- Corresponding Author:
- Effat souri
Department of Medicinal Chemistry, Faculty of Pharmacy and Drug Design and Development Research Center, Tehran University of Medical Sciences, Tehran-14155-6451
E-mail: souri@sina.tums.ac.ir
| Date of Submission | 12 May 2014 |
| Date of Revision | 10 January 2015 |
| Date of Acceptance | 30 May 2015 |
| Indian J Pharm Sci 2015;77(3):348-351 |
This is an open access article distributed under the terms of the Creative Commons Attribution−NonCommercial−ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non−commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
In this study a simple and efficient stability-indicating HPLC method with short run time was developed for the determination of nitisinone. The stress degradation of nitisinone was studied in different acidic, basic, oxidative, thermal and photolytic conditions. The chromatographic separation was achieved on a Nova-Pak C18 column using a mixture of 50 mM NaH 2 PO 4 (pH 2.5) and acetonitrile (45:55, v/v) as mobile phase. UV detection was performed at 280 nm. Good linearity was observed over the concentration range of 0.5-50 μg/ml with r 2 >0.999. The within-day and between-day precision values were less than 2%. The proposed method could be used for the determination of nitisinone in the presence of its degradation products and also dosage form excipients for the quality control purposes.
Keywords
Nitisinone, stability-indicating, stress degradation, HPLC, UV detection
Nitisinone (NTBC), 2-(2-nitro-4-trifluoromethyl benzoyl)-1,3-cyclohexanedione (fig. 1), is a reversible inhibitor of 4-hydroxyphenylpyruvate dioxygenase, which is used for the treatment of hereditary tyrosinemia type I [1]. Only a few articles have been published which reported the HPLC determination of nitisinone in biological fluids [2,3]. Capillary electrophoresis [4] and LC-MS/MS methods [5-9] have also been reported for the determination of nitisinone in biological samples. There is no pharmacopeial monograph for nitisinone or any reported HPLC method for the determination of nitisinone in pharmaceutical dosage forms.
In this study an HPLC method has been developed and validated for the determination of nitisinone in pharmaceutical formulations, which is necessary for quality control purposes. The stability of nitisinone was also studied under different stress conditions and the proposed method was shown to be stability-indicating.
Nitisinone bulk powder (batch No: 9009011) and nitisinone capsules (2 mg) (Batch No: 007) were kindly provided by Osvah Pharmaceutical Company, Tehran, Iran. All the analytical grade chemicals and HPLC grade solvents were from Merck (Darmstadt, Germany). Water was purified by using a Milli-Q purification system (Millipore, Milford, MA, USA). Stock standard solution of nitisinone at the concentration level of 2500 µg/ml was prepared in methanol. Working standard solutions were freshly prepared by consecutive dilution in mobile phase during the analysis day.
A Waters HPLC system (Milford, USA) was consisted of a Model 515 pump, a Model 710 plus autosampler and a Model 480 variable UV/Vis detector. A multi-channel Chrom and Spec software for chromatography (version 1.5×) was used for data processing. A dry air oven (Melag, Germany) and a Memmert water bath (Gmb+Co. KG, Germany) were used for heating. A 100 W tungsten lamp and a low pressure Mercury lamp 200 W were used as visible and UV light sources.
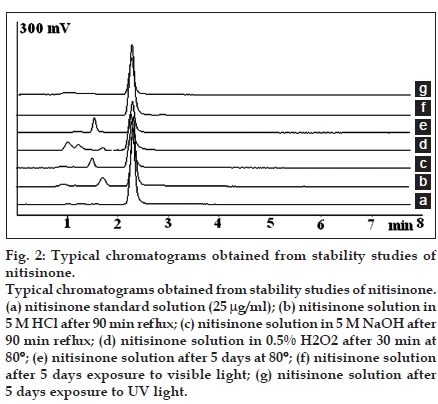
A Nova-Pak C18 4 µm column, 150×3.9 mm, (Waters, Milford, USA) was used for chromatographic separation. The mobile phase was consisted of 50 mM NaH2PO4 with pH adjusted to 2.5 with o-phosphoric acid and acetonitrile (45:55, v/v) and delivered isocratically at a flow rate of 1 ml/min. The mobile phase was degassed by sonication for 10 min. The injection volume was 20 µl and the UV detection was performed at 280 nm. Acceptable peak symmetry and retention time were observed without any interference from capsule excipients or degradation products. Representative chromatograms are presented in fig. 2. System suitability parameters were obtained from six replicate injections. The USP theoretical plates and tailing factor were 3200 and 0.98, respectively. The retention time and peak area repeatability were also 0.82 and 0.74%, respectively. All the parameters were within the acceptable range.
Fig. 2:Typical chromatograms obtained from stability studies of nitisinone. Typical chromatograms obtained from stability studies of nitisinone. (a) nitisinone standard solution (25 µg/ml); (b) nitisinone solution in 5 M HCl after 90 min reflux; (c) nitisinone solution in 5 M NaOH after 90 min reflux; (d) nitisinone solution in 0.5% H2O2 after 30 min at 80°; (e) nitisinone solution after 5 days at 80°; (f) nitisinone solution after 5 days exposure to visible light; (g) nitisinone solution after 5 days exposure to UV light.
Linearity of the method was studied by injecting six series of nitisinone standard solutions in mobile phase in the range of 0.5-50 µg/ml, at eight different concentration levels (0.5, 1, 2, 5, 10, 20, 40 and 50 µg/ml). The peak area versus nitisinone concentration was treated by least-squares linear regression analysis and the statistical data were calculated. Excellent linearity was observed over the concentration range of 0.5-50 µg/ml, with a high value of correlation coefficient. The slope and intercept of the calibration curves were 74.99±0.76 and 10.12±3.37, respectively. The quantification limit (LOQ) and detection limit (LOD) were calculated using the following Eqns [10], LOQ=10σ/s and LOD=3.3σ/s, where s is the standard deviation of intercept and s is the slope of the calibration graph. According to the results obtained the LOQ and LOD was 0.45 and 0.15 µg/ml, respectively.
To evaluate the accuracy and precision of the method, nitisinone standard solutions at three different concentration levels (0.5, 5.0, and 50.0 µg/ml) in the calibration range were prepared and injected to the HPLC system. The concentration of nitisinone was calculated using calibration curves. This study was performed in triplicate in one day and three different days. The within-day and between-day accuracy and precision data for the proposed method are demonstrated in Table 1. It was shown that the proposed method is reproducible over the studied concentration range. Assay results for nitisinone capsules by two analysts using two different HPLC systems did not show significant variations (CV<2%) which shows the ruggedness of the method.
| Concentration added (µg/ml) | Concentration | CV (%) | Error (%) |
|---|---|---|---|
| Within day (n=3) | |||
| 0.50 | 0.51±0.01 | 1.96 | 2.00 |
| 5.00 | 5.01±0.06 | 1.20 | 0.20 |
| 50.00 | 50.30±0.46 | 0.91 | 0.60 |
| Between day (n=9) | |||
| 0.50 | 0.50±0.01 | 2.00 | 0.00 |
| 5.00 | 5.02±0.04 | 0.80 | 0.40 |
| 50.00 | 50.28±0.41 | 0.82 | 0.56 |
Table 1: Precision and accuracy of the method for determination of nitisinone
Robustness of the analytical method is an indication of its reliability and the method robustness should evaluate to find out the effect of small variations in the method conditions on the analytical parameters. The influence of the organic phase composition (55±3) and pH value (2.5±0.2) of the buffer solution of the mobile phase were studied. No significant variations in peak area (CV<1%) were observed under different conditions. The retention time was dependent to the organic phase composition, but no problem was observed for peak resolution or quantification of nitisinone.
Stability of nitisinone standard stock solutions in methanol kept in refrigerator and also working standard solutions in the mobile phase kept in room temperature were evaluated. The standard stock solutions of nitisinone in methanol were stable after 5 days storage in refrigerator (recovery>99.5%). On the other hand, the working standard solutions of nitisinone in the mobile phase was relatively stable for 8 h at room temperature (recovery>99%). After 24 h a significant decrease (recovery<75%) was observed in the nitisinone solutions kept at room temperature.
The proposed method was applied for the determination of nitisinone content in pharmaceutical dosage forms. The content of ten nitisinone capsules (2 mg) were combined and weighed. An accurately weighed amount of the powder equivalent to one capsule was transferred to a 100 ml volumetric flask. After addition of 70 ml of the mobile phase, the solution was sonicated for 15 min. The flask made up to volume by the mobile phase. After filtration and two times dilution, 20 µl of the resulted solution was injected to the HPLC system. The peak area was compared with a nitisinone standard solution at the same concentration level and the nitisinone amount in capsules was calculated. Excellent agreement with the labeled amount (2.00±0.02) was observed.
To find out the relative recovery of nitisinone, one ml of nitisinone standard solution (2000 µg/ml) was added to an assay sample equivalent to one capsule and analyzed according to the assay method. Recovery was calculated by comparing the peak area of this solution and a standard solution at the same concentration value. The relative recovery of nitisinone using standard addition method was found to be 100.28±1.00 which shows no significant interferences from capsule excipients.
For forced degradation studies, the solid form or a solution of nitisinone at the initial concentration of 250 µg/ml was subjected to different degradation conditions. After the degradation, a sample of solid forms or a diluted solution of degraded samples at the concentration level of 25 µg/ml was prepared in mobile phase and injected to the HPLC system. A freshly prepared standard solution of nitisinone (25 µg/ml) was used to calculate the percentage of degradation. All the experiments were performed in triplicate.
Acid degradation studies was carried out in a mixture of 2 M HCl or 5 M HCl and methanol (85:15) and reflux for 90 min. Methanol was used as co-solvent. The degradation samples were neutralized by appropriate amount of sodium hydroxide before dilution and injection to the HPLC system. Basic degradation studies were carried out in a mixture of 2 M NaOH or 5 M NaOH and methanol (85:15) and reflux for 90 min. The samples were neutralized by hydrochloric acid before dilution and injection to the HPLC system. In the presence of 2 M HCl or 2 M NaOH, nitisinone was relatively stable (degradation<10%). Using 5 M HCl or 5 M NaOH the degradation rate was increased. The results are shown in Table 2. A small degradation peak at the retention time between 1 and 2 min was observed for both degradation conditions (figs. 2b and c).
| Stress test condition | Solvent | Temperature | Time | % of nitisinone |
|---|---|---|---|---|
| Acidic | 2 M HCl−methanol | Reflux | 90 min | 90.0 |
| (85:15) | ||||
| 5 M HCl−methanol | Reflux | 90 min | 76.4 | |
| (85:15) | ||||
| Basic | 2 M NaOH−methanol | Reflux | 90 min | 94.3 |
| (85:15) | ||||
| 5 M NaOH−methanol | Reflux | 90 min | 60.8 | |
| (85:15) | ||||
| Oxidative | 1% H2O2−methanol | 80° | 30 min | 7.1 |
| (85:15) | ||||
| 0.5% H2O2−methanol | 80° | 30 min | 43.5 | |
| (85:15) | ||||
| Photolytic | ||||
| UV | Solid form | RT* | 5 days | 92.6 |
| UV | Water−methanol (85:15) | RT* | 5 days | 31.7 |
| Visible | Solid form | RT* | 5 days | 93.2 |
| Visible | Water−methanol (85:15) | RT* | 5 days | 83.8 |
| Heat | Solid form | 80° | 5 days | 96.8 |
| Water−methanol (85:15) | 80° | 5 days | 27.4 |
Table 2: Stress degradation tests on nitisinone bulk powder using different conditions
A 1% or 0.5% hydrogen peroxide solution and methanol (85:15) was used for oxidative degradation studies and the solution was kept in a water bath at 80° for 30 min. Nitisinone was very labile in oxidative conditions and more than 92% degradation was observed after 30 min at 80° using 1% H2O2. Using 0.5% H2O2, the degradation rate was decreased and about 66% degradation was observed after 30 min at 80°. Two small peaks were observed in this condition (fig. 2d).
For thermal degradation condition studies, bulk powder of nitisinone in the solid form (in a thin layer) was placed in a dry oven at 80° for 5 days. A nitisinone solution in water and methanol (85:15) was also exposed to thermal degradation in a dry oven at 80° for 5 days. For photolytic degradation studies, bulk powder of nitisinone (in a thin layer) or a solution of nitisinone in water and methanol (85:15) was exposed to visible or UV light at a distance of about 20 cm from the light source at room temperature for 5 days. Slight degradation of nitisinone in solid form was observed upon exposure to visible light, UV light or heat. On the other hand, nitisinone solutions in water-methanol (85:15) were extensively degraded in these conditions as shown in Table 2. A major degradation peak was observed after heating of nitisinone solutions at the retention time of about 1.5 min (fig. 2e). No new peak was detected upon exposure to visible or UV light (figs. 2f and g).
Aknowledgements
This study was part of a Pharm D thesis supported by Tehran University of Medical Sciences (grant No: 23049- 92-02-92).
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- de Laet C, Dionisi-Vici C, Leonard JV, Mckiernan P, Mitchell G, Monti L, et al.Recommendations for the management of tyrosinaemia type 1. Orphanet J Rare Dis 2013;8:8.
- Hall MG, Wilks MF, McLean Provan W, Eksborg S, Lumholtz B. Pharmacokinetics and pharmacodynamics of NTBC (2-(2-nitro-4-fluoromethylbenzoyl)-1,3-cyclohexanedione) and mesotrione, inhibitors of 4-hydroxyphenyl pyruvate dioxygenase (HPPD) following a single dose to healthy male volunteers. Br J ClinPharmacol 2001;52:169-77.
- Bielenstein M, Astner L, Ekberg S. Determination of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione in plasma by direct injection into a coupled column liquid chromatographic system. J Chromatogr B Biomed SciAppl 1999;730:177-82.
- Cansever MS, Aktuglu-Zeybek AC, Erim FB. Determination of NTBC in serum samples from patients with hereditary tyrosinemia type I by capillary electrophoresis. Talanta 2010;80:1846-8.
- Herebian D, Spiekerkotter U, Lamshoft M, Thimm E, Laryea M, Mayatepek E. Liquid chromatography tandem mass spectrometry method for the quantitation of NTBC (2-(nitro-4-trifluoromethyl benzoy)1,3-cyclohexanedione) in plasma of tyrosinemia type I patatients. J Chromatogr B 2009;877:1453-9.
- Prieto JA, Andrade F, Lage S, Aldamiz-Echevarria L. Comparison of plasma and dry blood spots as samples for the determination of nitisinone (NTBC) by high-performance liquid chromatography-tandem mass spectrometry. Study of the stability of the samples at different temperatures. J Chromatogr B AnalytTechnol Biomed Life Sci 2011;879:671-6.
- Sander J, Janzen N, Terhardt M, Sander S, Gokcay G, Demirkol M, et al. Monitoring tyrosinaemia type I: Blood spot test for nitisinone (NTBC). ClinChimActa 2011;412:134-8.
- La Marca G, Malvagia S, Materazzi S, Della Bona ML, Boenzi S, Martinelli D, et al. LC-MS/MS method for simultaneous determination of a dried blood spot of multiple analytes relevant for treatment monitoring in patients with tyrosinemia type I. Anal Chem 2012;84:1184-8.
- Davit-Spraul A, Romdhane H, Poggi -Bach J. Simple and fast quantification of nitisone (NTBC) using liquid chromatography-tandem mass spectrometry method in plasma of tyrosinemia type I patients. J ChromatogrSci 2012;50:446-9.
- Shabir GA. Validation of high-performance liquid chromatography methods for pharmaceutical analysis. Understanding the differences and similarities between validation requirements of the US Food and Drug Administration, The US Pharmacopeia and the International Conference on Harmonization. J Chromatogr A 2003;987:57-66.