- *Corresponding Author:
- Jun Tang
Department of Neurology, Chongqing Hospital of Traditional Chinese Medicine, Chongqing 400021, China
E-mail: juntang12306@163.com
| Date of Received | 25 November 2021 |
| Date of Revision | 14 August 2022 |
| Date of Acceptance | 29 July 2023 |
| Indian J Pharm Sci 2023;85(4):863-883 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Vascular dementia is a serious neurological disease associated with reduced perfusion of the brain. No western drugs have been approved for the treatment of vascular dementia. Oral Chinese drugs are commonly employed for vascular dementia in China. The purpose of this scope review is to review the recent evidence from clinical trials regarding the benefits of traditional oral Chinese medicines and to outline their effects on vascular dementia. Seventeen Chinese herbal medicines used in vascular dementia were systematically searched from 7 databases, such as EMBASE, Cochrane, PubMed, China Sci-Tech journal database, China National Knowledge Infrastructure, Chinese Biomedical Database as well as Wanfang database. Data were obtained from guidelines, consensus, meta-analyses and systematic reviews and randomized controlled trials. A revised Cochrane Risk Assessment Instrument was applied to evaluate the risk of bias with respect to the intention-to-treat effect on eligible randomized controlled trials. Microsoft Excel 2019, GraphPad Prism and RSTUDIO statistics were employed for data integration and process. As the data show, the number of research published increased between 2009 and 2014, while the most studied medicines were TianZhi, Ginkgo biloba leaf and NaoXinTong. A low overall bias risk of intention-to-treat was achieved in only 7 trials. More consistent benefits were observed with Ginkgo biloba leaf and TianZhi for amelioration of mini-mental state examination score deficits and activities of daily living impairments. The overall reporting quality of randomized controlled trials of traditional oral Chinese medicines for vascular dementia was poor and the transparency of these randomized controlled trials must be urgently improved. Oral Chinese drugs may be beneficial to patients with vascular dementia, but they need to be further assessed.
Keywords
Clinical evidence, oral Chinese traditional medicine, scoping review, vascular dementia.
Dementia caused by vascular disease is the second most common type, which accounts for over 20 % of all dementia cases worldwide[1]. A clinical syndrome known as Vascular Dementia (VaD) is characterized by the following features including severe impairment of cognitive function, behavioral and motor abnormalities due to hypoxic brain tissue death caused by reduced cerebral vascular perfusion[2]. The current management strategies for VaD patients mainly include symptomatic treatment of VaD and management of risk factors[3]. VaD risk factors have primarily been characterized as modifiable risk factors and have been validated and standardized as clinical, biological and neuroimaging biomarkers[4]. Western medicines licensed to treat VaD are limited[5], and there is no clear therapeutic approach to VaD[3,6]. Thus, it is essential to develop treatments that are complementary or alternative for VaD.
Traditional Chinese Medicines (TCMs) are commonly employed for the treatment of VD in China[7,8]. However, the interventions assessed and conditions studied in TCM have been diverse[9]. Due to the heterogeneous nature of the interventions as well as the quality of the latest research, a comprehensive review of current clinical evidence is needed.
Oral Chinese medicine has been defined as a form of TCM that contains some dosage form of TCMs[10]. The Chinese National Health Commission and the National Medical Products Administration have approved a TCM product as a commercial product.
Traditional oral Chinese medicinal products are categorized by their nature (essential characteristics) [11]. Even though symptoms were described as dementia, memory loss and cognitive deficits, there is rarely a specific indication for 'VaD' in the drug instructions[11]. Nevertheless, a growing number of researches on VaD treated by oral TCMs have suggested the potential of their applications for VaD and this needs to be further explored[12]. Therefore, the scope of this review is replied upon the PRISMAScR Checklist[13]. There are 17 oral Chinese medicines for VaD covered by this systematic review, including guidelines, consensus, meta-analyses, Systematic Reviews (SRs), as well as Randomized Controlled Trials (RCTs).
Traditional oral Chinese medicines for VaD were searched according to drug instructions in the Chinese Patent Medicinal Prescriptions Database (https://data.yaozh.com/), which is an on-line database of Chinese traditional medicines, which has been approved by the Chinese National Medical Products Administration and the National Health Commission of the People's Republic of China. Reviews and guidelines of TCM for VaD have also been reviewed for the collection of medicines[12,14,15]. Drugs were collected if they met all of the following criteria including oral medication, major ingredients composed of Chinese medicines or herbal medicines, an indication containing any of the words dementia, cognition or memory and inclusion in the Chinese National Essential Health Insurance Program (2020 version, CNEHIP), the Chinese National Essential Medicines List (2018 version, CNEML), or the Chinese Pharmacopoeia (2020 version, CP). The traditional oral Chinese medicines included are listed below. In addition to Bushen Yinao, Shenwu Jiannao (formerly known as Kang-nao-shuai), Congrong Yishen, Dengzhan Shengmai, Fufang Congrong Yizhi (formerly known as Congsheng capsule), Fufang Danshen, Gui Ling Ji, Jian Nao, Naomaitai, Naoxintong, Tianma Xingnao, Tianzhi, Tong-xinluo, Xin Nao Jian, Yixin Ningshen, Yin-Dan Xin- Nao Tong, as well as Ginkgo biloba leaves were also included.
This scoping review aimed to conduct a systematic comprehensive review of 17 universally applicable TCMs for VaD and categorized in the CNEHIP, CNEML, or CP; to provide a concise summary of current clinical findings supporting the efficacy of oral Chinese traditional medicines and to outline the effects of these medicines on VaD.
Materials and Methods
Protocol and registration:
As outlined by Arksey et al.[16] as well as Levac et al.[17], a methodology based on the scoping research was used in conducting this review. The framework provides guidance for the development of a standardized and systematic approach to the conduct of scoping research. Since Chinese medicine encompasses a wide range of clinical evidence, including the formulation, dosage and combination therapy used in clinical trials, this approach is appropriate. All five phases of the scope review have been carried out for identification of the research issue(s), identification of relevant research, selection of the study, data collection and statistical analysis, summarization, and publishing the findings. The referral was registered with INPLASY (Registration No: INPLASY202170057) as the scope review is currently not approved for inclusion in the PROSPERO database.
Criteria for eligibility:
The review included all guidelines, consensus statements, meta-analyses, SRs and RCTs related to oral TCMs for VaD. The included medicines were Bushen Yinao (Kang-nao-shuai (Name previously used)), Congrong Yishen, Dengzhan Shengmai, Fufang Congrong Yizhi (formerly known as Congsheng capsule), Fufang Danshen, Gui Ling Ji, Jian Nao, Naomaitai, Naoxintong, Tianma Xingnao, TianZhi, Tong-xin-luo, Xin Nao Jian, Yixin Ningshen, Yin-Dan Xin-Nao Tong. A study is defined as an RCT if it is described as randomized (including terms like 'random', 'randomly' or 'randomized'). In the absence of a more detailed approach description, this study was included, but not evaluated because the Cochrane Risk of Bias for Randomized Trials (Rob 2.0) did not provide information on allocation sequence randomization or allocation sequence concealment (signaling questions 1.1 and 1.2). Language, publication year, or publication status were not restricted. In the absence of details, conference abstracts were excluded.
The guidelines, consensus, meta-analyses and SRs included in this review should address the medicinal product with regard to the search strategy (indicated as a single medicinal product in the heading and keywords) and describe the clinical use of the medicinal product within VaD.
The following guidelines were used to diagnose patients with VaD in RCTs Version III, III-R, IV, IV-TR, or V; the National Institute for Neurological Disorders as well as Stroke (NINDS-AIREN); the International Classification of Diseases (ICD) 9 or 10; or the Hachinski or modified Hachinski Ischemic score. In order to differentiate VaD from other types of dementia, we require an imaging technique. In this study, Participants who had a diagnosis of mixed dementia or who did not use imaging techniques to determine VaD were excluded from the study. Participants were not restricted in terms of their age or gender. A study intervention was defined as one that included one or more experimental interventions that included the medicine as part of the research process. The conventional pharmacological interventions, placebos, and conventional Routine Therapy (RT) for controlling the cerebrovascular risk factors have been regarded as a combination treatment or control interventions. RT is usually based on pharmacological agents such as antihypertensive agents, antidiabetic medicines, anti-platelets, lipidlowering medicines and medicines that improve blood flow. No pharmacological agents are also accepted as RT when the authors clearly describe them as being suitable for this purpose. The study was excluded if the search strategy included medicine in all interventions. No dose, duration, or outcome was imposed.
Sources of information:
From the start of the project to June 2021, we searched for EMBASE, Cochrane, PubMed, China Sci- Tech Journal Database, China National Knowledge Infrastructure, Chinese Biomedical Database and Wanfang Database. The final search was conducted on June 19, 2021.
Search strategy:
A total of 17 kinds of oral Chinese drugs were searched separately in this paper. Taking Gui Ling Ji to be the example, we used the following search strategy. #1 (CADASIL) OR (cerebrovascular disorders) OR (dementia, multi-infarct) OR (dementia, vascular) OR (neurocognitive disorders) (MeSH terms); #2 (subcortical ischemic vascular disease*) OR (vascular cognit*) OR (VaD*) OR (dement*) OR (cerebrovascular) OR (VaD) OR (VCI) (text words); #3 (guilingji) OR (gui ling ji) (title/abstract); #4 (guilingji) OR (gui ling ji) (text word); #5 (guilingji) OR (gui ling ji) (transliterated title); #6 (random*) (text word); #7 (meta) OR (system* review) OR (review*) OR (guideline*) OR (consensus*) (Title/ abstract); #8 #1 OR #2; #9 #3 OR #4 OR #5; #10 #6 OR #7 and #11 #8 AND #9 AND #10
Selection of study:
During the review process, the authors (X. Yu and Li Ran) examined the titles and abstracts of all references retrieved, as well as retrieving the full text for further analysis. Research on all eligible topics was conducted by Xiujuan Mi and Xiaoqiong Luo and any differences were discussed with others. Prior to data extraction, the reviewers agreed that trials should be included.
Information charting process:
X. Yu, Li Ran, Xin Xiong, Chenyu Li, Y. Guan, Xiaofei Gao, Wenqiang Tao, J. Shu, Xiaoli Qu, Xiujuan Mi, Yuhua Zhao as well as Z. Lian were used to extract data from structured tables, which were verified by Jun Tang, Jian Yang as well as Xing Liao. A detailed explanation of how to use data mining tables has been provided to all investigators in advance in order to ensure accurate and consistent data mining and evaluation. By examining the original papers and conducting a discussion, discrepancies have been resolved.
Data items:
Additionally, three different kinds of data extraction tables have been developed for guidelines as well as consensus; analysis of meta-data as well as reports and RCTs obtaining the necessary data from various researches. Generally, the characteristics of the extracted data for the various types of included research are described below.
Consensus and guidelines: Title, author, publication year, fund and therapeutic effects of VaD.
SRs and meta-analyses: Title, author, publication year, fund, size of the sample, type of population, intervention, comparison as well as therapeutic effects on VaD.
RCTs: The characteristics of the study (title, author, publication year, fund, multiple/single center, hospital level for research performed in China (1, 2 (a), 2 (b), 3 (a) and 3(b)), the characteristics of the study (size of the sample, classification of TCM syndromes), details of the intervention and comparison (formulation, dosage, frequency, time period, combination intervention, and comparison), measures of outcome (cognition [Alzheimer's Disease Assessment Scale-Cognitive subscale (ADASCog), VaD AS-cog, Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), Clinical Dementia Rating (CDR), Clock Drawing Test free-hand format (CDT), Hasegawa Dementia Scale (HDS), Wechsler Memory Scale (WMS), Behavioral Syndromes Scale for Dementia (BSSD), and Trail Making Test (TMT), global performance (Activities of Daily Living (ADL), Board of Behavioral Sciences (BBS), Frequently Asked Questions (FAQ), Neuropsychiatric Inventory (NPI), Sandoz Clinical Assessment-Geriatric (SCAG), Brief Psychiatric Rating Scale (BPRS), National Institutes of Health Stroke Scale (NIHSS), and Clinician Interview-Based Impression of Change-plus), electrophysiology (Electroencephalography (EEG), Evidence-Based Medicine (EBM), and Event- Related Potentials (ERP)), structural examination of brain and vessels (Intima Media Thickness (IMT), Magnetic Resonance Imaging (MRI), and Transcranial Doppler (TCD), blood tests (Amyloid-β peptide, Brain Derived Neurotrophic Factor, blood lipids, c-Reactive Protein, Interleukin-6, Nitric Oxide, Neuron Specific Enolase (NSE), S100 Calcium Binding Protein B (S100B), Superoxide dismutase (SOD), tumor necrosis factor-α, Vascular endothelial growth factor (VEGF), Homocysteine (Hcy), Malondialdehyde (MDA) and Nerve Growth Factor (NGF), TCM syndrome, Syndromes of VaD (SDSVD)) and adverse events.
Assessment of the risk of bias in RCTs:
The clinical trials of oral TCM for VaD usually aim to evaluate the application of the medicine in clinical treatment. The effect of assignment to an intervention is one of the critical factors for RCT screening in reviews with such an objective[18]. In order to estimate the impact of the inclusion of RCTs, Xinyuan Yu and Li Ran used a modified version of the Cochrane Risk Assessment Tool (RB-2) as indicated in the Cochrane Handbook for SRs[18]. By answering a series of questions ('signaling questions') in a template, we evaluated and recorded the following aspects like randomization process; deviation from planned interventions; lack of results; measurement of results and choice of reported results. The whole risk of bias judgment was used as the algorithm result of the tool. We resolved our differences with reference to the original publication and discussions with Xing Liao.
Data synthesis:
We have summarized the basic data of 17 oral Chinese medicines based on the drug indications, the Chinese Patent Medicine Prescription Database including the CNEHIP, the CNEML and the DRUGDATAEXPY. Data and quantitative descriptions were generated using Microsoft Excel 2019 (Redmond, WA, US) and GraphPad Prism software (GraphPad; San Diego, CA, US) and the RSTUDIO statistical software (RSTUDIO; Boston, MA, US). A scope review was performed and a report was prepared according to the Preferred Reporting Items for SRs and Meta- Analyses Extension for Scoping Reviews (PRISMASCRs) checklist (supplementary material: Appendix S3)[13].
Results and Discussion
A brief description of the 17 traditional oral Chinese medicines included in the CNEHIP, CNEML, or CP is presented in Table 1. According to the classification of the CNEHIP and CNEML, seven medicines were defined as QY drugs, four were FZ drugs, one was an AS drug, and one was a ZF drug in traditional medicine. Four drugs that were only listed in the CP were not classified based on the basic properties since the classification in the CP depends on the Chinese characters for drug names. There were fifteen medicines containing a combination of herbal ingredients, whereas two medicines contained a single herb as the main ingredient. Definitions of terminologies regarding traditional medicine were summarized.
| Name | Catalog (Corresponding basic properties [section/division/group])* | Approved year | Main components | Actions | Indications | Dosage (formulation; dose; frequency) |
|---|---|---|---|---|---|---|
| BuShenYiNao | CNEHIP(FZ/QXSB/BSYX) | 1998 | Ginseng Radix Et Rhizoma Rubra, Cervi Pantotrichum Cornu, Ziziphi spinosae semen, Radix Rehmanniae Praeparata, Poria, Scrophulariae Radix, Polygalae Radix, Ophiopogonis Radix, Schisandra chinensis fructus, Angelicae sinensis Radix, Chuanxiong Rhizoma, Achyranthis bidentatae radix, Rhizoma Dioscoreae, Psoraleae fructus, Lycii Fructus, Cinnabaris | Tonify kidney and replenish qi, tonify blood and engender essence | Palpitations, shortness of breath, insomnia, forgetfulness, night sweating, waist soreness, tinnitus, deafness caused by dual deficiency of qi and blood and insufficiency of kidney essence | Pill: 1.60-2.40g each time (i.e., 8-12 pills), twice daily; tablet: 1.32-1.98 g each time (i.e., 4-6 tablets), twice daily; capsule: 0.81-1.08 g each time (i.e., 3-4 capsules), twice daily |
| ShenWuJianNao(KangNaoShuai[former name]) | CP | 2002 | Ginseng Radix Et Rhizoma, Polygoni Multiflori Radix Praeparata, Codonopsisradix, Astragali Radix, Radix Rehmanniae Praeparata, Rhizoma Dioscoreae, Salviae miltiorrhizae Radix Et Rhizoma, Lycii Fructus, Paeoniae Radix Alba, Polygalae Radix, Tuckahoe with pine, Acori Tatarinowii Rhizoma, Scutellariae radix, Pueraria lobata Radix, Radix Puerariae thomsonii, Ziziphi spinosae semen, Ophiopogonis radix, Dragon bones, Cyperi Rhizoma, Flos Chrysanthemi Indici, Lecithin, Vitamin E | Tonify the kidney essence, replenish qi and nourish the blood | Lassitude of spirit, insomnia, profuse dreaming, dizziness, dizzy vision, lack of strength, forgetfulness caused by insufficiency of kidney essence, liver qi deficiency and liver blood deficiency | Capsule: 1.50-1.80 g each time (i.e., 5-6 capsules), 3 times daily |
| CongRongYiShen | CNEHIP(FZ/ZY/ZBSY); CP | 2003 | Schisandra chinensis Fructus, Cistanches Herba, Poria, Cuscutae Semen, Plantaginis semen, Morindae Officinalis Radix | Tonif the kidney essence | Lack of physical strength, forgetfulness, dizziness, tinnitus caused by kidney qi deficiency | Granule: 2.00 g each time (i.e., 1 pack), twice daily |
| DengZhanShengMai | CNEHIP(QY/HYTM/n.a);CNEML(QY/YQHX/n.a); CP | 2002 | Herba Erigerontis, Radix et Rhizoma Ginseng, Schisandra chinensis Fructus, Ophiopogonis Radix | Replenish qi and tonify yin | Chest impediment, sequela of wind stroke, dementia, forgetfulness, numbness in limbs caused by dual deficiency of qi and yin and stasis obstructing the brain collateral; coronary heart disease with angina pectoris, ischemic cardio-cerebrovascular disease, hyperlipidemia with syndrome of stasis obstructing the brain collateral and dual deficiency of qi and yin | Capsule: 0.36 g each time (i.e., 2 capsules), 3 times daily |
| Fu Fang Cong Rong YiZhi (Cong Sheng Capsule [formername]) | CNEHIP(FZ/YYSB/n.a) | 2008 | Polygoni Multiflori Radix Praeparata, Folium nelumbinis, Cistanches Herba, Pheretima, Rhapontici Radix | Emolliate the liver, activate blood and resolve turbidity | Mild to moderate vascular dementia with stasis obstructing the brain collateral and blood stasis-phlegm | Capsule: 1.20 g each time (i.e., 4 capsules), 3 times daily |
| FuFangDanShen | CNEHIP(QY/XQHX/n.a);CNEML(QY/LQHX/n.a); CP | 2002 | Salvia miltiorrhiza Radix Et Rhizoma, Notoginseng Radix Et Rhizoma, borneolum syntheticum | Activate blood and resolve stasis, move qi to relieve pain | Chest impediment caused by blood stasis due to qi stagnation | Granule: 1.00 g each time (i.e., 1 pack), 3 times daily; pill: 1.00 g each time (i.e., 5 pills), 3 times daily; drop pill: 0.27 g each time (i.e., 10 drop pills), 3 times daily; oral spray: 1-2 sprays each time, 3 times daily; capsule: 0.90 g each time (i.e., 3 capsules), 3 times daily; tablet: 0.96 g each time (i.e., 3 tablets), 3 times daily |
| GuiLingJi | CP | 2002 | Ginseng Radix Et Rhizoma Rubra, Cervi Pantotrichum Cornu, Hippocampus, Lycii Fructus, Caryophylli Flos, pangolin scales, sparrow brains, Achyranthis Bidentatae Radix, Cynomorii Herba, Radix Rehmanniae Praeparata, Psoraleae fructus, Cuscutae Semen, Cortex Eucommiae, Fossil shell of spirifer, Cistanches Herba, Glycyrrhizae radix Et Rhizoma, Asparagi Radix, Epimedii Folium, Halitum, Amomi fructus | Tonify qi and secure kidney | Forgetfulness, profuse dreaming, waist soreness, qi deficiency, fifth-watch sloppy diarrhea, loss of appetite caused by kidney yang deficiency | Capsule: 0.60g each time (i.e., 2 capsules), once daily |
| JianNao | CP | 2002 | Angelicae sinensis Radix, Bambusae concretio silicea, Cistanches Herba, Dragon's Teeth, Rhizoma Dioscoreae, Succinum, Schisandra Chinensis Fructus, Gastrodiae Rhizoma, Semen Platycladi, Salviae miltiorrhizae Radix Et Rhizoma, Sharpleaf Galangal Fruit, Ginseng Radix Et Rhizoma, Polygalae Radix, Chrysanthemi Flos, Irkutsk Anemone Rhizome, Haematitum, Arisaema Cum Bile, Ziziphi Spinosae Semen, Lycii Fructus. | Tonify the kidney, nourish the blood to tranquilize. | Mild cognitive impairment with forgetfulness, dizziness, dizzy vision, palpitations, insomnia, waist soreness caused by kidney and heart deficiency. | Pill: 0.75 g each time (i.e., 5 pills), 2-3 times daily; capsule: 0.60 g each time (i.e., 2 capsules), 3 times daily. |
| NaoMaiTai | CNEHIP(QY/YQHX/n.a);CP | 1998 | Ginseng Radix Et Rhizoma Rubra, Notoginseng Radix Et Rhizoma, Angelicae sinensis Radix, Salviae miltiorrhizae Radix Et Rhizoma, Spatholobi caulis, Carthami flos, Ginkgo Folium, Crataegi Fructus, Chrysanthemi Flos, Concha Haliotidis, Polygoni multiflori Radix Praeparata, Acori Tatarinowii Rhizoma, Pueraria lobata Radix | Tonify qi and activate blood, extinguish wind and resolve phlegm | Ischemic stroke with qi deficiency, wind-phlegm and blood stasis obstructing the brain collateral | Capsule: 1.00 g each time (i.e., 2 capsules), 3 times daily |
| NaoXinTong | CNEHIP(QY/YQHX/n.a);CNEML(QY/YQHX/n.a); CP | 2002 | Astragali Radix, Paeoniae Radix Rubra, Salviae miltiorrhizae Radix Et Rhizoma, Angelicae sinensis Radix, Chuanxiong Rhizoma, Persicae Semen, Carthami flos, Olibanum, Myrrha, Spatholobi caulis, Achyranthis Bdentatae Radix, Ramulus Cinnamomi, Mori Ramulus, Pheretima, Scorpio, Hirudo | Tonify qi and activate blood, resolve stasis and free the collateral vessels | Syndrome of wind striking the meridians and collaterals, and angina pectoris with qi deficiency with blood stasis and stasis obstructing the brain collateral | Capsule: 0.80-1.60 g each time (i.e., 2-4 capsules), 3 times daily |
| TianMaXingNao | CNEHIP(FZ/ZY/ZBGS); CP | 2002 | Gastrodiae Rhizoma, Pheretima, Acori Tatarinowii Rhizoma, Polygalae Radix, Radix Rehmanniae Praeparata, Cistanches Herba | Enrich the kidney and nourish the liver, free the collateral vessels and relieve pain | Headache, dizziness, forgetfulness, insomnia, slow response, tinnitus, waist soreness caused by liver-kidney depletion | Capsule: 0.80 g each time (i.e., 2 capsules), 3 times daily |
| TianZhi | CNEHIP(ZF/PGQY/n.a); CP | 2004 | Gastrodiae Rhizoma, Uncariae Ramulus Cum Uncis, Concha Haliotidis, Cortex Eucommiae, Taxilli Herba, Tuckahoe with pine, Polygoni Multiflori Caulis, Flos Sophorae, Gardeniae fructus, Scutellariae radix, Radix Cyathulae, Leonur Iherba | Pacify the liver to subdue yang, enrich the kidney and nourish the liver, tranquilize | Mild to moderate vascular dementia with syndrome of ascendant hyperactivity of liver yang | Granule: 5.00 g each time (i.e., 1 pack), 3 times daily |
| TongXinLuo | CNEHIP(QY/YQHX/n.a);CNEML(QY/HYTM/n.a); CP | 1998 | Ginseng Radix Et Rhizoma, Hirudo, Scorpio, Paeoniae Radix Rubra, Cicadae Periostracum, Eupolyphaga, Steleophaga, Scolopendra, Santali albi Lignum, Dalbergia Odorifera Lignum, Olibanum, Ziziphi spinosae semen, borneolum syntheticum | Tonify qi and activate blood, free the collateral vessels and relieve pain | Angina pectoris and wind stroke with qi deficiency and blood stasis obstructing the brain collateral | Capsule: 0.52-1.04 g each time (i.e., 2-4 capsules), 3 times daily |
| XinNaoJian | CP | 2002 | Camellia sinensis extract | Open the orifices, resolve turbidity | Dizziness, dizzy vision, oppression in the chest, shortness of breath, lack of strength, lassitude of spirit, forgetfulness caused by cardiovascular disease with high fibrinogen and atherosclerosis, and leukopenia caused by radiotherapy and chemotherapy | Tablet: 0.20 g each time (i.e., 2 tablets), 3 times daily; capsule: 0.20 g each time (i.e., 2 capsules), 3 times daily |
| YiXinNingShen | CNEHIP(AS/YXAS/n.a); CP | 2002 | Panax ginseng Leaves Extract, Fructus Celastri orbiculati, Schisandrae chinensis fructus, Ganoderma | Tonify qi and engender fluid, nourish the heart to tranquilize | Palpitations, shortness of breath, profuse dreaming, insomnia, forgetfulness, neurasthenia | Tablet: 1.56 g each time (i.e., 3 tablets), 3 times daily |
| YinDanXingNaoTong | CNEHIP(QY/XQHX/n.a); CNEML(QY/HXQY/n.a); CP | 2002 | Ginkgo Folium, Salviae miltiorrhizae Radix Et Rhizoma, Erigerontis Herba, Herba Gynostemmatis Pentaphylli, Crataegi fructus, Allii sativi bulbus, Notoginseng Radix Et Rhizoma, Ɩ-Borneolum. | Activate blood and resolve stasis, move qi to relieve pain, promote digestion and remove food stagnation. | Chest impediment, angina pectoris, hyperlipidemia, cerebral atherosclerosis, wind stroke, sequela of wind stroke with blood stasis due to qi stagnation | Capsule: 0.80-1.60 g each time (i.e., 2-4 capsules), 3 times daily |
| Ginkgo biloba Leaf | CNEHIP(QY/HYTM/n.a); CNEML(QY/HXQY/n.a); CP | 2002 | Ginkgo Leaves Extract (EGb761). | Activate blood and resolve stasis, free the collateral vessels. | Chest impediment, heart pain, angina pectoris, ischemic stroke with blood stasis obstructing the brain collateral. | Juice: 10ml each time, 3 times daily; pill: 0.30g each time (i.e., 5 pills), 3 times daily; tablet: 0.48 g each time (i.e., 2 tablets), 3 times daily; capsule: 0.25g each time (i.e., 1 capsule), 3 times daily; soft capsule: 0.5 g each time (i.e., 2 capsule), 3 times daily. |
Table 1: The Basic Information for Seventeen Oral Chinese Traditional Medicines For Vascular Dementia
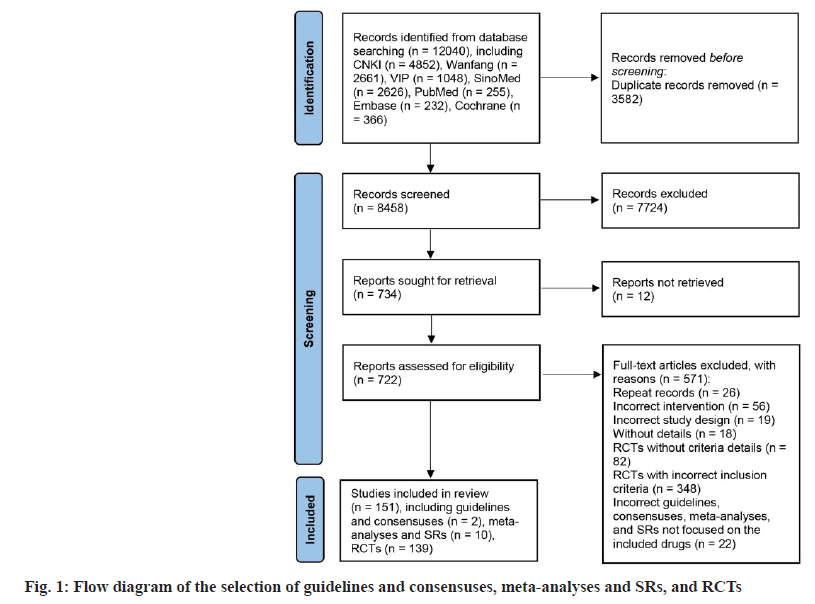
The flow chart in fig. 1 illustrates the procedure for selecting documents. Initially, seven databases were searched, resulting in the identification of 12 040 records. Upon removing duplicates and references from titles as well as abstracts, a total of 722 fulltext articles were accessed, and 571 references were omitted for a variety of reasons (as shown in the flow chart). In the end, 151 researches were included in the scope, comprising 2 consensus research, 10 metaanalyses and reviews and 139 RCTs. The numbers of enrolled trials for 17 oral TCMs are presented in Table 2.
| Name of the oral Chinese traditional medicines | No. of guidelines/consensuses (%) | No. of SRs/meta-analyses (%) | No. of RCTs (%) |
|---|---|---|---|
| TianZhi | 0 (0) | 3 (30) | 36 (25.9) |
| Ginkgo biloba Leaf | 0 (0) | 3 (30) | 30 (21.6) |
| NaoXinTong | 1 (50) | 2 (20) | 28 (20.1) |
| NaoMaiTai | 0 (0) | 0 (0) | 14 (10.1) |
| FuFangCongRongYiZhi | 0 (0) | 1 (10) | 10 (7.2) |
| TongXinLuo | 0 (0) | 1 (10) | 9 (6.5) |
| YinDanXingNaoTong | 0 (0) | 0 (0) | 6 (4.3) |
| DengZhanShengMai | 0 (0) | 0 (0) | 3 (2.2) |
| FuFangDanShen | 0 (0) | 0 (0) | 2 (1.4) |
| TianMaXingNao | 1 (50) | 0 (0) | 1 (0.7) |
| BuShenYiNao | 0 (0) | 0 (0) | 0 (0) |
| ShenWuJianNao | 0 (0) | 0 (0) | 0 (0) |
| CongRongYiShen | 0 (0) | 0 (0) | 0 (0) |
| GuiLingJi | 0 (0) | 0 (0) | 0 (0) |
| JianNao | 0 (0) | 0 (0) | 0 (0) |
| XinNaoJian | 0 (0) | 0 (0) | 0 (0) |
| YiXinNingShen | 0 (0) | 0 (0) | 0 (0) |
| Total | 2 (100) | 10 (100) | 139 (100) |
Table 2: Number of Included Studies on Seventeen Oral Chinese Traditional Medicines for Vascular Dementia
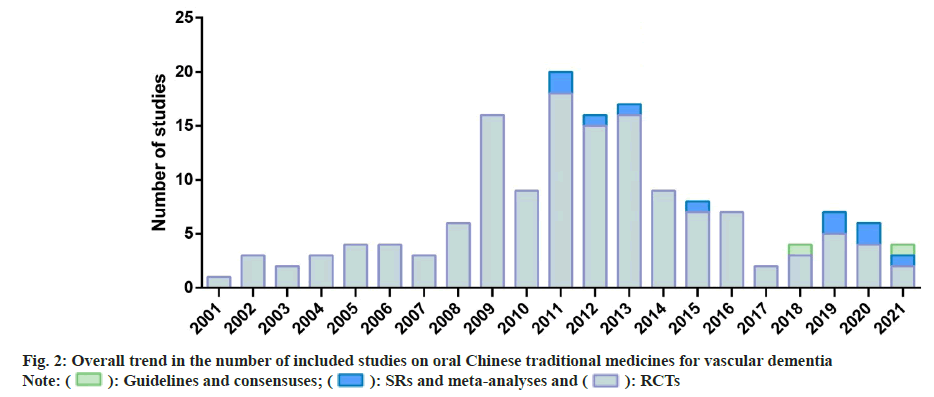
Three of the most commonly used medicines based on the RCTs were TianZhi (36, 25.9 %), Ginkgo biloba leaf (30, 21.6 %), and Naoxintong (28, 20.1 %) and the top three medicines in the SRs and meta-analyses were also Tianzhi (3, 30 %), Ginkgo biloba leaf (3, 30 %) and Naoxintong (2, 20 %). Two medicines in consensuses were Naoxintong (1, 50 %) and Tianma Xingnao (1, 50 %), while no guidelines on any of the seventeen medicines were included. Seven medicines did not have any evidence that was eligible viz., Bushen Yinao, Shenwu Jiannao, Congrong Yishen, Gui Ling Ji, Jian Nao, Xin Nao Jian and Yixin Ningshen. The remainder of the analysis covers only 10 medicines. Fig. 2 shows a general trend in the number of research. Prior to 2008, only sporadic RCTs were performed. Since 2008, the number has grown rapidly, with between 9 and 18 new researches published each year and then progressively reduced since 2014. The SRs and metaanalyses were not reported until 2011, with up to 2 newly published reports annually. The 2 consensuses were published in 2018 and 2021.
The geographical distribution was uneven for RCTs of ten traditional oral Chinese medicines for which there was eligible evidence according to the data depicted in fig. 3. In total, 24 provinces and municipalities participated in the published research. There were 34 RCTs conducted in Henan Province (n=34), including 21 research on TianZhi; therefore, Henan Province possessed the highest number of RCTs conducted on a single medicine.
Fig. 3: The geographical distribution of the primary investigators of RCTs on oral Chinese traditional medicines for vascular dementia
Note:  : DengZhanShengMai;
: DengZhanShengMai;  :FuFangCongRongYiZhi ;
:FuFangCongRongYiZhi ;  : FUFangDanShen;
: FUFangDanShen;  : NaoMaiTai;
: NaoMaiTai;  : NaoXinTong;
: NaoXinTong;  : TianMaXingNao;
: TianMaXingNao;  : TianZhi ;
: TianZhi ;  : TongXinLuo;
: TongXinLuo;  : YinDanXingNaoTong and
: YinDanXingNaoTong and  : Ginkog Biloba Leaf
: Ginkog Biloba Leaf
A total of 13 010 VaD participants were enrolled in the RCTs, and the sample size was generally between 51 and 100 (96, 69.1 %) (Table 3). Level 3a hospitals managed most of the RCTs, accounting for 86 trials (61.9 %), while TCM syndromes were only mentioned in 46 research (33.1 %). Regarding interventions, conventional pharmacological interventions were used as combined therapy in 77 RCTs (55.4 %). There were 21 types of controls included in the RCTs, of which piracetam was the most prevalent (15.8 %). A medication duration of 61 to 120 d accounted for most of the RCTs, including 62 trials (44.6 %). With respect to the methodology of the submitted RCTs, 131 RCTs (94.2 %) were conducted at a single center, as well as 18 research (13.7 %), including single-blind trials in one study and doubleblind trials in seventeen research. Although reported as RCTs, only 33 papers (23.7 %) explicitly reported the details of randomization methods (stratified and blocked randomization, computer-generated random sequence, table of random numbers, and simple randomization), while randomization methods were not specified for 98 research (70.5 %), and predictable allocation sequences for randomization (allocated by treatment, sorting, or admission time) were described in the remaining 8 research (5.8 %). 11 RCTs (7.9 %) reported funding sources, of which 7 received support from national sources, 2 received provincial funding, and 2 received municipal funding. Regarding the outcome measures, the top three were cognitive assessments, which were applied in 132 research (95.0 %); global performance assessments, which were conducted in 99 research (71.2 %); and structural examinations of the brain and vessels, which were reported in 28 research (20.1 %). Safety data were reported in 74 trials (53.2 %), while adverse reactions were described in 73 trials (52.5 %).
| Items | Details | N (% of the 139 RCTs) |
|---|---|---|
| Study setting | Single-center study | 131 (94.2) |
| Multicenter study | 8 (5.8) | |
| Hospital setting | 1 | 3 (2.2) |
| 2a | 37 (26.6) | |
| 2b | 1 (0.7) | |
| 3a | 86 (61.9) | |
| 3b | 9 (6.5) | |
| Clinic | 1 (0.7) | |
| Unknown | 2 (1.4) | |
| Sample size | ≤50 | 12 (8.6) |
| 51-100 | 96 (69.1) | |
| 101-200 | 26 (18.7) | |
| >200 | 5 (3.6) | |
| TCM syndrome | Reported | 46 (33.1) |
| Not reported | 93 (66.9) | |
| Interventions | Combined with conventional pharmacological interventions | 77 (55.4) |
| Combined with placebo and RT | 33 (23.7) | |
| Oral Chinese traditional medicine only | 29 (20.9) | |
| Duration (d) | ≤60 | 46 (33.1) |
| 61-120 | 62 (44.6) | |
| 121-180 | 28 (20.1) | |
| >180 | 3 (2.2) | |
| Controls | Piracetam | 22 (15.8) |
| Donepezil | 21 (15.1) | |
| Nimodipine | 17 (12.2) | |
| Almitrine and Rubasine | 14 (10.1) | |
| Dihydroergotoxine Mesylate | 11 (7.9) | |
| Oxiracetam | 8 (5.8) | |
| Nicergoline | 5 (3.6) | |
| Aniracetam | 4 (2.9) | |
| Butylohthalide | 4 (2.9) | |
| Huperzine | 4 (2.9) | |
| Memantine | 3 (2.2) | |
| Citicoline | 2 (1.4) | |
| Aspirin | 1 (0.7) | |
| Buflomedil | 1 (0.7) | |
| Cerebroprotein hydrolysate | 1 (0.7) | |
| Flunarizine | 1 (0.7) | |
| Idebenone | 1 (0.7) | |
| Pyritinol | 1 (0.7) | |
| TCM medicine (Ginkgo, FuFangCongRongYiZhi, FuFangDanShen) | 5 (3.6) | |
| Acupuncture | 1 (0.7) | |
| Placebo | 2 (1.4) | |
| Outcome measures | Cognition (ADAS-Cog, VADAS-cog, MMSE, MoCA, CDR, CDT, HDS, WMS, BSSD, TMT) | 132 (95) |
| Global performance (ADL, BBS, FAQ, NPI, SCAG, HIS, BPRS, NIHSS, CIBIC-plus) | 99 (71.2) | |
| Electrophysiology (EEG, EBM, ERP) | 9 (6.5) | |
| Structural examination of brain and vessels (IMT, MRI, TCD) | 28 (20.1) | |
| Blood test (Aβ, BDNF, Blood lipids, CRP, ET, IL-6, NO, NSE, S100B, SOD, TNF-α, VEGF, HCY, MDA, NGF) | 17 (12.2) | |
| TCM syndrome (SDSVD) | 7 (5) | |
| No details | 5 (3.6) | |
| Safety data reported | Reported | 74 (53.2) |
| Not reported | 65 (46.8) | |
| Adverse reactions | Reported | 73 (52.5) |
| Not reported | 66 (47.5) | |
| Random sequence generation | Stratified and blocked randomization | 2 (1.4) |
| Computer-generated random sequence | 3 (2.2) | |
| Table of random numbers | 27 (19.4) | |
| Simple randomization | 1 (0.7) | |
| Allocated by treatment | 3 (2.2) | |
| Allocated by sortition | 3 (2.2) | |
| Allocated by admission time | 2 (1.4) | |
| No details | 98 (70.5) | |
| Blinding | Double blinding | 18 (12.9) |
| Single blinding | 1 (0.7) | |
| No blinding | 121 (87.1) | |
| Funding source | National funding | 7 (5) |
| Provincial funding | 2 (1.4) | |
| Municipal funding | 2 (1.4) | |
| Not reported | 128 (92.1) |
Table 3: The Clinical and Methodological Characteristics Reported In the Included RCTs of Oral Chinese Traditional Medicines for Vascular Dementia
As shown in Table 4, the 2 included consensuses reported the beneficial medical effects of two traditional oral Chinese medicines on VaD and received no funding sources. In total, 10 eligible SRs and meta-analyses included 10 149 participants, with sample sizes ranging from 536 to 1419, of which more than 1000 participants distributed in 6 reviews (60.0 %). One of the reviews (10.0 %) included both VCI and VaD participants, while the other 9 reviews (90 %) focused on participants only diagnosed with VaD. Half (5 reviews, 50 %) of the reviews included conventional pharmacological interventions as a combined therapy, yet the other half (5 reviews, 50 %) contained traditional oral Chinese medicine as the only intervention. It is worth mentioning that conventional pharmacological interventions were adopted as eligible comparators in 9 (90 %) of the 10 reviews, while only one review (10.0 %) limited the comparators to placebo or RT.
| Items | Details | N (%) |
|---|---|---|
| Guidelines and consensuses | ||
| Publication type | Consensuses | 2 (100 %) |
| Medical effects on VaD | Beneficial effect | 2 (100 %) |
| Funding source | Not reported | 2 (100 %) |
| Systematic reviews and meta-analyses | ||
| Sample size | ≤1000 | 4 (40 %) |
| >1000 | 6 (60 %) | |
| Populations | VCI and VaD | 1 (10 %) |
| VaD | 9 (90 %) | |
| Interventions | Combined with conventional pharmacological interventions | 5 (50 %) |
| Oral Chinese traditional medicine only | 5 (50 %) | |
| Comparators | Conventional pharmacological interventions | 9 (90 %) |
| Placebo or RT | 1 (10 %) | |
Table 4: The Clinical Characteristics of the Included Guidelines, Consensuses, Systematic Reviews, and Meta-Analyses of Oral Chinese Traditional Medicines for Vascular Dementia.
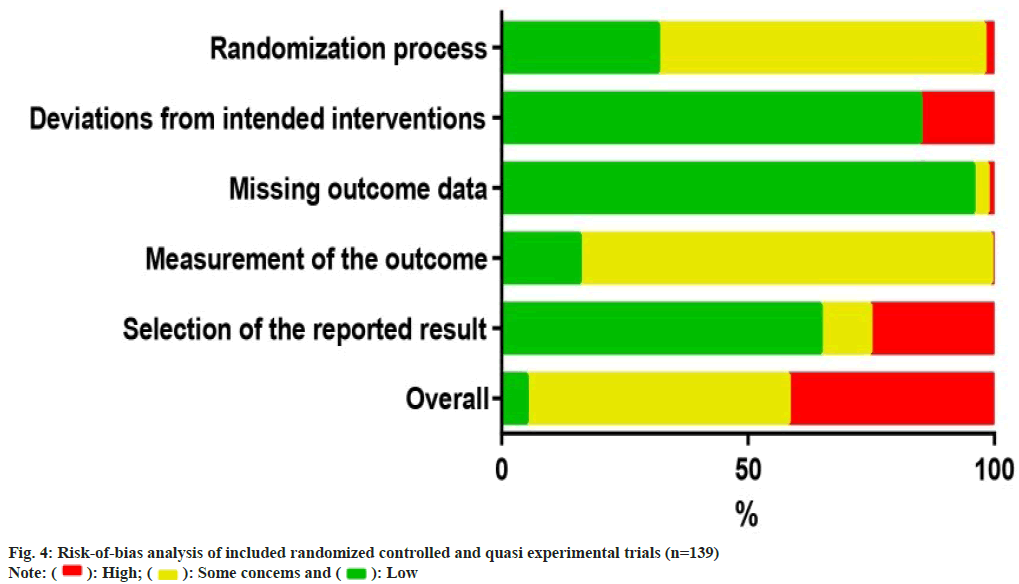
The risk of bias analysis (fig. 4) for 139 RCTs showed that 44 research (31.7 %) were conducted with proper randomizations, and they were comprehensively evaluated by the randomizations and concealments of allocation sequence and baseline differences between groups. The bias caused by deviations from the intended interventions was evaluated in the context of the trial, in accordance with the protocol, as well as the effect on outcome. 118 research (84.9 %) found a low risk of bias, whereas 21 research (15.1 %) indicated a high risk of bias in this field. In the assessment of the bias risk caused by missing outcome data, 133 trials (95.7 %) were found to have a low risk of bias after examining the proportions and causes of missing outcome data, with the lowest risk bias across all domains. Bias in outcome measures showed that 116 trials (83.5 %) were at risk of some concern, mainly because of un blinded data on the allocation of interventions which might affect the evaluation of results. Bias in the choice of outcomes was the highest rated bias in the included articles (25.2 %, n=35), as many trials only reported the overall efficacy calculated as a combination of the results of the protocol rather than showing specific details of those results. For the overall risk-of-bias judgment, 74 trials (53.2 %) with judgments of 'some concerns' accounted for the largest proportion. Only 7 research (5.0 %) had a low risk of general bias, whereas 58 (41.7 %) had a high risk of general bias because of a high risk of bias in one or more structural domains. An overview of the risk of bias analysis for every article is shown in fig. 5.
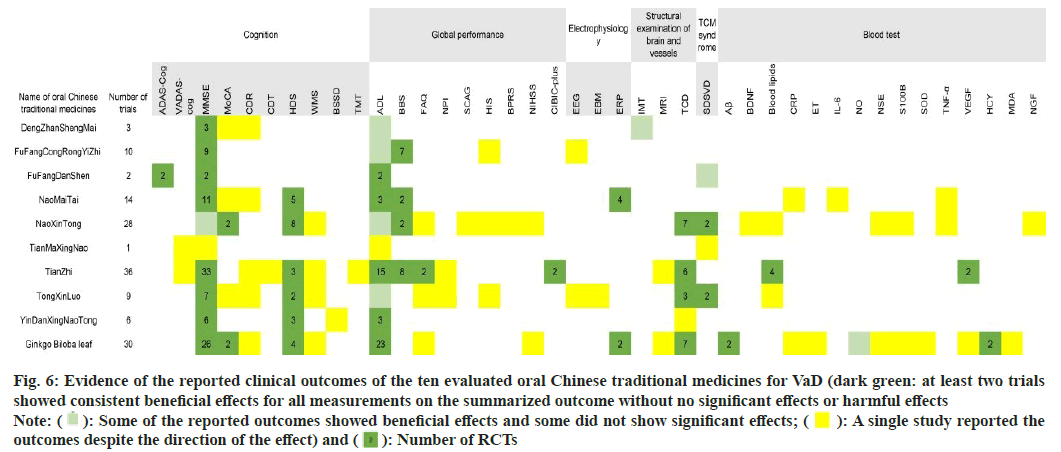
In fig. 6, the clinical outcome and drug measurements are presented. Consistently benefits (at least two researches have showed beneficial effects with no significant or adverse effects) based on published RCTs are represented in dark green, and the number of RCTs per outcome is displayed in cells.
Fig. 6: Evidence of the reported clinical outcomes of the ten evaluated oral Chinese traditional medicines for VaD (dark green: at least two trials
showed consistent beneficial effects for all measurements on the summarized outcome without no significant effects or harmful effects
Note:  : Some of the reported outcomes showed beneficial effects and some did not show significant effects;
: Some of the reported outcomes showed beneficial effects and some did not show significant effects;  : A single study reported the
outcomes despite the direction of the effect) and
: A single study reported the
outcomes despite the direction of the effect) and  : Number of RCTs
: Number of RCTs
Three RCTs were identified to treat VaD using Dengzhan Shengmai. The MMSE scores of VaD participants were consistently improved when Dengzhan Shengmai combined with oxiracetam (1 RCT), donepezil hydrochloride (1 RCT) or idebenone (1 RCT).Ten RCTs compared Fufang Congrong Yizhi to conventional pharmacological interventions, including dihydroergotoxine mesylate (6 RCTs), donepezil hydrochloride (2 RCTs), piracetam (1 RCT), and nimodipine (1 RCT). Beneficial effects on MMSE and BBS scores were reported. One meta-analysis noted that Fufang Congrong Yizhi significantly improved MMSE, MoCA, and ADL scores in vascular cognitive impairment and VaD.
Two RCTs of Fufang Danshen were conducted with comparisons to placebos. Both of the clinical outcomes showed consistent beneficial effects on MMSE, ADAS-Cog, and ADL scores after 24 w of therapy.
In the 14 RCTs of NaoMaiTai, 4 RCTs were conducted with combined therapy using conventional pharmacological interventions, including memantine hydrochloride (2 RCTs), donepezil hydrochloride (1 RCT), and oxiracetam (1 RCT), all of which were compared to the corresponding conventional pharmacological interventions. Another RCT combined a TCM decoction and compared it to Ginkgo biloba capsule treatment. The remaining 9 RCTs used NaoMaiTai as the only intervention and compared it to almitrine and rubasine, piracetam, nicergoline, dihydroergotoxine mesylate, or cerebroprotein hydrolysate. The outcomes indicated consistent beneficial effects on MMSE, HDS, ADL, BBS, and ERP scores.
Fourteen of the 28 RCTs that tested NaoXinTong for VaD reported combined therapies, including conventional pharmacological interventions, such as donepezil hydrochloride (4 RCTs), nimodipine (5 RCTs), butylohthalide (1 RCT), memantine hydrochloride (1 RCT), almitrine and rubasine (1 RCT), and buflomedil hydrochloride (1 RCT), as well as TCM combined therapy with acupuncture (1 RCT). These combined therapies were also set as the corresponding comparisons in the RCTs, except for one RCT comparing combined therapy of Naoxintong with acupuncture to another combined treatment of nimodipine and piracetam. The other 14 RCTs without combined therapy compared Naoxintong to nimodipine (1 RCT), almitrine and rubasine (4 RCTs), FuFangDanShen (1 RCT), flunarizine hydrochloride (1 RCT), oxiracetam (1 RCT), pyritinol hydrochloride (1 RCT), aniracetam (1 RCT), butylohthalide (1 RCT), or routine therapies (3 RCTs). Consistent beneficial effects were indicated in MoCA, HDS, BBS, TCD, and SDSVD scores. The consensus on the clinical application of Naoxintong implied a beneficial effect on learning and memory. In addition, two meta-analyses reported that NaoXinTong significantly improved the MMSE and ADL scores of patients with VaD.
Only one RCT demonstrated the clinical effects of Tianma Xingnao compared with Fufang Congrong Yizhi, implying beneficial effects of Tianma Xingnao on VaDAS-cog, MMSE, ADL and SDSVD outcomes. The same study was also mentioned in the consensus on Tianma Xingnao.
Thirteen of 36 RCTs evaluated TianZhi with combined pharmacological therapies, including donepezil hydrochloride (5 RCTs), oxiracetam (3 RCTs), dihydroergotoxine mesylate (1 RCT), huperzine A (1 RCT), butylohthalide (1 RCT), nimodipine (1 RCT), and Ginkgo biloba extract (1 RCT), while nonpharmacological combined therapy with Transcranial Magnetic Stimulation (TMS) was also employed in one RCT that used donepezil hydrochloride as a comparison. For the other 22 RCTs assessing the clinical effects of TianZhi without combined therapy, 18 RCTs conducted comparisons of conventional pharmacological interventions, such as almitrine and rubasine (7 RCTs), piracetam (5 RCTs), aniracetam (2 RCTs), donepezil hydrochloride (2 RCTs), oxiracetam (1 RCT), and nimodipine (1 RCT). One RCT compared Tianzhi to a combined treatment of almitrine, rubasine and nimodipine, and the remaining 3 RCTs adopted only routine therapies in the control groups. The outcomes suggested consistent beneficial effects on MMSE scores, HDS scores, FAQ scores, ADL scores, BBS scores, CIBIC-plus outcomes, TCD results, blood lipid levels, and VEGF levels. Two meta-analyses reported a beneficial effect of TianZhi for VaD without further details, while one meta-analysis indicated that TianZhi significantly contributed to improved MMSE scores in VaD.
Combined therapies of Tong-xin-luo were used in 4 of 9 RCTs, in which donepezil hydrochloride (1 RCT), dihydroergotoxine mesylate (1 RCT), piracetam (1 RCT), or butylohthalide (1 RCT) was used as both combined therapies and comparators. Three RCTs compared Tong-xin-luo to huperzine A (1 RCT), piracetam (1 RCT), or donepezil hydrochloride (1 RCT), while the remaining 2 RCTs evaluated Tongxin- luo in comparison with blank controls. The beneficial effects were consistent in the MMSE, HDS, TCD, and SDSVD outcomes. One meta-analysis of TongXinLuo demonstrated significant beneficial effects on MMSE and HDS scores in patients with VaD.
All 6 RCTs tested Yin-Dan Xin-Nao Tong with donepezil hydrochloride, Huperzine A, oxiracetam, nicergoline, or nimodipine as both combined interventions and comparators. The beneficial effects were consistent in the MMSE, HDS, and ADL outcomes.
Eighteen of 30 RCTs of Ginkgo biloba leaves conducted combined therapy with treatments identical to the comparators, including citicoline (2 RCTs), piracetam (3 RCTs), donepezil hydrochloride (2 RCTs), nicergoline (3 RCTs), butylohthalide (1 RCT), huperzine A (1 RCT), nimodipine (5 RCTs), and acupuncture (1 RCT). Six RCTs used piracetam as a comparator to combined therapies of Ginkgo biloba leaf with citicoline (1 RCT), huperzine A (1 RCT), aniracetam (1 RCT), dihydroergotoxine mesylate (1 RCT), vincamine (1 RCT), or electropuncture (1 RCT). One RCT compared the combination of Ginkgo biloba leaf and nimodipine to a blank control group. For the other 4 RCTs without any combined therapies, the effects of Ginkgo biloba leaf were appraised with comparisons of piracetam (2 RCTs), aniracetam (1 RCT), or Fufang Danshen (1 RCT). The consistent beneficial effects of Ginkgo biloba leaf on VaD were reported regarding MMSE, MoCA, HDS, ADL, ERP, TCD, HCY, and NO outcomes. In addition, three meta-analyses of Ginkgo biloba leaves published consistent beneficial effects on MMSE, HDS, and ADL outcomes in patients with VaD.
This scoping review was based on 2 consensuses, 10 meta-analyses and SRs and 139 RCTs; Furthermore, the review provided a comprehensive overview of the available evidence on traditional oral Chinese medicines for VaD. More than half of the RCTs were for TianZhi, Ginkgo biloba leaf, and Naoxintong. The number of RCTs in the last 20 y gradually increased and peaked in 2011. Although the number decreased after that time, SRs and meta-analyses have been published since 2011. The majority of the available evidence has examined the clinical effects of traditional oral Chinese medicines with combined therapies. A low risk of overall bias was achieved in only 7 trials in the bias risk assessment of interpolated twitch technique. The outcomes and effect metrics showed generally similar effects of traditional oral Chinese medicines for patients with VaD. More consistent benefits in terms of improved MMSE and ADL outcomes were observed in response to Ginkgo biloba leaf and Tianzhi. The outcomes of ADAS-Cog were only reported consistently for Fufang Danshen based on two individual RCTs. The results of this review may have several implications for future research on traditional oral Chinese medicine for the treatment of VaD.
First, little clinical evidence was found for some of the medicines evaluated. Fewer than 10 eligible researches were found for 5 medicines in this scoping review, while no eligible research was identified for 7 medicines. Although listed in the CNEHIP, CNEML, or CP, the medicines were defined as eliciting actions with TCM syndrome differentiation, instead of targeted for specific diseases, and only Tianzhi and Fufang Congrong Yizhi were licensed treatments for VaD across the indications of 17 medicines. Despite clinical research of these medicines having been conducted for multiple diseases[9,19-22], the clinical evidence for their application in VaD remains limited. Second, the quality of the reporting of RCTs was unsatisfactory, indicating an urgent need to improve transparency in RCTs. We were not successful in contacting authors to clarify aspects of RCT design that were partially reported, e.g., details of randomization, completeness of the follow-up by treatment arm, reasons for dropout or exclusion from analysis, scope of safety evaluations and safety outcomes and missing data for meta-analyses. In order to promote better reporting as well as improved methodology for obtaining evidence, it is highly desirable that a prospective trial protocol registration (e.g. in the Chinese Clinical Trials Registry), as well as compliance with the CONSORT Consolidated Standards for Reporting Trials for reporting the results of RCTs on TCM medicinal products. It is also recommended to adhere to best practices for designing RCTs for investigating Chinese traditional medicines, such as those proposed by Witt et al.[23], is recommended as well. In spite of the quality of the consensuses, the included SRs as well as metaanalyses in this scoping review were not assessed. The existing professional procedures for guidelines, consensuses, SRs and meta-analyses of Chinese traditional medicines are desirable for future research[24,25].
Third, the research rarely reported differentiation of symptoms. All the medicinal products covered used terms in TCM theory to describe the indications, indicating that the oral Chinese medicine in VaD needs to be syndrome differentiated. Notably, 66.9 % of the research lack of the reporting of TCM syndrome, which was found to be a limiting factor.
Fourth, in most of the research covered, oral Chinese traditional medicines were used combined with conventional pharmacological therapies. While some research reported these findings as adjunctive therapy and discussed side effects, these were insufficient to clarify the impact of herb-drug interactions. In order to prevent adverse reactions that may lead to undesirable adverse reactions or treatment failure, further clinical trials on pharmacokinetics and pharmacodynamics effects of combination therapy should be carried out with respect to the combination of traditional oral Chinese medicinal products and conventional pharmacological treatments[26].
Finally, the mechanisms underlying the majority of TCMs are unknown. It is necessary to further study the mechanism using modern scientific methods and methods to emphasize the therapeutic possibility of traditional Chinese drugs for VaD. Well-designed animal research and RCTs are also needed to confirm their physiological and pathologic role in treating VaD patients. The consistent benefits observed in several medicines could shed light on future drug development to treat VaD. However, although this scoping review reported the consistent benefits of several outcomes, they were only alternative outcomes and could not fully represent clinical benefits. Adequately designed RCTs are needed to establish the consistency of the beneficial effects on cognition, global performance, TCM syndrome, and other clinical performances.
Regarding English publications that comprehensively review oral TCM or herbs for VaD, a number of SRs have been published[8,12]. However, because of the poor quality of the evidence, the conclusions are limited. In 2018, one High Quality RCTs on TCM for VaD were carried out, but only for the integration of efficacy and safety of TCM[15]. Cocolane published a review of VaD, which compared the effectiveness and safety of TCM in treating VaD with different databases by May 2015[12], as well as a number of network meta-analyses of certain Chinese VaD medicines[27-29]. However, because the overall quality of the RCTs was unsatisfactory, the conclusions of these analyses of efficacy and safety were different and reflect the limitations of this research. We conducted this scoping review until June 2021.
We aimed to conduct a systematic, comprehensive analysis of 17 TCMs that are commonly employed for VaD and categorized in the CNEHIP, CNEML, or CP; to provide an overview of recent clinical evidence concerning the efficacy of TCMs; and to outline the effects of these medicines on VaD. Scope reviews are conducted in order to determine how much, what type, and what kind of evidence exists in a particular research area, to examine its value systematically, to draw conclusions from knowledge that is methodologically or disciplinarily distinct, or to identify research gaps to aid in future research planning as well as determination. Consequently, we did a scoping assessment of OCT for VaD using the PRISMA-ScR checklist to determine what evidence is now available and what is lacking. We included guidelines, consensuses, meta-analyses, SRs, and RCTs among the 17 OCTs for VaD. Nevertheless, there have been no scoping reviews of traditional oral Chinese medicines for VaD approved by the CNEHIP, CNEML, or CP.
There were several limitations of the research. Firstly, it was not possible to fully assess the quality of the study for all included RCTs, SRs, and metaanalyses; thus, only the main components were used as a compromise. Secondly, while the purpose of the scope review was to review recent knowledge on 17 oral TCMs, only 10 medicinal products were eligible. This outcome could be the result of the expanded search strategy for drug instructions in the preliminary work. By searching for drugs with terms such as ‘dementia’, ‘cognition’ and ‘memory’, traditional oral Chinese medicines for VaD could be selected extensively. However, it is inevitable that some drugs that are not employed for VaD would be included. The eligibility criteria applied in this review could be another reason for the lack of evidence about the seven medicines based on the possibility of eliminating publications with other diagnostic criteria or without diagnostic details. Despite no eligible evidence being shown in this scoping review, the 7 drugs with indications containing the terms ‘dementia’, ‘cognition’, or ‘memory’ are still worth investigating for the VaD therapy. Third, we were unable to contact all the authors of the trials in order to get a better understanding of the unclear risk of bias in trial conduct. In addition, certain lowbias research may not be included due to insufficient reporting; hence, the scope review may not adequately show the overall quality of the research throughout this domain. Fourth, the included research was all conducted in China, although we did not set limitations on population types in eligibility criteria. Therefore, the effects of these traditional oral Chinese medicines for patients with VaD in this review might not be universally applied for all populations.
The present scope review provides a summary of current knowledge and benefits of TCM oral therapy in VaD patients. The overall reporting quality of RCTs of traditional oral Chinese medicines for VaD was poor, and the transparency of these RCTs must be urgently improved. Consistent benefits were observed in several outcomes of traditional oral Chinese medicines for VaD. Given the promising findings in this scoping review, the applications of these traditional oral Chinese medicines could be broader if they were based on a number of properly designed, large-scale trials or researches conducted in the real world.
Acknowledgements:
Fund Project: This research was funded by The National Administration of Traditional Chinese Medicine: 2019 Project of building evidence-based practice capacity for TCM (No. 2019XZZX-NB014), Natural Science Foundation of Chongqing Municipal Science and Technology Commission, China (No. cstc2019jcyj-msxmX0047), Chongqing Talent Plan Project (No.[2021]5) and Chongqing Municipal Key Specialty Construction Project Traditional Chinese Medicine (No.[2022]4).
Conflict of interests:
The authors declared no conflict of interests.
Conclusion
GRDDS is considered a formulative strategy to enhance the absorption and bioavailability of the active pharmaceutical ingredient by retaining it in the absorptive window. It is a popular technique that has been widely studied for the past few decades. It offers effective drug delivery by improving patient compliance and minizing dosing frequency. It is firmly concluded that GRDDS has the potential to enhance the therapeutic efficacy of drugs that exhibit active absorption in the gastric region. It is obvious that clinical assessments of GRDDS still lack a solid foundation. Various new gastroretentive approaches using combinations of techniques remain less explored. It is anticipated that further investigation will reveal the extended potential of GRDDS.
Acknowledgements:
The authors thank Karpagam Academy of Higher Education for their support.
Conflict of interest:
The authors declared no conflict of interests.
References
- Wolters FJ, Ikram MA. Epidemiology of vascular dementia: Nosology in a time of epiomics. Arterioscler Thromb Vasc Biol 2019;39(8):1542-9.
[Crossref] [Google Scholar] [PubMed]
- Kalaria RN. The pathology and pathophysiology of vascular dementia. Neuropharmacology 2018;134:226-39.
[Crossref] [Google Scholar] [PubMed]
- Sun MK. Potential therapeutics for vascular cognitive impairment and dementia. Curr Neuropharmacol 2018;16(7):1036-44.
[Crossref] [Google Scholar] [PubMed]
- Tariq S, Barber PA. Dementia risk and prevention by targeting modifiable vascular risk factors. J Neurochem 2018;144(5):565-81.
[Crossref] [Google Scholar] [PubMed]
- Lagunin AA, Ivanov SM, Gloriozova TA, Pogodin PV, Filimonov DA, Kumar S, et al. Combined network pharmacology and virtual reverse pharmacology approaches for identification of potential targets to treat vascular dementia. Sci Rep 2020;10(1):257.
[Crossref] [Google Scholar] [PubMed]
- He W, Li M, Han X, Zhang W. Acupuncture for mild cognitive impairment and dementia: An overview of systematic reviews. Front Aging Neurosci 2021;13:647629.
[Crossref] [Google Scholar] [PubMed]
- Bai X, Zhang M. Traditional Chinese medicine intervenes in vascular dementia: Traditional medicine brings new expectations. Front Pharmacol 2021;12:689625.
[Crossref] [Google Scholar] [PubMed]
- Kim TH, Kang JW. Herbal medicine for vascular dementia: An overview of systematic reviews. Curr Vasc Pharmacol 2020;18(4):394-409.
[Crossref] [Google Scholar] [PubMed]
- Duan Y, Xu Z, Deng J, Lin Y, Zheng Y, Chen J, et al. A scoping review of cohort studies assessing traditional Chinese medicine interventions. BMC Complement Med Ther 2020;20(1):361.
[Crossref] [Google Scholar] [PubMed]
- World Health Organization. WHO international standard terminologies on traditional medicine in the western pacific region. 2007.
- Shorter E, Segesser K. Traditional Chinese medicine and western psychopharmacology: Building bridges. Phytother Res 2013;27(12):1739-44.
[Crossref] [Google Scholar] [PubMed]
- Chan ES, Bautista DT, Zhu Y, You Y, Long JT, Li W, et al. Traditional Chinese herbal medicine for vascular dementia. Cochrane Database Syst Rev 2018;12:CD010284.
- Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med 2018;169(7):467-73.
[Crossref] [Google Scholar] [PubMed]
- Zhang BL, Chen KY, Gao XM, Tian JZ, Li YP, Chen XM. The clinical application guideline of chinese traditional medicine in the treatment of vascular dementia. Chin J Integr Traditional Western Med 2021;41:273-9.
- Xu QQ, Shan CS, Wang Y, Shi YH, Zhang QH, Zheng GQ. Chinese herbal medicine for vascular dementia: A systematic review and meta-analysis of high-quality randomized controlled trials. J Alzheimers Dis 2018;62(1):429-56.
[Crossref] [Google Scholar] [PubMed]
- Arksey H, O'Malley L. Scoping studies: Towards a methodological framework. Int J Soc Res Methodol 2005;8(1):19-32.
- Levac D, Colquhoun H, O'Brien KK. Scoping studies: Advancing the methodology. Implement Sci 2010;5:69.
[Crossref] [Google Scholar] [PubMed]
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane; 2021.
- Yang XY, Wang LQ, Li JG, Liang N, Wang Y, Liu JP. Chinese herbal medicine Dengzhan Shengmai capsule as adjunctive treatment for ischemic stroke: A systematic review and meta-analysis of randomized clinical trials. Complement Ther Med 2018;36:82-9.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Ma H, Nan T, Li Y, Zheng W, Zhou Z, et al. Comparative efficacy of oral Chinese patent medicine for chronic prostatitis/chronic pelvic pain syndrome with sexual dysfunction: A Bayesian network meta-analysis of randomized controlled trials. Front Pharmacol 2021;12:649470.
- Min L, Wang X, Yang L, MA W, Yuan J, Liu X. Effect of compound danshen on neural function defect and free radicals in patients with cerebral infarction. Chin J Tissue Eng Res 2005;53(9):241-3.
- Wang X, Liu Y, Cheng L, Dong Y. Effectiveness of Yindan Xinnaotong capsule in the treatment of cardiovascular and cerebrovascular diseases: A meta-analysis. Chin J Evid Based Med 2017;17:429-39.
- Epidemiology of vascular dementia: Nosology in a time of epiomics
- Xie R, Xia Y, Chen Y, Li H, Shang H, Kuang X, et al. The RIGHT extension statement for traditional Chinese medicine: Development, recommendations, and explanation. Pharmacol Res 2020;160:105178.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Tan R, Lam WC, Yao L, Wang X, Cheng CW, et al. PRISMA (preferred reporting items for systematic reviews and meta-analyses) extension for Chinese herbal medicines 2020 (PRISMA-CHM 2020). Am J Chin Med 2020;48(6):1279-313.
[Crossref] [Google Scholar] [PubMed]
- Auxtero MD, Chalante S, Abade MR, Jorge R, Fernandes AI. Potential herb–drug interactions in the management of age-related cognitive dysfunction. Pharmaceutics 2021;13(1):124.
[Crossref] [Google Scholar] [PubMed]
- Ren FQ, Chen L, Cheng MM, Hao HF, Chen ZG, Han G. Bayesian network meta-analysis of 12 oral Chinese patent medicines combined with donepezil in the treatment of dementia. World Chin Med 2022;17:2587-94.
- Li FS, Liu ZY, Qiao ML, Cheng JH, Meng Y. Bayesian network meta-analysis on the efficacy and safety of oral Chinese drugs in the efficacy and safety of oral Chinese patent treatment of vascular dementia. Chin Pharm 2022;25:655-63.
- Shi ML, Zhao M, Zhang J, Sun TY, Jiang YN, Dong ZBM. Network Meta-analysis of oral Chinese patent medicine in adjuvant treatment of vascular dementia. Chin J Med 2020;22(3):12-8.
 : Guidelines and consensuses;
: Guidelines and consensuses;  : SRs and meta-analyses and
: SRs and meta-analyses and  : RCTs
: RCTs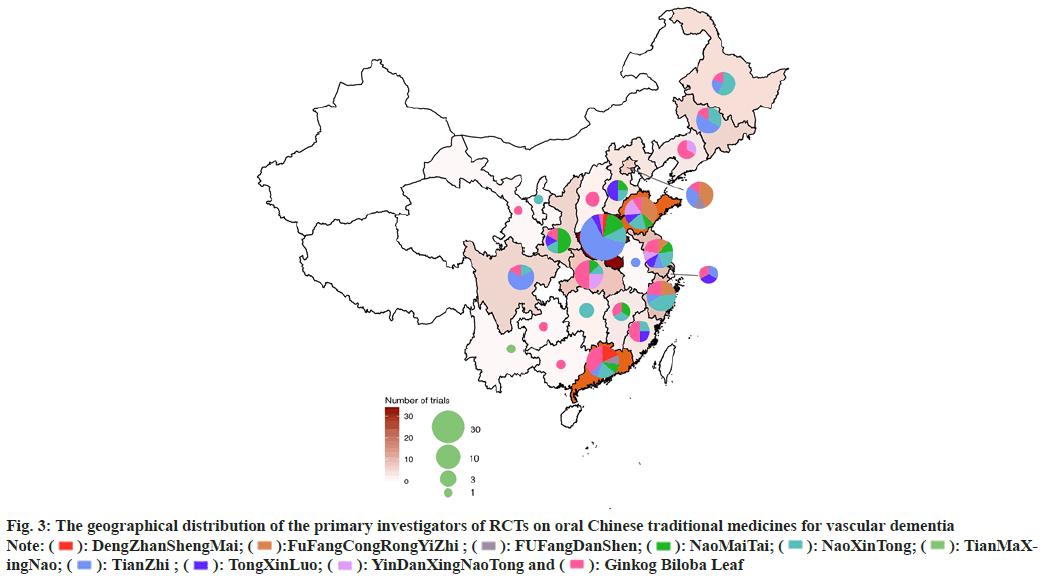
 : High;
: High;  : Some concems and
: Some concems and  : Low
: Low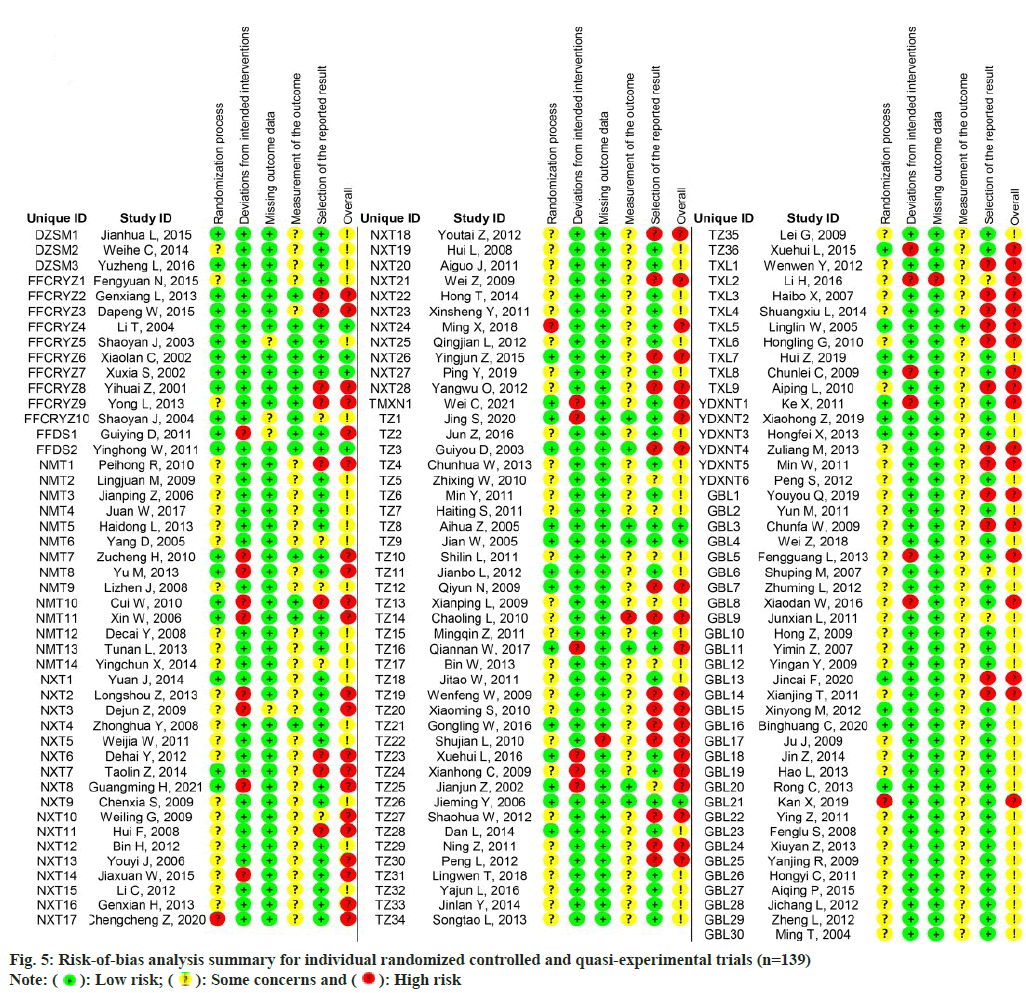
 : Low risk;
: Low risk;  : Some concerns and
: Some concerns and  : High risk
: High risk