- *Corresponding Author:
- K. N. Killari
Department of Pharmacology,
AU College of Pharmaceutical Sciences,
Andhra University,
Visakhapatnam 530003,
India,
E-mail: kishorenaidu.killari@gmail.com
| Date of Received | 03 June 2020 |
| Date of Revision | 08 March 2021 |
| Date of Acceptance | 01 July 2021 |
| Indian J Pharm Sci 2021;83(4):634-647 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Polyalthia longifolia (Annonaceae family) is native to the drier areas of India and is locally called “Ashoka.” It is also cultivated in Southeast Asia, Africa, Australia and New Zealand. Polyalthia longifolia is also known as Buddha tree, mast tree, cemetery tree, false Ashoka or green Champa. Generally, Polyalthia longifolia is viewed as a street tree because of its effectiveness in combating noise pollution. Macroscopically, the versatile Polyalthia longifolia can reach over 15.0 m high with symmetrical pyramidal growth and weeping pendulous branches. The term Polyalthia is derived from Greek roots, with “poly” meaning many and “althia" meaning cure, indicating that this plant has been used to treat various diseases/disorders. In traditional and indigenous systems of medicine, Polyalthia longifolia has been commonly used in the treatment of fever, helminthiasis, diabetes and various cardiac problems. Pharmacological investigations have shown that Polyalthia longifolia possesses significant biological and pharmacological activity, which may include antibacterial, antifungal, antitumor, anti-ulcer, antidiabetic and antioxidant properties. To date, more than 30 studies have analyzed extracts from bark, leaves, roots, seeds, etc. of the plant and reported a total of approximately 100 compounds, including steroids, flavonoids, clerodane diterpenes, cleroda-oic acids and alkaloids. In context of the broad medicinal potential of Polyalthia longifolia, this review compiles a detailed exploration of currently available knowledge of the phytochemical and their pharmacological properties of Polyalthia longifolia. Its potential applications in the treatment of various conditions are also discusseds.
Keywords
Polyalthia longifolia, pharmacological applications, therapeutic applications, phytochemicals
The Polyalthia genus belongs to Annonaceae family, which consists of approximately 350 species distributed mostly in the regions of Southeast Asia, Africa, Australia and New Zealand [1]. Polyalthia longifolia (P. longifolia) is also known as Buddha tree, mast tree, cemetery tree, false Ashoka or green Champa. Generally, P. longifolia is viewed as a street tree because of its effectiveness in combating noise pollution [2]. Macroscopically, the versatile P. longifolia can reach over 15.0 m high with symmetrical pyramidal growth and weeping pendulous branches. There are two varieties of P. longifolia, namely “var. pendula” which has a straightslim trunk and short branches and “var. angustifolia” which has wide spreading branches forming a pyramidal crown with grey and smooth bark [1].
The term Polyalthia is derived from Greek roots, with “poly” meaning many and “althia" meaning cure, indicating that this plant has been used to treat various diseases/disorders. In Ayurveda, herbal preparations of P. longifolia have been mainly used to treat duodenalulcers while decoctions of the plant have been used in the treatment of fever, diabetes and skin diseases in various traditional medicine systems [1,3,4]. Furthermore, the bark and leaves of P. longifolia have been used to treat microbial infection, inflammation, diabetes and multiple diseases of the digestive system [1,3].
Earlier reviews on the genus Polyalthia [3,4] and plant P. longifolia [1]summary the general phytochemistryand pharmacological activities of Polyalthia extracts. To our knowledge, there is no review on the therapeutic applications of chemical constituents of P. longifolia, till date. Therefore, in the current study, we aim to provide an overview of the phytochemistry and their pharmacological investigations to describe the many uses of this medicinal plant.
Phytochemistry
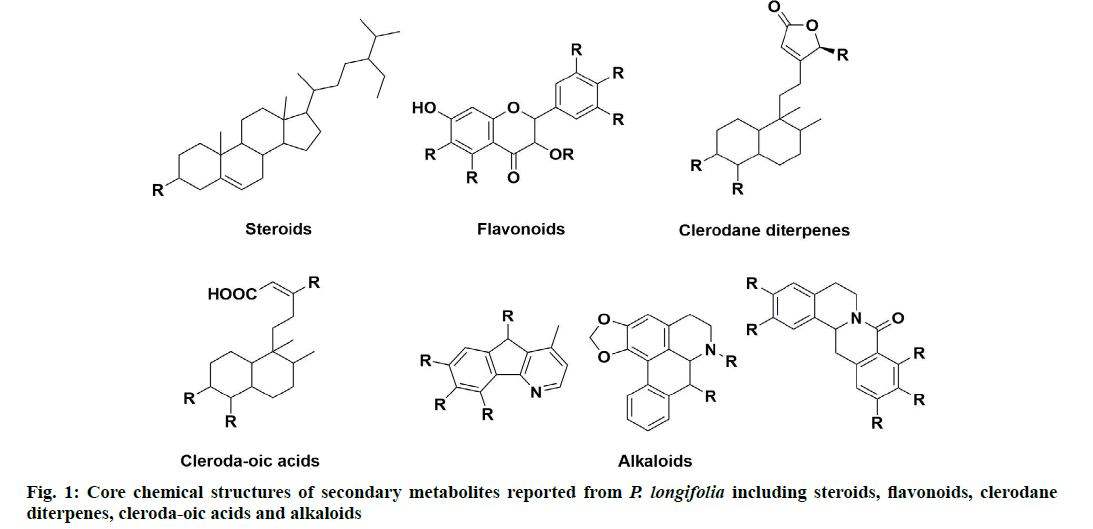
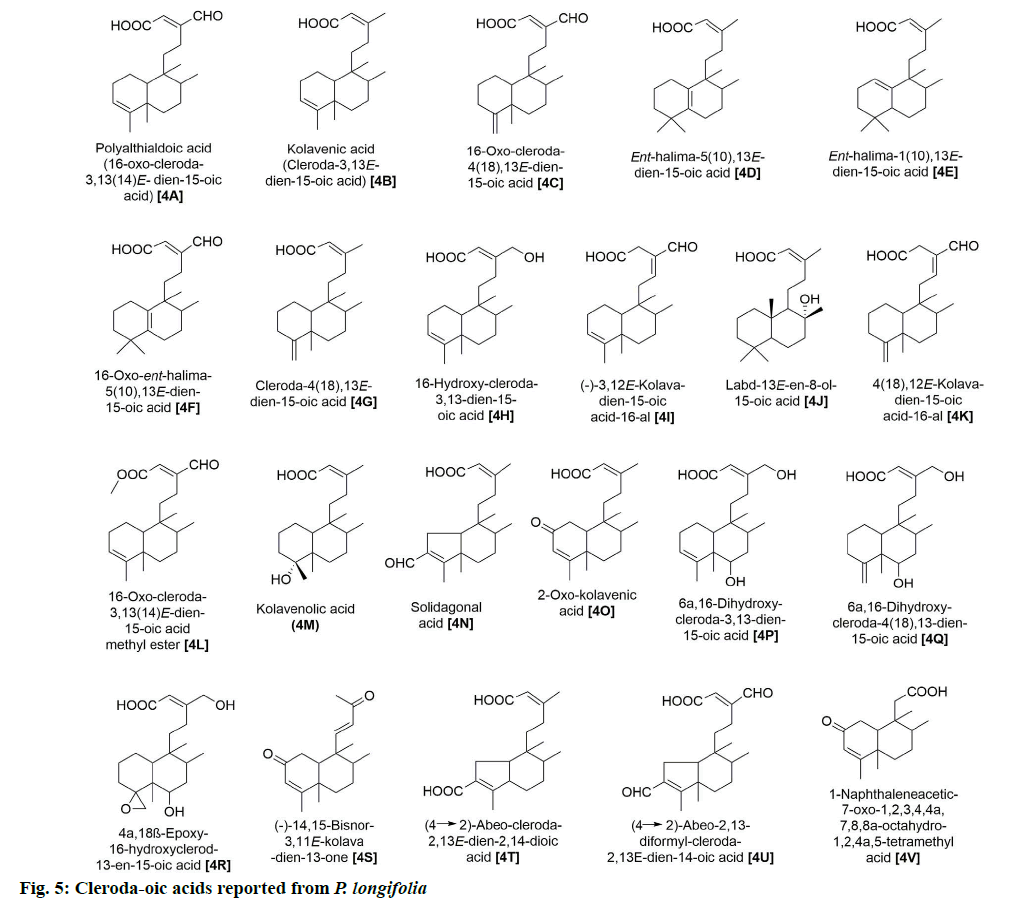
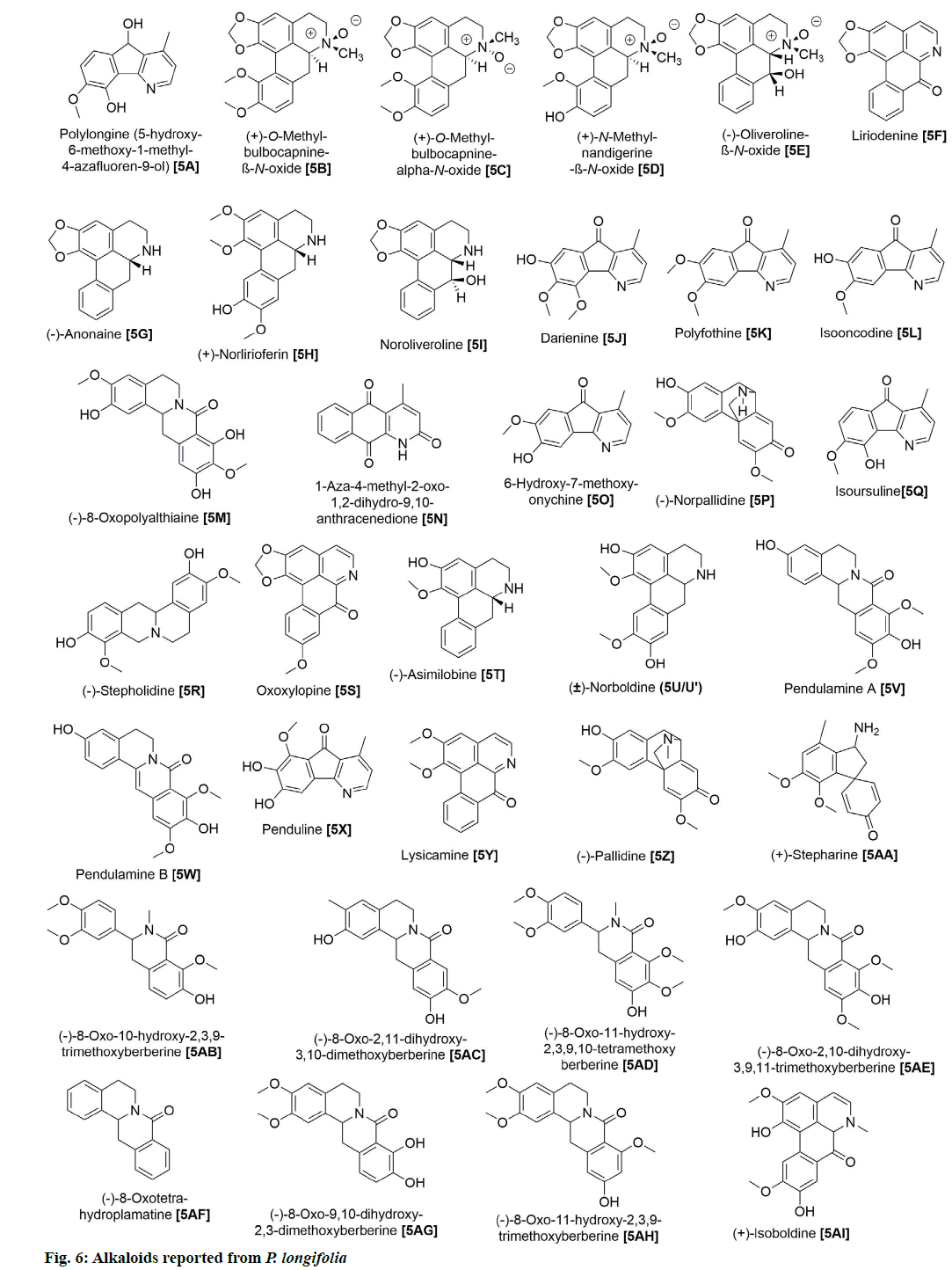
P. longifolia extract consists mostly of steroids,flavonoids, clerodane diterpenes, cleroda-oic acids and alkaloids (fig. 1) [5]. To date, more than 30 studies have analyzed extracts from bark, leaves, roots, seeds, etc., of the plant and reported a total of approximately 100 compounds (Table 1). The main compounds of the plant that have been identified and reported in multiple studies are beta (β)-sitosterol (1A), leucocyanidin (2A), proanthocyanidin (2B), 16 Alpha (α)-hydroxycleroda-3,13(14)Z-dien-15,16-olide (3A), (4→2)-abeo-16(R and S)-2,13Z-kolava-dien-15,16-olide-3-al (3K), 16-oxo-cleroda-3,13(14)E-dien-15-oic acid (4A), cleroda-3,13E-dien-15-oic acid (4B), liriodenine (5F), solidagonal acid (4N) and labd-13E-en-8-ol-15-oic acid (4J) (Table 1).
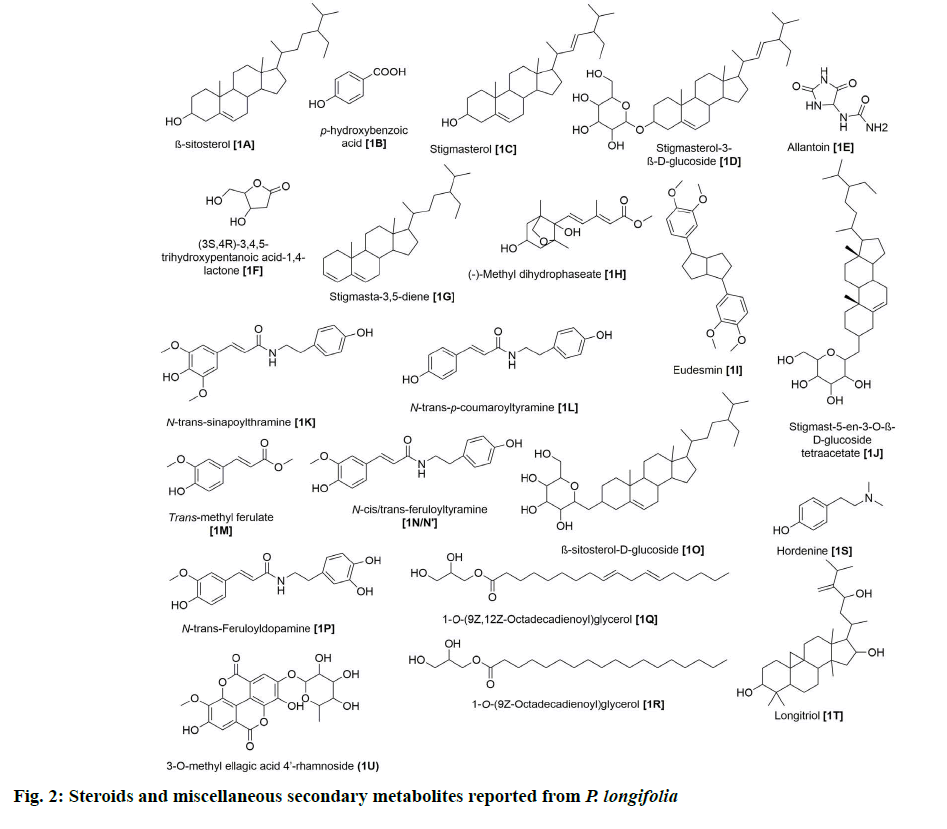
Seven steroids have been isolated from this plant (fig. 2). 1A was one of the earliest steroids to be identified from the ethanolic extract of stem bark [6].
Afterward, several other groups analyzed extracts from leaves [7,8], stems [9,10], roots [11] and bark [12] and identified six others, namely stigmasterol (1C), stigmasterol-3-β-D-glucoside (1D), stigmasterol-3,5-diene (1G), stigmast-5-en-3-O-β-D-glucoside tetraacetate (1J), β-sitosterol-D-glucoside (1O) and longitriol (1T). None of these compounds have been reported for their bioactivities.
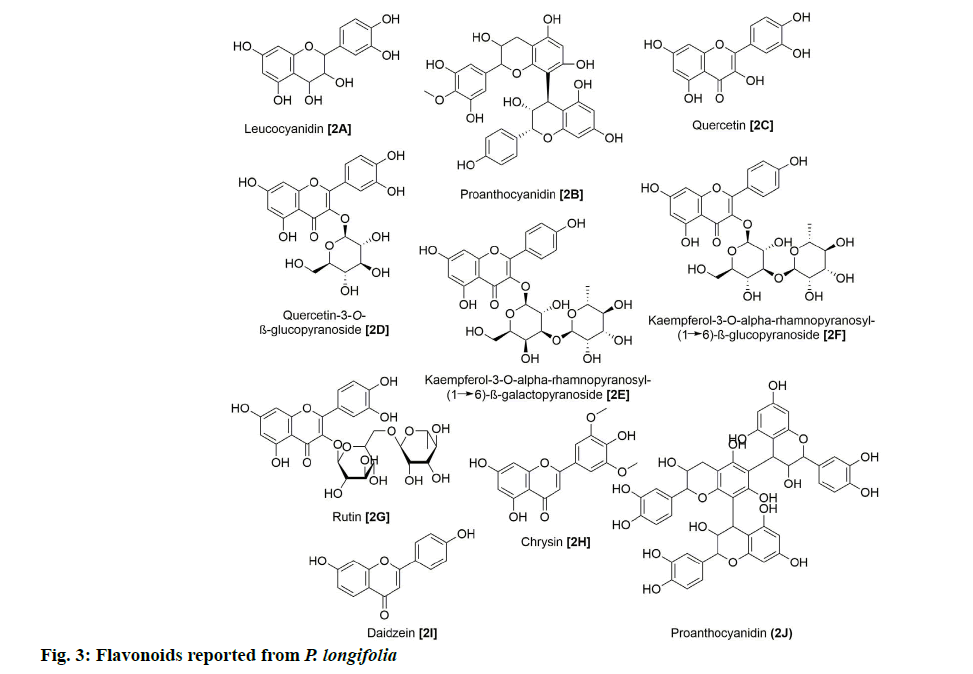
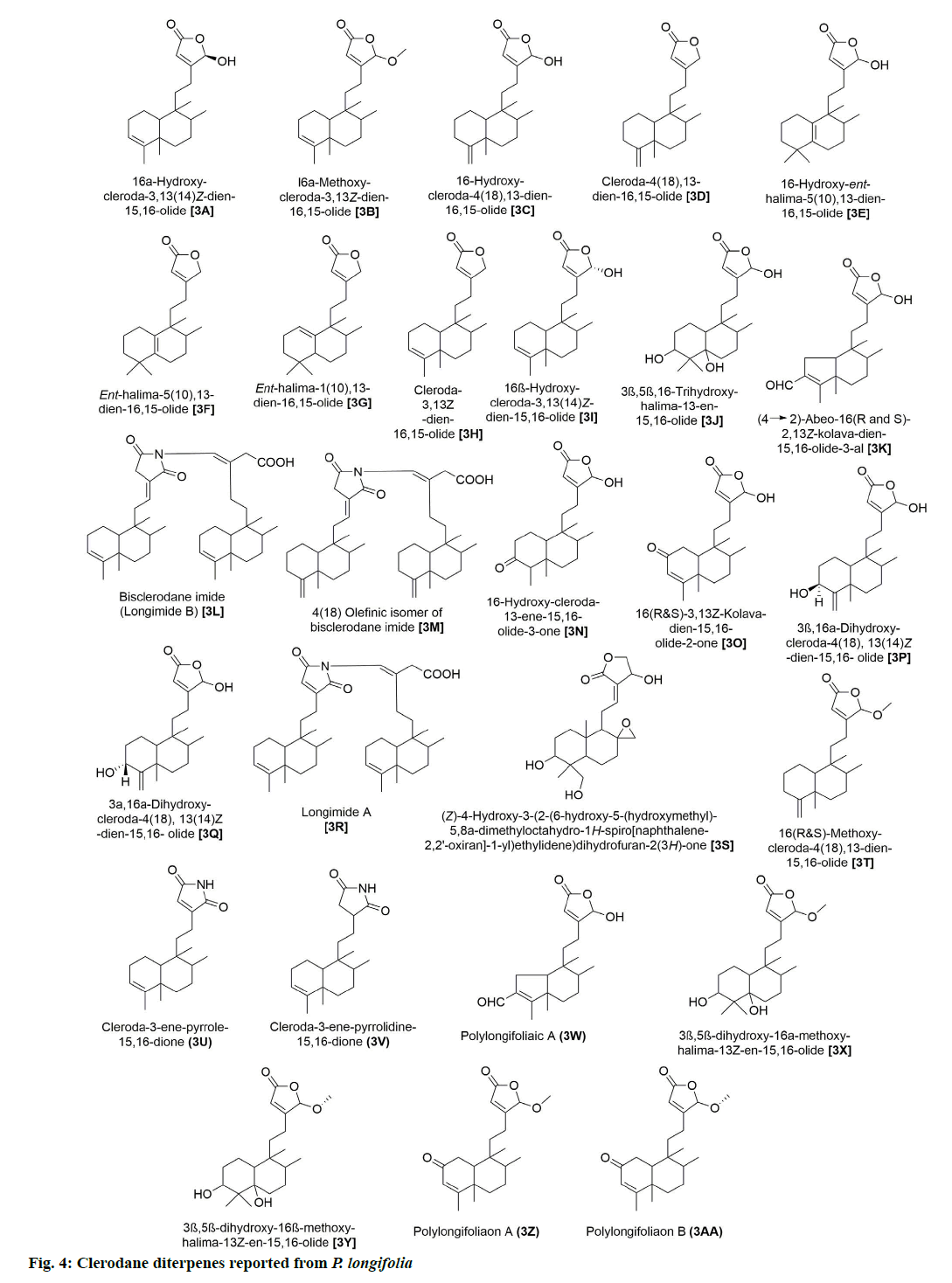
In addition, 9 flavonoids have been isolated and reported from P. longifolia (fig. 3). Of these, 2A and 2B were among the earliest flavonoids identified from the stem bark extract of this plant [6]. Later, chemical examination of butanol fraction of leaf extract yielded 5 known compounds (2C, 2D, 2E, 2F and 2G) with good scavenging of free radicals [13]. The other study on the ethanolic extract of P. longifolia leaves, using thin-layer chromatography (TLC) and reverse phase high-performance liquid chromatography (RP-HPLC), identified 3 more flavonoids (2G, 2H and 2I), which showed antimicrobial, antioxidant and anti-cancer potential [14]. 27 clerodane diterpenes have also been isolated from P. longifolia (fig. 4). Two major compounds that have been repeatedly identified in the extract of leaves, stem bark, root bark and berries of this plant were 3A and 3K (Table 1). The remaining compounds were reported by several other studies [7,8,22,23,12,15–21].
Moreover, 22 cleroda-oic acids with diverse bioactivities have also been identified (fig. 5), of which 4A, 4B, 4N and 4J have been repeatedly reported (Table 1). These compounds have been noted for their antifeedant [24], antioxidant [25], antibiotic [19,20,26] and anti-cancer activities [8,21,27].
| Part used | Solvent Used | Isolated compound(s) | Biological activity | Reference |
|---|---|---|---|---|
| Bark | Petroleum ether | 3B | - | [15] |
| Methanol | 1A:1C (3:1), 1D:1O (1:4), 1H-N/N’, 3A, 3C, 3K, 3N-O, 4J, 4L-N, 5A, 5F, 5J, 5R, 5Y, 5Z, 5AA | 3A, 3C and 3O showed significant cytotoxicity activity and 4L showed better anti-inflammatory activity, compared to other compounds. | [12] | |
| 3S | - | [23] | ||
| Berries | Methanol | 1E | - | [19] |
| Leaves | Acetone | 3A, 4A | Both exhibited significant antifeedant activity | [24] |
| Butanol fraction | 2C-G, IE | Only 2C, 2D, and 2G showed significant antioxidant activity compared to ascorbic acid. | [13] | |
| Ethanol | 1S, 3A, 3K, 3P, 4A, 4I-J, 4S, 5F-G, 5T, 5AI | Among all, 3A was found to be most potent agent (MIC = 6.25 μg/mL against S. aureus and Sporothrix schenckii). | [20] | |
| 3A | 3A was found to be a potential antileishmanial activity. | [30] | ||
| 1T, 3L, 3R | 1T and 3R were active against C33A cell lines. | [8] | ||
| 2G-I | Only 2G showed significant antibacterial, antiradical, and cytotoxicity activity compared to the standards. | [14] | ||
| 3A, 3P, 3W, 4A | Only 3A showed pronounced lipid lowering activity. | [31] | ||
| Ethyl Acetate | 3A, 4A-B | 4A and 4B had better apoptotic potentiality against HL-60 cells than 3A. | [32] | |
| Hexane fraction | 3A, 3P-Q, 4A | All are effective cytotoxic agents. | [21] | |
| Methanol | 5A-H | - | [28] | |
| 1A-C, 3A, 3J-K, 4B, 4H-J, 5F-G, 5M-U/U’ | Only 3A, 5Q, and 5G were tested for cytotoxicity and found to be active against HA59T cells. | [7] | ||
| 4H | 4H significantly inhibited the release of elastase in FMLP/CB-activated human neutrophils (IC50 = 3.30±0.48 μM). | [25] | ||
| 3A | 3A exhibited significant anti-MRSA activity compared to a β-lactam antibiotic. | [33] | ||
| 4V | 4V did not exhibit significant inhibitory effect against HL-60 cells. | [34] | ||
| Leaves and berries | Methanol | 3A, 3K, 3O-P, 4A, 4L, 4O | Except 4L and 4O, all are active antimicrobial agents with MIC values ranging between 7.8-500 μg/mL. | [19] |
| Root | Methanol | 1D-E, 4B, 5Q, 5V-X | Only 5V and 5W showed good antimicrobial activity with MIC values of 0.02-20 μg/mL. | [11] |
| Root bark | Methanol | 3L, 3M, 4B, 4I, 4K, 5F, 5Y | At 30 mg/kg, only 4B produced a 22% fall in the MABP. | [18] |
| 3A, 4B | 3A had higher antimicrobial potential than 4B. | [19] | ||
| Root-wood | Methanol | 4B, 4N | Both 4B and 4N found to have mild antimicrobial activity. | [19] |
| Seeds | Hexane | 3A, 4A | Both 3A and 4A showed significant antimicrobial activity as compared to nystatin and Dithane M-45. | [26] |
| Methanol | 3A, 3C, 3Q, 4A, 4H | 3A, 3C, and 3Q were exhibited to be potent dual inhibitors towards COX-1/2 and LOX enzymes | [42] | |
| 3C, 3Q | Both exhibited potent xanthine oxide inhibitory activity | [56] | ||
| Stem | Ethanol | 1F, 5F | Only 1F showed significant antibacterial activity compared to amphicilin. | [9] |
| Methanol | 1D:1O (1:2), 1P-R, 4A, 4O-R, 5M, 5AB-AH | Among all, 4A exhibited cytotoxicity against A549 and MCF-7 cell lines. | [10] | |
| Stem bark | Bioassay guided | 3A, 4A, 4B | 4A found to be most efficient in inhibition of crown gall tumours. | [27] |
| Ethanol | 1A, 2B, 2C | - | [6] | |
| Hexane | 3A, 3C-H, 4A-G | - | [16] | |
| Petroleum ether | 4A-B, 3I | All showed significant antibacterial activity compared to kanamycin. | [17] | |
| Stem parts | Methanol | 5E, 5F, 5I-L | Among all, only 5F showed potent cytotoxicity. | [29] |
| Unripe fruit | Methanol | 3A, 3C, 3J, 3T, 4A, 4N, 4T-U | At 10 μM, compounds 3A and 4A exhibited promising NO inhibitory activity. | [22] |
Note: “-”: Not tested for any biological activity; A549: Lung adenocarcinoma; C33A: Cervical carcinoma cell lines; FMLP/CB: formyl-methionyl-leucyl-phenilalanine/cytochalasin B; HA59T: Hepatoma cells; HL-60: Human leukemia cell line; IC50: Half maximal inhibitory concentration; MABP: Mean arterial blood pressure; MCF-7: Human breast cancer cell line; MIC: Minimum inhibitory concentration; MRSA: Methicillin-resistant S. aureus; NO: Nitric oxide
Table 1: Secondary Metabolites Isolated and Reported from Different Parts of P. longifolia and their Biological Importance
Lastly, 35 alkaloids have also been isolated while exploiting different parts of this plant (fig. 6). Among those, 5F was repeatedly found in various parts of the plant [7,12,18,20,28,29]. Bioactivity studies of these alkaloid compounds revealed the cytotoxicity properties of 5F [29], 5A and 5G [7] and the antibacterial capacities of 5V, 5W and 5X [11].
Antimicrobial Activity
Bioassay of the metabolites isolated from P. longifolia extracts has revealed several with strong antibacterial activity, including 1F [9], 2G [14], 3A [19,20,26], 4A [17,26], 5V, 5W and 5X [11].
Rashid et al. [17] performed an isolation work on the petroleum ether extract of P. longifolia stem bark and discovered 3 metabolites comprising 2 cleroda (4A and 4B) and a clerodane diterpene (3I). The total extract and metabolites were then tested for their antibacterial activities against 7 gram-positive (Gram+ve) and 12 gram-negative (Gram-ve) bacterial strains and 7 fungal strains and it was found that both the extract and those metabolites had significant antimicrobial activity. Among the 3 compounds, 4A had the highest activity against most of the tested microorganisms and had better inhibition potential as compared to kanamycin on several strains; diterpene 3I had the highest activity against Bacillus polymyxa and Shigella shiga; and 4B was found to be the least active [17].
Murthy et al. [26] analyzed the extract of seeds and found 2 metabolites, 3A and 4A. Antibacterial and antifungal activities of 3A and 4A were checked for 9 Gram+ve and 7 Gram-ve bacterial strains and 8 fungal strains using the two fold serial dilution method and the paper disc method, respectively. Similar to the previous study, this study also revealed significant antibacterial activity of MTT f 4A. Moreover, 4A has the ability to act against fungi Candida and Saccharomyces with comparable potency as compared to the standard fungicides dithane M-45 and nystatins, respectively. On the other hand, 3A was found to be highly active against most Gram-ve bacteria, such as Escherichia coli (E. coli), Klebsiella aerogenes, Pseudomonas species and Sarcina lutea and one Gram+ve species (Bacillus) for which it showed a stronger effect than standard gentamycin [26].
The antibacterial activity of 3A and/or 4A has also been reported by several later studies [19,20,33]. Specifically, Faizi et al. [11] isolated 10 metabolites (1E, 3A, 3K, 3O, 3P, 4A, 4B, 4L, 4N and 4O) from methanolic extracts of different parts of P. longifolia [30-34] and tested them for antifungal and antibacterial potential against 11 fungal and 21 bacterial strains, using the disc diffusion technique. Among those, 5 clerodanes (3A, 4A, 3K, 3O and 3P) were found to be bioactive antimicrobial compounds and 3A emerged as the best one [19]. Later, Gupta et al. successfully isolated 7 metabolites (3A, 3K, 3P, 4A, 4I, 4J and 4S) from this variant. All these metabolites were tested against 6 fungal and 4 bacterial strains and most of the tested compounds were found to be active against E. coli. Of those, diterpene 3A was most active against Staphylococcus aureus (S. aureus) and fungi Sporothrix schenckii.
Additionally, in vitro and in vivo assays revealed 3A’s ability to work against methicillin-resistant S. aureus with a minimum inhibitory concentration (MIC) of 15.625–31.25 mg/l; while the MICs were significantly lower for the reference and clinical isolated strains of S. aureus. The combination of 3A with 7.5 % sodium chloride (NaCl) caused a significant decrease in microbial count within 24 h, signifying the loss of the salt tolerance capacity of S. aureus. Furthermore, application of 3A in infected mice significantly dropped the systemic microbial load in spleen, liver, lung, blood and kidney tissues and it did not show any significant toxicity at 100 mg/kg dose [33]. Bhattacharya et al. [35] obtained metabolite 3A from methanolic extract of P. longifolia leaves and reported its antifungal activity against Cryptococcus neoformans (a human pathogen), Candida albicans and Neurospora crassa (saprophyte) [35]. The mechanism of action of 3A in C. albicans was suggested to be compromised cellmembrane permeability and/or cell wall structures or reactive oxygen species (ROS) generation as antifungal mechanisms [35]. Zhang et al. [36] isolated 3A, 4A and 4B from stem bark extracts of P. longifolia and tested for antibacterial activity against methicillin-resistant Staphylococcus aureus and S. aureus. Of these, 4B wasreported to have better activity than 3A [36].
Faizi et al. [19] analyzed an ethanolic extract of stems of P. longifolia and the obtained metabolite 1F. This metabolite was tested for its antibacterial, antifungal, anti-mycobacterial and anticandidal activities using the disc diffusion method and was reported for its weak activity against these organisms [11]. Contemporaneously, this group investigated the antibacterial activity of petroleum ether and methanolic extract of roots of P. longifolia and its metabolites (5V, 5W and 5X) andshowed that methanolic extract had better antibacterial activity as compared to petroleum ether extract and 5V and 5W were more active than 5X [9]. Jain et al. [37] isolated 1U from a butanol fraction of P. longifolia stem bark and both were tested against aerobic bacterial strains. 1U exhibited higher antibacterial potential than the butanol fraction against almost all examined bacterial strains, but the potency was lower compared to standards (erythromycin, vancomycin, oxacillin and ciprofloxacin) [37].
Later, two flavonoids (F1 and F2, names not mentioned) were isolated from the ethanolic extract of bark of P. longifolia and reported for their antimicrobial andantifungal activities. F1 showed a strong inhibition against B. subtilis and a moderate inhibition against E. coli, B. thuringiensis and P. aeruginosa; whereasF2 showed moderate activity against E. coli, B. thuringiensis, B. subtilis and P. aeruginosa [38].
Multiple extracts of different parts of P. longifolia were also tested for their antimicrobial activity. Accordingly, the petroleum ether extract was displayed to be more effective in antimicrobial activity than other solvent extracts [39,40].
Anti-Inflammatory Activity
Several studies have investigated the anti-inflammatory activity of compounds isolated from extracts of P. longifolia and found 2 clerodane diterpenes (3Aand 3K) and 3 cleroda-oic acids (4A, 4L, 4H) to be active. 4L and 3K were the first compounds isolated from this plant to be reported for inhibitory activity against formyl-L-methionyl-L-leucyl L-phenylalanine/ cytochalasin B (fMLP/CB)-induced superoxide generation by neutrophils [12]. After that, Chang et al. [12] established the anti-inflammatory functions of 4H in isolated human neutrophils. 4H induced the discharge of elastase in fMLP-activated human neutrophils in a concentration-dependent mode. Its suppressive effects on degranulation and human neutrophil respiratory burst were at least partly facilitated by inhibition of protein kinase B (PKB/AKT), calcium and p38 signaling pathway [25]. Later, Wu et al. [28] isolated and investigated the inhibitory effect of 3A and 4A on nitric oxide production in lipopolysaccharide (LPS)-stimulated macrophage (RAW 264.7) cells by the Griess reaction and half maximal inhibitory concentration (IC50) values of 3A and 4A were found to be ~1 µm [22]. Similarly, Shih et al. found that 3A reduced the expression of cyclooxygenase 2 (COX-2), inducible nitric oxide synthase (iNOS), nuclear factor kappa-light-chain-enhancer of activated B cells protein 65 (NF-κBp65) and a glycosylated 91-kDa glycoprotein (gp91phox), while it boosted the expression of HO-1, an anti-inflammatory and cytoprotective enzyme. Moreover, 3A reduced LPS-activated microglia-induced cell death, suggesting 3A as a potential candidate to treat inflammation related to microglia and neuronal cell death [41].
More recently, our group identified five clerodane diterpenes (3A, 3C, 3Q, 4A and 4H) from methanolic extract of seeds of P. longifolia and reported on their anti-inflammatory activities. Of these, compounds 3A, 3C and 3Q were exhibited to be potent dual inhibitors towards COX-1/2 and lipoxygenases (LOX) enzymes with IC50 values lower than or similar to those of the indomethacin and allopurinol [42].
Cytotoxicity
Several compounds isolated from this plant have also been tested for their cytotoxicity and a few of them, including 3A and 4A, have been repeatedly reported for their anti-cancer properties. Wu et al. [43] investigated the bioassay-guided isolation of methanolic extract of P. longifolia stem parts using the KB cytotoxicity assay.Specifically, metabolite 5F obtained from this extract demonstrated great potential cytotoxicity against adenocarcinomic human alveolar basal epithelial cell (A549), tebu-bio's knockdown (KB), human cancer cell lines (HCT-8), mouse lymphocytic leukemia cell line (L-1210) and leukemia cell (P-388) cells [29]. In another study, 3 metabolites (3A, 4A and 4B) obtained from stem bark of P. longifolia were tested for cytotoxicity by brine shrimp bioassay. These compounds were found to have a strong anti-growth capacity against crown gall tumors (A-549, MCF-7 and HT-29) on potato discs [27].
Later, Chen et al. [29] assessed the petroleum ether extract of leaves of P. longifolia and reported that several of the isolated compounds (3A, 5A and 5G) were highly active against different cancer cell lines via 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide, a tetrazole (MTT) assay. Particularly, 5A was active against Hepatoma cell line (HA59T) while 3A and 5G displayed significant cytotoxicity against adenocarcinoma gastric cell line (AGS) and HA59T and AGS, Colorectal Adenocarcinoma cell line (DLD1), human hepatoma cell lines (HepG2) and HA59T, respectively [7]. Similarly, another group isolated 13 metabolites (1L, 1N’, 3A, 3C, 3K, 3O, 4L, 4N, 5A, 5F, 5R, 5Y and 5AA). All these compounds were tested against breast cancer cell line (MCF-7) and an epithelial, human breast cancer cell line (MDA-MB-231), while 3A, 3C, 3O, 4L and 4J were further tested on human hepatoma (Hep 3B and Hep G2) cell lines. Their results exposed significant inhibitory activity against Hep 3B and Hep G2 of 3A, 3C and 3O [12]. Misra et al. [30]. isolated 3 compounds 3A, 3P and 3Q. The activity of 3A on J774A.1 macrophages was assessed by MTT reduction assay and no cytotoxic effect was found even at 200 mg/ml concentration [30]. Wu et al. [43] obtained 12 diterpenes (3B, 3K, 3O, 3P, 3Q, 3W, 3X, 3Y, 3Z, 3AA, 4O and 4L) from the methanolic extract of unripe fruit of P. longifolia and tested for cell viability in human neuroblastoma (SKNMC) cells using amyloid-beta (Aβ)-induced neurotoxicity. Of these, compounds 3P, 3O, 3W, 3AA and 4O improved the ability of SKNMC cells. Among the active compounds, 3W and 3AA revealed the most powerful activity toward SKNMC cells. Additionally, the isolated compounds were found to have strong acetylcholinesterase inhibitory actions in TLC bioautographic assay [43].
In addition, several other compounds have been isolated and tested for their cytotoxicity on different cell lines [14,21,32]. 3P and 3Q were examined for their growth inhibitory actions on ovarian teratocarcinoma cell line (PA1), MCF-7, KB and cervical cancer cells (C33A) using MTT assay and both of them exhibited good inhibitory activity against C33A and PA1 and moderate inhibitory activity against the remaining cell lines. They have also been tested against Vero (African green monkey kidney) cell lines, in which 3Q was more active than 3P [21]. 2G was isolated from ethanolic extract of P. longifolia leaves and examined against A549 using MTT-based cytotoxicity activity and the cytotoxic concentration (CTC50) values of ethanol extract, preparative thin layer chromatography (PTLC) isolate and 2G were found to be 210±5.14, 230±10.62 and 270±3.001 μg/ml, respectively [14].
Compounds 3A, 4A and 4B isolated from ethyl acetate extract of P. longifolia leaves were tested for cytotoxicity on promyelocytic cell line (HL-60) using MTT assay. The IC50 values of 3A, 4A and 4B were reported to be 25.0, 12.5 and 6.25 μm, respectively [32]. Compound 3A exhibited a potential effect on cell viability on mouse neuroblastoma (N18) and rat glioma (C6) cell lines by MTT assay and cell cycle arrest in the resting phase- growth 1 phase (G0-G1) phase. Treatment with compound 3A raised pro-apoptotic proteins and reduced anti-apoptotic proteins [44]. The involvement of 3A-induced autophagy in N18 and C6 via activation of active extracellular-signal-regulated kinase 1/2 (pERK 1/2) and phosphorylated-p38 mitogen-activated protein kinase expression (P-p38 MAPK) pathway has also been studied. It was found that 3A significantly induced p-ERK-1/2 and P-p38 MAPK proteins, implying that 3A acts as a potential anticancer compound that employs its action via inducing ROS-mediation [45]. The IC50 values of compounds 4V and 3A isolated from ethyl acetate fraction of P. longifolia leaves were 71.1 and 44.8 μm, respectively [46]. Multiple compounds (1D:1O (1:2), 1P-R, 4A, 4O-R, 5M and 5AB-AH) have also been successfully isolated by Lee et al. [11], but among those, only 4A exhibited cytotoxicity against A549 and MCF-7 cancer cells [10].
Recently, Liu et al. investigated the antitumor activity of 3A in two human clear-cell type renal (786O and A498) carcinoma lines and stated that 3A induced cell necrosis via ROS overproduction [47]. Also, Chen et al. [48] investigated the governing mechanisms of 3A-induced programmed cell death in 786O and A498 cells and reported that 3A-induced cell phase arrest, focal adhesion complex break down and the inactivation of signaling pathways (migratory-related) to prompt apoptosis [48].
In addition, two fractions (F1 and F2) were isolated from seed proteins of P. longifolia using trypsin. The MTT assay revealed a significant cytotoxicity of F2 against immortal cell line (HeLa) and A-549 cells at 30 and 10 µg/ml, respectively. Further, flow cytometry analyses found that F2 augmented apoptotic cells in sub-G0 phase of HeLa and A549 at 30 and 10 µg/ml, respectively. This suggested that F2 peptide is an active inducer of programmed cell death of cancer cells [49].
Antioxidant Properties
The ability of 4H to scavenge O2·− and free radicals was assayed using 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging test and cell-free xanthine oxidase system. Up to 10 μm concentration, 4H failed to modify DPPH and water soluble tetrazolium salts-1 (WST-1) reduction, indicating that the inhibitory effect by O2·− release shown by 4H occurs via scavenging of free radicals and O2·−. In addition, 4H did not induce the elimination of O2·− by superoxide dismutase (0.5 U/ml), meaning that 4H did not have superoxide anion-scavenging or antioxidant activity [25]. Sashidhara et al. isolated and tested three compounds 2C, 2D and2G for scavenging of free radicals and reported that 2C had the highest antioxidant activity (4.10 mM) of all, followed by 2G (2.38 mM) and 2D (1.91 mM) [13]. Additionally, 2G metabolite (IC50 value=14.67 ±0.023 μg/ml) from the ethanolic extract of leaves of P. longifolia was reported for its antioxidant ability [14].
Two flavonoids (F1 and F2) obtained from the ethanol (95 %) extract of bark of P. longifolia were tested for DPPH radical scavenging, nitric oxide scavenging, metal chelating and reducing power activities. The results showed that F1 had stronger antioxidant activity than F2 [38]. Chen et al. [43] isolated 2J from acetone extract of leaves of P. longifolia and reported DPPH, 2,2'-azino-bis(3- ethylbenzothiazoline-6-sulfonic acid (ABTS) and ferric ion reducing activities. The IC50 values were 89.32±12.07 and 76.79±5.88 mg/ml against DPPH and ABTS free radicals respectively and the ferric ion reducing power value was 710.54 ±142.82 mg ascorbic acid equivalent/g dry weight [50].
The compound 1U from butanol fraction of P. longifolia stem bark was also tested against DPPH free radicals by Jain et al. [37]. The highest antioxidant activity of 1U and butanol fraction was 57.95 % and 66.05 % respectively, at 40 μg/ml. Accordingly, 1U exhibited better antioxidant properties than the standard drug, vitamin C [37].
Miscellaneous Activities
Two isolated metabolites (3A and 4A) from acetone extract of leaves of P. longifolia were tested for their antifeedant activity against casterlooper i.e., Achara janata and antifeedant activity was noted in both ofthem [24].
Metabolites 3L, 3U and 3V were isolated from bark of P. longifolia and reported for their anti-plasmodial property with IC50 values ranging 1.51-3.37 μg/ml [51]. Gbedema et al. [52] identified 1C, 3A, 3Q, 4A, 5J and 5R from steam barks of P. longifolia and tested for anti-plasmodial activity using Plasmodium falciparum K1 strain. Among those, 3A, 3Q and 4A were found to have potent IC50 values ranging from 3-6 μg/ml, while others showed mild anti-plasmodial activity [52].
Clerodane diterpene 3A from hexane extract of P. longifolia leaves was tested for in vitro and in vivo antileishmanial activities. The antileishmanial activity of 3A (IC50 value: 5.79±0.31 µg/ml) was found to be similar to that of the standard drug (miltefosine, 5 µg/ml). At 250 mg/kg dose, a higher efficacy of 3A administered by the oral route was observed in the liver (87.5±1.8 %), bone marrow (89.1±2.1 %) and spleen (91±2 %); while at 100 mg/kg, the percentages of inhibition were found to be 86.88±1.3, 83.7±1.5 and 84.2±2.2 %, respectively [30]. Similarly, Zhang group tested 3A, 4A and 4B isolated from P. longifolia stem bark extract for their antileishmanial activity against Leishmania donovani (L. donovani) axenic amastigote, L. donovani amastigote and L. donovani promastigotein human acute monocytic leukemia cell line (THP-1) macrophage cultures. Among all of these, only 3A was reported to have activity, with IC50 values of <1.60, 2.34 and 4.50 µg/ml, respectively [36].
Amongst 7 isolated compounds (4A, 4B, 4C, 3L, 3M, 5F and 5Y) of defatted extract of root bark of P. longifolia in methanol (50 %), 4B (30 mg/kg dose)was claimed to be the one that confers hypotensive activity as it caused a 22 % reduction in the mean arterial blood pressure [18].
3A and 3K were isolated from methyl chloride (CH3Cl) soluble fraction from 70 % hydroalcoholic extract of leaves of P. longifolia and tested for antihistaminic activity. 3A (IC50 value of 29.7 µg/ml) showed a dose-dependent inhibition of histamine discharge, while 3K (IC50=189.2 µg/ml) showed very weak activity [53].
The two metabolites 3A and 3K were also tested for anti-helicobacter property against H. pylori and it was found that 3A completely inhibited the growth of H. pylori with MIC of 31.25 µg/ml. 3K(MIC value>125 µg/ml), on the other hand, showed a weak inhibitory action [53].
Four clerodane diterpenes (3A, 3K, 3P and 4A) were obtained from ethanolic extract of P. longifolia leaves and reported for their lipid lowering strength in the high fat diet hamster model, at 25 mg/kg dose. Among these 4, only 3A showed pronounced activity comparable to lovastatin, while others did not show any activity. The mechanistic studies of 3A “privileged structure” exposed that it inhibited the DNA topoisomerase I of L. donovani and proposed as a potential 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitor [31].
Ebiloma et al. [55] isolated 3A, 4A and 4B from the leaves of P. longifolia and tested for anti-trypanosomatid activity using Trypanosoma brucei (T. brucei) and T. congolense and promastigotes of L. mexicana. Amongthose, 3A was found to be more potent against the above pathogens, with EC50 value of below 0.38 µg/ml [54] and the mechanism of action of 3A for anti-trypanosomatid activity was found to be multi-targeted [55]. Moreover, besides anti-oxidant activity, 2J obtained from acetone extract of leaves of P. longifolia also showed a potent antityrosinase activity with IC50 value of 773.09 ±1.47 mg/ml [50].
More recently, Nuygen group screened compounds 3C and 3Q for their xanthine oxidase inhibitory assay using both in vitro and in silico methods and found that both showed potent inhibitory activity against xanthine oxidase enzyme, compared to standard drug allopurinol [56].
The broad and ongoing use of P. longifolia in a system of traditional medicine suggests its safety for human use at appropriate doses. This has been confirmed in the lab via toxicology studies of leaf extracts in Wistar albino rat [57]. Moreover, treatment with leave extracts of P. longifolia reversed the adverse effects in the cadmium-induced tissues via increased antioxidant activity. This effect could partly due to a rich source of phenolic compounds and other effective antioxidants present in the leaf extract of this plant [1,13].
Conclusion
Plants used in traditional systems of medicine have often been a starting point in the search for new and effective pharmaceuticals. This is partly because their history of human use suggests their safety and partly because a significant portion of traditional medicines have been shown to be effective. The investigations into P. longifolia summarized in this review have reinforcedthat it is worthy of further, more in-depth study in terms of potential drug discovery. Future research into P. longifolia and its extracts is needed to determine theirdegree of benefit in the treatment of specific conditions and their mechanisms of action.
Acknowledgements
The authors sincerely thank the Department of Science and Technology (DST) for funding the project with the grant number (DST/DISHA/SoRF-PM/032/2015/01/G).
Conflict of interests:
The author declares no conflict of interest.
References
- Katkar KV, Suthar AC, Chauhan VS. The chemistry, pharmacologic and therapeutic applications of Polyalthia longifolia. Pharmacogn Rev 2010;4(7):62-8.
- Thang TD, Hoi TM, Ogunwande IA. Essential oil constituents of Desmos cochinchinensis Lour, and Polyalthia longifolia var. pendula Hort from Vietnam. Plant 2014;1(4):45-9.
- Yao LJ, Jalil J, Attiq A, Hui CC, Zakaria NA. The medicinal uses, toxicities and anti-inflammatory activity of Polyalthia species (Annonaceae). J Ethnopharmacol 2019;229:303-25.
- Paarakh PM, Khosa RL. Phytoconstituents from the genus Polyalthia-a review. J Pharm Res 2009;2(4):594-605.
- Ghosh A, Das BK, Chatterjee SK, Chandra G. Antibacterial potentiality and phytochemical analysis of mature leaves of Polyalthia longifolia (Magnoliales: Annonaceae). South Pacific J Nat Appl Sci 2008;26(1):68-72.
- Agrawal S, Misra K. Proanthocyanidin from Polyalthia longifolia stem bark. Curr Sci 1979;48:141-3.
- Chen CY, Chang FR, Shih YC, Hsieh TJ, Chia YC, Tseng HY, et al. Cytotoxic Constituents of Polyalthia longifolia var. pendula. J Nat Prod 2000;63(11):1475-8.
- Sashidhara KV, Singh SP, Kant R, Maulik PR, Sarkar J, Kanojiya S, et al. Cytotoxic cycloartane triterpene and rare isomeric bisclerodane diterpenes from the leaves of Polyalthia longifolia var. pendula. Bioorganic Med Chem Lett 2010;20(19):5767-71.
- Faizi S, Mughal NR, Khan RA, Khan SA, Ahmad A, Bibi N, et al. Evaluation of the antimicrobial property of Polyalthia longifolia var. pendula: isolation of a lactone as the active antibacterial agent from the ethanol extract of the stem. Phyther Res 2003;17(10):1177-81.
- Lee TH, Wang MJ, Chen PY, Wu TY, Wen WC, Tsai FY, et al. Constituents of Polyalthia longifolia var. pendula. J Nat Prod 2009;72(11):1960-3.
- Faizi S, Khan RA, Azher S, Khan SA, Tauseef S, Ahmad A. New antimicrobial alkaloids from the roots of Polyalthia longifolia var. pendula. Planta Med 2003;69(04):350-5.
- Chang FR, Hwang TL, Yang YL, Li CE, Wu CC, Issa HH, et al. Anti-inflammatory and cytotoxic diterpenes from formosan Polyalthia longifolia var. pendula. Planta Med 2006;72(14):1344-7.
- Sashidhara KV, Singh SP, Srivastava A, Puri A. Identification of the antioxidant principles of Polyalthia longifolia var. pendula using TEAC assay. Nat Prod Res 2011;25(9):918-26.
- Sampath M, Vasanthi M. Isolation, structural elucidation of flavonoids from Polyalthia longifolia (Sonn.) Thawaites and evaluation of antibacterial, antioxidant and anticancer potential. Int J Pharm Pharm Sci 2013;5(1):336-41.
- Chakrabarty M, Nath AC. A new clerodane-type butenolide diterpene from the bark of Polyalthia longifolia. J Nat Prod 1992;55(2):256-8.
- Hara N, Asaki H, Fujimoto Y, Gupta YK, Singh AK, Sahai M. Clerodane and ent-halimane diterpenes from Polyalthia longifolia. Phytochemistry 1995;38(1):189-94.
- Rashid MA, Hossain MA, Hasan CM, Reza MS. Antimicrobial diterpenes from Polyalthia longifolia var. pendulla (Annonaceae). Phyther Res 1996;10(1):79-81.
- Saleem R, Ahmed M, Ahmed SI, Azeem M, Khan RA, Rasool N, et al. Hypotensive activity and toxicology of constituents from root bark of Polyalthia longifolia var. pendula. Phytother Res 2005;19(10):881-4.
- Faizi S, Khan RA, Mughal NR, Malik MS, Sajjadi KE, Ahmad A. Antimicrobial activity of various parts of Polyalthia longifolia var. pendula: isolation of active principles from the leaves and the berries. Phytother Res 2008;22(7):907-12.
- Sashidhara KV, Singh SP, Shukla PK. Antimicrobial evaluation of clerodane diterpenes from Polyalthia longifolia var. pendula. Nat Prod Commun 2009;4(3):1934578X0900400305.
- Sashidhara KV, Singh SP, Sarkar J, Sinha S. Cytotoxic clerodane diterpenoids from the leaves of Polyalthia longifolia. Nat Prod Res 2010;24(18):1687-94.
- Wu TH, Cheng YY, Chen CJ, Ng LT, Chou LC, Huang LJ, et al. Three new clerodane diterpenes from Polyalthia longifolia var. pendula. Molecules 2014;19(2):2049-60.
- Ghosh G, Subudhi BB, Banerjee M, Mishra SK. A new clerodane-type γ-hydroxybutenolide diterpene from the bark of Polyalthia longifolia var. angustifolia. Indian J Chem B 2011;50(10):1510-12.
- Phadnis AP, Patwardhan SA, Dhaneshwar NN, Tavale SS, Row TN. Clerodane diterpenoids from Polyalthia longifolia. Phytochemistry 1988;27(9):2899-901.
- Chang HL, Chang FR, Chen JS, Wang HP, Wu YH, Wang CC, et al. Inhibitory effects of 16-hydroxycleroda-3, 13 (14) E-dien-15-oic acid on superoxide anion and elastase release in human neutrophils through multiple mechanisms. Eur J Pharmacol 2008;586(1-3):332-9.
- Murthy MM, Subramanyam M, Bindu MH, Annapurna J. Antimicrobial activity of clerodane diterpenoids from Polyalthia longifolia seeds. Fitoterapia 2005;76(3-4):336-9.
- Zhao G, Jung JH, Smith DL, Wood KV, McLaughlin JL. Cytotoxic clerodane diterpenes from Polyalthia longifolia. Planta Med 1991;57(04):380-3.
- Wu YC. Azafluorene and aporphine alkaloids from Polyalthia longifolia. Heterocycles 1989;29(3):463-75.
- Wu YC, Duh CY, Wang SK, Chen KS, Yang TH. Two new natural azafluorene alkaloids and a cytotoxic aporphine alkaloid from Polyalthia longifolia. J Nat Prod 1990;53(5):1327-31.
- Misra P, Sashidhara KV, Singh SP, Kumar A, Gupta R, Chaudhaery SS, et al. 16α?Hydroxycleroda?3, 13 (14) Z?dien?15, 16?olide from Polyalthia longifolia: a safe and orally active antileishmanial agent. Br J Pharmacol 2010;159(5):1143-50.
- Sashidhara KV, Singh SP, Srivastava A, Puri A, Chhonker YS, Bhatta RS, et al. Discovery of a new class of HMG-CoA reductase inhibitor from Polyalthia longifolia as potential lipid lowering agent. Eur J Med Chem 2011;46(10):5206-11.
- Sari DP, Ninomiya M, Efdi M, Santoni A, Ibrahim S, Tanaka K, et al. Clerodane diterpenes isolated from Polyalthia longifolia induce apoptosis in human leukemia HL-60 cells. J Oleo Sci 2013;62(10):843-8.
- Gupta VK, Verma S, Pal A, Srivastava SK, Srivastava PK, Darokar MP. In vivo efficacy and synergistic interaction of 16α-hydroxycleroda-3, 13 (14) Z-dien-15, 16-olide, a clerodane diterpene from Polyalthia longifolia against methicillin-resistant Staphylococcus aureus. Appl Microbiol Biotechnol 2013;97(20):9121-31.
- Afolabi S, Olorundare O, Ninomiya M, Babatunde A, Mukhtar H, Koketsu M. Comparative antileukemic activity of a tetranorditerpene isolated from Polyalthia longifolia leaves and the derivative against human leukemia HL-60 cells. J Oleo Sci 2017;66:1169-74.
- Bhattacharya AK, Chand HR, John J, Deshpande MV. Clerodane type diterpene as a novel antifungal agent from Polyalthia longifolia var. pendula. Eur J Med Chem 2015;94:1-7.
- Zhang J, Jain SK, Jacob MR, Tekwani BL, Hufford CD, Muhammad I. Antileishmanial and Antimicrobial Clerodane Diterpenes from Polyalthia longifolia. Planta Med 2013;79(05):P88.
- Jain PK, Patra A, Satpathy S, Jain S, Khan S. Antibacterial and Antioxidant Activities of 3-O-methyl Ellagic Acid from Stem Bark of Polyalthia longifolia Thw. Chiang Mai J Sci 2018;45(2):858-67.
- Bose S, Byahatti VV, Souza MD, Bose A. Antioxidant and antimicrobial activities of isolated constituents from the bark of Polyalthia longifolia. Int J Green Pharm 2010;4(2):93.
- Ghosh G, Subudhi BB, Badajena LD, Ray J, Mishra MK, Mishra SK. Antibacterial activity of Polyalthia longifolia var. angustifolia stem bark extract. Int J PharmTech Res 2011;3(1):256-60.
- Satish S, Mohana DC, Ranhavendra MP, Raveesha KA. Antifungal activity of some plant extracts against important seed borne pathogens of Aspergillus sp. J Agric Technol 2007;3(1):109-19.
- Shih YT, Hsu YY, Chang FR, Wu YC, Lo YC. 6-Hydroxycleroda-3, 13-dien-15, 16-olide protects neuronal cells from lipopolysaccharide-induced neurotoxicity through the inhibition of microglia-mediated inflammation. Planta Med 2010;76(02):120-7.
- Nguyen HT, Vu TY, Chandi V, Polimati H, Tatipamula VB. Dual COX and 5-LOX inhibition by clerodane diterpenes from seeds of Polyalthia longifolia (Sonn.) Thwaites. Sci Rep 2020;10(1):1.
- Wu TH, Cheng YY, Liou JR, Way TD, Chen CJ, Chen YH, et al. Clerodane diterpenes from Polyalthia longifolia var. pendula protect SK-N-MC human neuroblastoma cells from β-amyloid insult. RSC Adv 2014;4(45):23707-12.
- Thiyagarajan V, Lin SH, Chia YC, Weng CF. A novel inhibitor, 16-hydroxy-cleroda-3, 13-dien-16, 15-olide, blocks the autophosphorylation site of focal adhesion kinase (Y397) by molecular docking. Biochim Biophys Acta-Gen Subj 2013;1830(8):4091-101.
- Thiyagarajan V, Sivalingam KS, Viswanadha VP, Weng CF. 16-hydroxy-cleroda-3, 13-dien-16, 15-olide induced glioma cell autophagy via ROS generation and activation of p38 MAPK and ERK-1/2. Environ Toxicol Pharmacol 2016;45:202-11.
- Afolabi SO, Olorundare OE, Babatunde A, Albrecht RM, Koketsu M, Syed DN, et al. Polyalthia longifolia extract triggers ER stress in prostate cancer cells concomitant with induction of apoptosis: insights from in vitro and in vivo studies. Oxid Med Cell Longev 2019;2019:6726312.
- Liu C, Lee WC, Huang BM, Chia YC, Chen YC, Chen YC. 16-Hydroxycleroda-3, 13-dien-15, 16-olide inhibits the proliferation and induces mitochondrial-dependent apoptosis through Akt, mTOR and MEK-ERK pathways in human renal carcinoma cells. Phytomedicine 2017;36:95-107.
- Chen YC, Huang BM, Lee WC, Chen YC. 16-Hydroxycleroda-3, 13-dien-15, 16-olide induces anoikis in human renal cell carcinoma cells: involvement of focal adhesion disassembly and signaling. Onco Targets Ther 2018;11:7679-90.
- Rupachandra S, Sarada DV. Anti-proliferative and apoptotic properties of a peptide from the seeds of Polyalthia longifolia against human cancer cell lines. Indian J Biochem Biophys 2014;51:127-134.
- Chen XX, Liang G, Chai WM, Feng HL, Zhou HT, Shi Y, et al. Antioxidant and antityrosinase proanthocyanidins from Polyalthia longifolia leaves. J Biosci Bioeng 2014;118(5):583-7.
- Annan K, Ekuadzi E, Asare C, Pistorius D, Oberer L, Gyan BA, et al. Antiplasmodial clerodane diterpene alkaloids from the stem bark of Polyalthia longifolia. Planta Med 2014;80(10):PD114.
- Gbedema SY, Bayor MT, Annan K, Wright CW. Clerodane diterpenes from Polyalthia longifolia (Sonn) Thw. var. pendula: Potential antimalarial agents for drug resistant Plasmodium falciparum infection. J Ethnopharmacol 2015;169:176-82.
- Edmond MP, Mostafa NM, El-Shazly M, Singab AN. Two clerodane diterpenes isolated from Polyalthia longifolia leaves: comparative structural features, anti-histaminic and anti-Helicobacter pylori activities. Nat Prod Res 2020:1-5.
- Ebiloma GU, Igoli JO, Katsoulis E, Donachie AM, Eze A, Gray AI, et al. Bioassay-guided isolation of active principles from Nigerian medicinal plants identifies new trypanocides with low toxicity and no cross-resistance to diamidines and arsenicals. J ethnopharmacol 2017;202:256-64.
- Ebiloma GU, Katsoulis E, Igoli JO, Gray AI, De Koning HP. Multi-target mode of action of a Clerodane-type diterpenoid from Polyalthia longifolia targeting African trypanosomes. Sci Rep 2018;8(1):1-9.
- Nguyen HT, Vu TY, Dakal TC, Dhabhai B, Nguyen XH, Tatipamula VB. Cleroda-4 (18), 13-dien-15, 16-olide as novel xanthine oxidase inhibitors: An integrated in silico and in vitro study. PloS one 2021;16(6):e0253572.
- Chanda S, Dave R, Kaneria M, Shukla V. Acute oral toxicity of Polyalthia longifolia var. pendula leaf extract in Wistar albino rats. Pharm Biol 2012;50(11):1408-15.