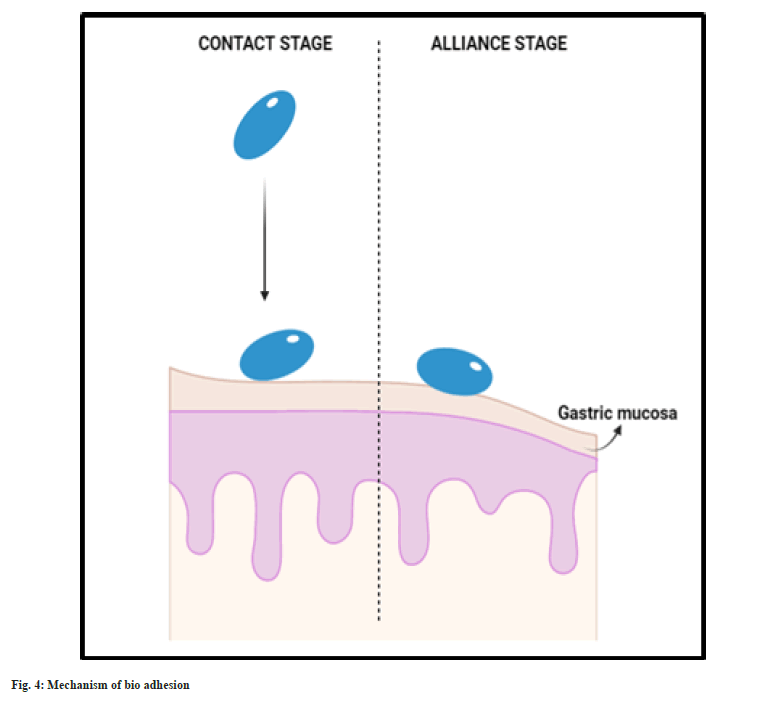
As the name confers, these formulations are made up of bio adhesive/mucoadhesive polymers which tend to bind to the mucosa of gastric lining and tend to hold the system in the stomach for longer time. Thus, the gastric residence is prolonged. Bio adhesive system is formulated using polymers along with mucoadhesive agents. The adhesion of the system and the gastric wall is achieved by two stages, namely, contact and alliance. Initially the system undergoes wetting and forms contact with the gastric mucosal layer (fig. 4). Then the system continues to form chemical bonding with the gastric mucosa with the hydrogen bond forming groups present in the hydrophilic polymer used. This system is usually made of hydrophilic polymers like carbopol 971P, ethyl cellulose, Eudragit RS-PO[39-41].
Magnetic systems:
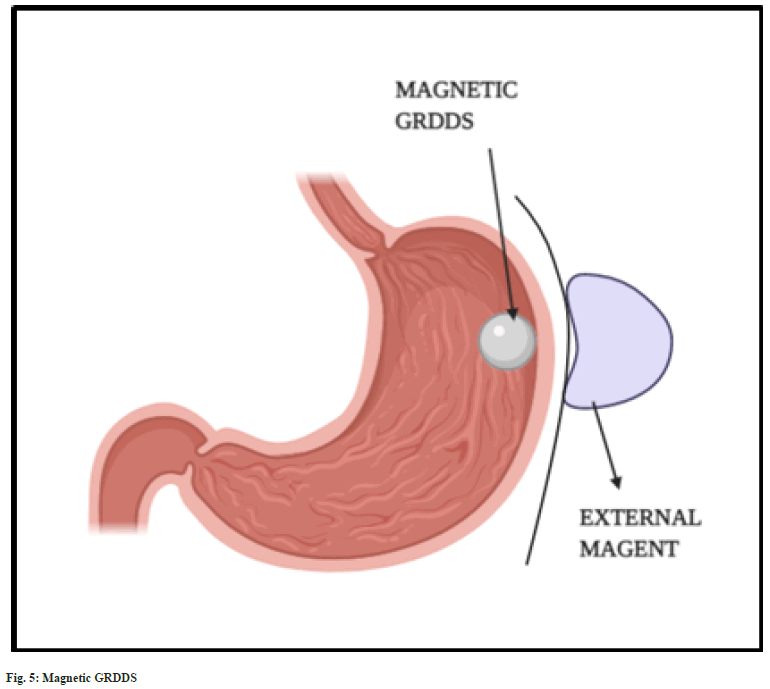
The magnetic systems are based on magnetic attraction force exerted between two magnets. The formulated dosage form is prepared using the mixture of API, excipients and a small quantity of magent[15]. Once it reaches the stomach, the dosage form is attracted towards the gastric wall by placing an external magnet over the stomach (fig. 5). This increases the gastric residence time and bioavailability[28,42]. An external magnet of strength 1700 G was found to retain magnetic granules in the gastric regions for more than 2 h[43]. The major disadvantage of magnetic systems is placement of external magnet. It is found to have very low patient compliance compared to other GRDDS approaches[18].
Challenges
GRDDS is a site-specific delivery system, that works in the stomach. The major challenge in designing a GRDDS is retaining the dosage form in the stomach and upper intestinal region for a longer period. Gastric mobility and peristaltic movement of the GIT are significant concerns contributing to the expulsion of the system[44]. High caloric and fatty diet, age, gender, pathological state, alter the gastric emptying rate[45]. Inter subject variation in pylorus size and peristaltic movement have to be taken into serious considerations. Another challenge is controlling the drug release with prolonging gastric retention before the drug gets metabolized[46].
Evaluation of GRDDS
Drug content, hardness, particle size analysis, friability, weight variation are some of the conventional tests for any dosage form[47]. Dissolution, buoyancy studies, swelling index, muco-adhesion, magnetic strength, x-ray studies have special notice[45,48].
Buoyancy and dissolution:
Ability of the dosage form to float is a based on its buoyancy. Floating time is estimated by dissolution. Float lagging time is the time taken for the dosage form to initiate floating. Dissolution can be carried out in United States Pharmacopeia dissolution apparatus using 900 ml of simulated gastric fluid or acidic buffer at 37-39°.
Swelling index:
The dosage form is placed in a determined medium and measured before and after. The difference in weight confers to the swelling capacity.
X-ray study:
As a part of in vivo analysis, X-Rays can be obtained to locate the position of the dosage form in gastric tract. Techniques like magnetic resonance imaging are widely used in in vivo evaluation. Evaluating complete in vivo efficacy is though a major setback in case of small animals. It is evident that in vitro studies are numerous compared to in vivo results of animals and human subjects[46].
Biological Aspect
The in vitro behavior of the formulation must correlate with the in vivo performance. Lin et al.[49] formulated nilotinib GRDDS to improve its oral solubility. According to the pharmacokinetic studies carried out on rabbits, they claimed that the improved oral bioavailability varied between 2.65-8.39 fold compared to the corresponding commercial formulation. Kim et al.[50] prepared pregabalin based non-effervescent floating and swelling GRDDS. They performed in vivo evaluations on beagle dogs and noticed dose dependent pharmacokinetics. It was evident that non effervescent floating system was preferrable for once-a-day delivery of pregabalin, in terms of obtained results[50]. Shishu and Aggarwal conducted an in vivo anti-tumor investigation to evaluate the therapeutic efficacy of floating calcium alginate beads of 5-flurouracil. In mice, it was discovered that the multiple unit floating system was able to reduce the occurrence of stomach tumours by 74 %, whilst a standard tablet dosage form was only able to lower this incidence by 25 %[51]. Famotidine mini-tablets developed by Zhu et al.[52] with HPMC K4M as a release-retarding and swelling polymer, carbopol 971P, and sodium bicarbonate were found to have better in vivo efficacy when floating and bio adhesion were combined. The mini-tablets were evaluated in rat models and may increase bioavailability by 1.62 %[52]. Despite of its challenges and drawbacks, few marketed GRDDS formulations are available, shown in Table 3[46,53,54].
| Approach | API | Brand | Manufacturer |
|---|---|---|---|
| Floating system | Alginic acid | Gaviscon | Reckitt Benckiser Healthcare, UK |
| Cefaclor | Cefaclor LP | Galenix, France | |
| Tramadol | Tramadol LP | ||
| Diazepam | Valrelease | Roche, UK | |
| Levodopa, Benserazide | Madopar | ||
| Ciprofloxacin hydrochloride | Cifran OD | Ranbaxy, India | |
| Aluminium and magnesiun | Topalkan | Pierre Fabre Medicament, France | |
| Swellable system | Baclofen | Baclofen GRS | Sun Pharma, India |
| Gabapentin | Gabapentin GR | Depomed Inc, USA | |
| Metformin hydrochloride | Glumetza | ||
| Gas generating system | Ofloxacin | Oflin OD | Ranbaxy, India |
| Bio adhesive system | Rifaximin | Xifaxan | Lupin, India |
Table 3: Marketed GRDDS Formulations.
Conclusion
GRDDS is considered a formulative strategy to enhance the absorption and bioavailability of the active pharmaceutical ingredient by retaining it in the absorptive window. It is a popular technique that has been widely studied for the past few decades. It offers effective drug delivery by improving patient compliance and minizing dosing frequency. It is firmly concluded that GRDDS has the potential to enhance the therapeutic efficacy of drugs that exhibit active absorption in the gastric region. It is obvious that clinical assessments of GRDDS still lack a solid foundation. Various new gastroretentive approaches using combinations of techniques remain less explored. It is anticipated that further investigation will reveal the extended potential of GRDDS.
Acknowledgements:
The authors thank Karpagam Academy of Higher Education for their support.
Conflict of interest:
The authors declared no conflict of interests.
References
- Tiwari S, Batra N. Oral drug delivery system: A review. Am J Life Sci Res 2014;2(1):27-35.
- Stomach articles from across Nature Portfolio. Nature.
- Czinn SJ, Blanchard SS, Arora S. Developmental anatomy and physiology of the stomach. In: Pediatric Gastrointestinal and Liver Disease 2006;349-357.
- Daroff RB, Aminoff MJ. Encyclopedia of the neurological sciences. Academic press; 2014.
- Marinaganti RK, Bonthu S, Nagakanyaka DP, Neerukonda V, Sheik M, Shaik IP, et al. A comprehensive review on gastro retentive drug delivery system. Acta Chim Pharm Indica 2013;3(2):149-64.
- Jassal M, Nautiyal U, Kundlas J, Singh D. A review: Gastroretentive drug delivery system (grdds). Indian J Pharm Biol Res 2015;3(1):82-92.
[Crossref] [Google Scholar] [PubMed]
- Swetha S, Allena RT, Gowda DV. A Comprehensive review on gastroretentive drug delivery systems. Int J Pharm Biomed Res 2012;3:1285-93.
- Rathod HJ, Mehta DP, Yadav JS. A review on gastroretentive drug delivery systems. PharmaTutor. 2016;4(7):29-40.
- Gradowski T. Principles of anatomy and physiology. New York; John Wiley and Sons; 2002.
- Shinde S, Tadwee I, Shahi S. Gastro retentive drug delivery system: A review. Int J Pharm Res All Sci 2012;1(1):01-13.
- Salessiotis N. Measurement of the diameter of the pylorus in man: Part I. Experimental project for clinical application. Am J Surg 1972;124(3):331-3.
[Crossref] [Google Scholar] [PubMed]
- Vinchurkar K, Sainy J, Khan MA, Sheetal MA, Mishra DK, Dixit P. Features and facts of a gastroretentive drug delivery system-A review. Turk J Pharm Sci 2022;19(4):476-87.
[Crossref] [Google Scholar] [PubMed]
- Chauhan MS, Kumar A, Pathak K. Osmotically regulated floating asymmetric membrane capsule for controlled site-specific delivery of ranitidine hydrochloride: Optimization by central composite design. AAPS PharmSciTech 2012;13:1492-501.
[Crossref] [Google Scholar] [PubMed]
- Clarke GM, Newton JM, Short MD. Gastrointestinal transit of pellets of differing size and density. Int J Pharm 1993;100(1-3):81-92.
- Prajapati VD, Jani GK, Khutliwala TA, Zala BS. Raft forming system-An upcoming approach of gastroretentive drug delivery system. J Control Release 2013;168(2):151-65.
- Prinderre P, Sauzet C, Fuxen C. Advances in gastro retentive drug-delivery systems. Expert Opin Drug Deliv 2011;8(9):1189-203.
[Crossref] [Google Scholar] [PubMed]
- Shaha SH, Patel JK, Pundarikakshudu K, Patel NV. An overview of a gastro-retentive floating drug delivery system. Asian J Pharm Sci 2009;4(1):65-80.
- Awasthi R, Kulkarni GT. Decades of research in drug targeting to the upper gastrointestinal tract using gastroretention technologies: Where do we stand? Drug Deliv 2016;23(2):378-94.
[Crossref] [Google Scholar] [PubMed]
- Meyer JH, Gu Y, Elashoff J, Reedy T, Dressman J, Amidon G. Effects of viscosity and fluid outflow on postcibal gastric emptying of solids. Am J Physiol 1986;250(2):G161-4.
[Crossref] [Google Scholar] [PubMed]
- Gandhi A, Verma S, Imam SS, Vyas M. A review on techniques for grafting of natural polymers and their applications. Plant Arch 2019;19:972-8.
- Feldman M, Barnett C. Fasting gastric pH and its relationship to true hypochlorhydria in humans. Dig Dis Sci 1991;36:866-9.
[Crossref] [Google Scholar] [PubMed]
- Nimmo WS. Drugs, diseases and altered gastric emptying. Clin Pharmacokinet 1976;1(3):189-203.
[Crossref] [Google Scholar] [PubMed]
- Makwana A, Sameja K, Parekh H, Pandya Y. Advancements in controlled release gastroretentive drug delivery system: A review. J Drug Deliv Ther 2012;2(3):12-21.
- Pandey A, Kumar G, Kothiyal P, Barshiliya Y. A review on current approaches in gastro retentive drug delivery system. Asian J Pharm Med Sci 2012;2(4):60-77.
- Kagan L, Hoffman A. Selection of drug candidates for gastroretentive dosage forms: Pharmacokinetics following continuous intragastric mode of administration in a rat model. Eur J Pharm Biopharm 2008;69(1):238-46.
[Crossref] [Google Scholar] [PubMed]
- Sharma AR, Khan A. Gastroretentive drug delivery system: An approach to enhance gastric retention for prolonged drug release. Int J Pharm Sci Res 2014;5(4):1095.
- Reddy BV, Navaneetha K, Deepthi PS. Gastroretentive drug delivery system: A review. J Glob Trends Pharm Sci 2013;4(1):1018-33.
- Tripathi J, Thapa P, Maharjan R, Jeong SH. Current state and future perspectives on gastroretentive drug delivery systems. Pharmaceutics 2019;11(4):193.
[Crossref] [Google Scholar] [PubMed]
- Singh BN, Kim KH. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Release 2000;63(3):235-59.
[Crossref] [Google Scholar] [PubMed]
- Lopes CM, Bettencourt C, Rossi A, Buttini F, Barata P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int J Pharm 2016;510(1):144-58.
[Crossref] [Google Scholar] [PubMed]
- Kumar S, Jamil F, Rajput M, Sharma S. Gastro retentive drug delivery system: Features and facts. Int J Res Pharm Biomed Sci 2012;3(1):125-36.
- Chawla G, Gupta P, Koradia V. A means to address intestinal drug absorption. Pharm Technol 2003;27(2):50-68
- Sharma A, Goyal AK, Rath G. Development and characterization of gastroretentive high-density pellets lodged with zero valent iron nanoparticles. J Pharm Sci 2018;107(10):2663-73.
[Crossref] [Google Scholar] [PubMed]
- Rubinstein A, Friend DR. Specific delivery to the gastrointestinal tract. Polymeric site-specific Pharmacotherapy, Wiley, Chichester. 1994:282-3.
- Ikura H, Suzuki Y, Nagai T, Machida Y. Cellulose ether, polyacrylic acid, foaming agent, drug; United States Patent US 4777033 A; 1988.
- Umezawa H, inventor; Microbial Chemistry Research Foundation, assignee. Pepstatin floating minicapsules. United States patent US 4101650; 1978.
- Mishra J, Kumar DA. Recent advances in gastro retentive drug delivery system: A review. Mintage J Pharm Med Sci 2013;2(2):25-7.
- Thapa P, Jeong SH. Effects of formulation and process variables on gastroretentive floating tablets with a high-dose soluble drug and experimental design approach. Pharmaceutics 2018;10(3):161.
[Crossref] [Google Scholar] [PubMed]
- Narang N. An updated review on: Floating drug delivery system (FDDS). Int J Appl Pharm 2011;3(1):1-7.
- Vinod KR, Vasa S, Anbuazaghan S, Banji D, Padmasri A, Sandhya S. Approaches for gastrotentive drug delivery systems. Int J Appl Biol Pharm Technol 2010;1(2):589-601.
- Smart JD. The basics and underlying mechanisms of mucoadhesion. Adv Drug Deliv Rev 2005;57(11):1556-68.
[Crossref] [Google Scholar] [PubMed]
- Murphy CS, Pillay V, Choonara YE, du Toit LC. Gastroretentive drug delivery systems: Current developments in novel system design and evaluation. Curr Drug Deliv 2009;6(5):451-60.
[Crossref] [Google Scholar] [PubMed]
- Ito R, Machida Y, Sannan T, Nagai T. Magnetic granules: A novel system for specific drug delivery to esophageal mucosa in oral administration. Int J Pharm 1990;61(1-2):109-17.
- Camilleri M, Iturrino J, Bharucha AE, Burton D, Shin A, Jeong ID, et al. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil 2012;24(12):1076-e562.
[Crossref] [Google Scholar] [PubMed]
- Shah SH, Patel JK, Patel NV. Stomach specific floating drug delivery system: A review. Int J Pharm Tech Res 2009;1(3):623-33.
- Mandal UK, Chatterjee B, Senjoti FG. Gastro-retentive drug delivery systems and their in vivo success: A recent update. Asian J Pharm Sci 2016;11(5):575-84.
- Ravichandiran V. Gastroretentive drug delivery systems: A review. J Pharm Res 2011;4(9):3232-6.
- Devi P, Rajamanickam V. The recent developments on gastric floating drug delivery systems: An overview. Int J Pharm Tech Res 2010;2(1):524-34.
- Lin HL, Chen LC, Cheng WT, Cheng WJ, Ho HO, Sheu MT. Preparation and characterization of a novel swellable and floating gastroretentive drug delivery system (sf GRDDS) for enhanced oral bioavailability of nilotinib. Pharmaceutics 2020;12(2):137.
[Crossref] [Google Scholar] [PubMed]
- Kim S, Hwang KM, Park YS, Nguyen TT, Park ES. Preparation and evaluation of non-effervescent gastroretentive tablets containing pregabalin for once-daily administration and dose proportional pharmacokinetics. Int J Pharm 2018;550(1-2):160-9.
[Crossref] [Google Scholar] [PubMed]
- Shishu, Gupta N, Aggarwal N. Stomach-specific drug delivery of 5-fluorouracil using floating alginate beads. AAPS Pharm Sci Tech 2007;8:E143-9.
[Crossref] [Google Scholar] [PubMed]
- Zhu X, Qi X, Wu Z, Zhang Z, Xing J, Li X. Preparation of multiple-unit floating-bioadhesive cooperative minitablets for improving the oral bioavailability of famotidine in rats. Drug Deliv 2014;21(6):459-66.
[Crossref] [Google Scholar] [PubMed]
- Prinderre P, Sauzet C, Fuxen C. Advances in gastro retentive drug-delivery systems. Expert Opin Drug Deliv 2011;8(9):1189-203.
[Crossref] [Google Scholar] [PubMed]
- Pawar VK, Kansal S, Asthana S, Chourasia MK. Industrial perspective of gastroretentive drug delivery systems: Physicochemical, biopharmaceutical, technological and regulatory consideration. Expert Opin Drug Deliv 2012;9(5):551-65.
[Crossref] [Google Scholar] [PubMed]
 .
.