- Corresponding Author:
- R. V. Nadh
Department of Biotechnology, Vignan University, Vadlamudi-522 213, Guntur, India
E-mail: doctornadh@yahoo.co.in
| Date of Submission | 25 December 2011 |
| Date of Revision | 8 December 2012 |
| Date of Acceptance | 10 December 2012 |
| Indian J Pharm Sci, 2012, 74 (6): 580-583 |
Abstract
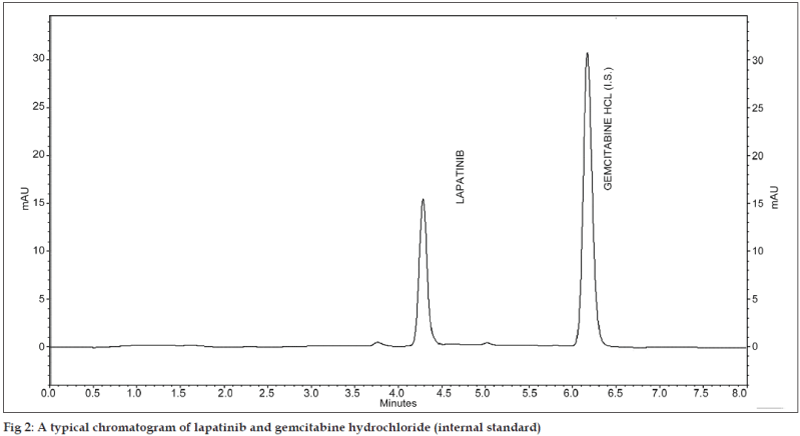
A simple, selective, rapid, precise and economical reverse-phase high-performance liquid chromatography method has been developed for the determination of lapatinib in tablet using gemcitabine hydrochloride as an internal standard. Chromatography was carried out on an ODS C-18 RP column (4.6 mm i.d. ×250 mm) using a mixture of acetonitrile and water (50:50 v/v) as the mobile phase at a flow rate of 1.0 ml/min. The drug was monitored at 232 nm. The retention times for lapatinib and gemcitabine hydrochloride were found to be 4.25±0.05 and 6.10±0.05 min, respectively. The method produced linear responses in the concentration range of 2-60 μg/ml of lapatinib. The limit of detection and limit of quantitation were 0.265 and 0.884 μg/ml, respectively.
Keywords
Gemcitabine hydrochloride, internal standard, lapatinib, reverse-phase high-performance liquid chromatography, validation
Lapatinib is a small molecule kinase inhibitor and derivative of 4-anilinoquinoline [1]. Lapatinib was approved by the US Food and Drug Administration on March 13, 2007 [2]. Its molecular formula is C29H26ClFN4O4S and chemically it is N- [3-chloro-4- [(3-fluorophenyl) methoxy] phenyl]-6- [5- [(2-methylsulfonylethylamino) methyl]-2-furyl] quinazolin-4-amine [3] (fig. 1). It is commonly prescribed for the treatment of breast cancer and other solid tumours. It reversibly inhibits ErbB1 and ErbB2 tyrosine kinases leading to inhibition of MAPK and PI3K signalling pathways [4,5].
Literature survey reveals that high-performance liquid chromatography (HPLC) [6], liquid chromatography-mass spectrometry (LC-MS)/MS [7-11] and ultraperformance liquid chromatography (UPLC)/ MS-MS [12] methods have been developed for the estimation of lapatinib. However, reverse-phase high-performance liquid chromatography (RP-HPLC) method employing internal standard (IS) for the quantification of lapatinib in pharmaceutical dosage form has not yet reported. IS methods are preferred for quality control of drugs since it overcomes errors from sample preparation and run to run variations, thus improving the accuracy and precision of the method. Moreover, IS methods are highly sensitive and specific [13]. Hence, in the present investigation, a new RP-HPLC method has been reported for the estimation of lapatinib in tablet dosage form using gemcitabine hydrochloride as an IS.
HPLC grade acetonitrile (E. Merk,Mumbai, India) was used. Triple distilled water was prepared by using a glass distillation apparatus. Agilent 1120 Compact LC-HPLC system consisting of a gradient liquid pump, UV/Vis spectrophotometric detector, ODS C-18 RP column (4.6×250 mm), 20 μl Hamilton injecting syringe and widow based single channel software was used. An Essae electronic balance was used for weighing the materials.
The mobile phase was filtered through 0.45 μm membrane filter and then used. The flow rate of the mobile phase was maintained at 1 ml/min. The mobile phase consists of acetonitrile and water in the ratio of 50:50 (v/v). Different compounds were tested as an IS for the chromatographic procedure. Among them, gemcitabine was eluted within 8 min of the analysis and has a better symmetry as well as resolution with respect to lapatinib. It shows that gemcitabine behaves in a similar way to the analyte under investigation. Hence, gemcitabine has been chosen as an IS. The detection was carried out at wavelength of 232 nm with a run time of 8 min. The retention times for lapatinib and gemcitabine hydrochloride were found to be 4.25±0.05 and 6.10±0.05 min, respectively (fig. 2). In the present method, the retention time for lapatinib is 4.25±0.05 min, which is lower compared to 6.5 min of the earlier HPLC validation method proposed by Haribabu et al. [6]. In simultaneous test for six tyrosine kinase inhibitors by LC-MS/MS by using a gradient of acetonitrile containing 1% formic acid with 20/10 mM ammonium formate, the retention times were 8.5/6.6 min for lapatinib detection [8,10], whereas, the retention time was only 1.8 min in UPLC-MS/MS method adopted by Bouchet et al. [12] under a gradient of combination of phase A (4 mM ammonium formate, pH 3.2) and phase B (90% acetonitrile and 10% phase A).
Standard stock solutions of lapatinib and gemcitabine hydrochloride were prepared by dissolving 25 mg of each in separate 25 ml of the mixture of acetonitrile and water (50:50 v/v) to get 1 mg/ml solutions. These standard stock solutions were suitably diluted with mobile phase (acetonitrile: water 50:50 v/v) to get the stock solution of concentration of 10 and 100 μg/ml.
The main validation parameters such as linearity and range, accuracy and precision, recovery, ruggedness, limit of detection (LOD) and limit of quantitation (LOQ) were evaluated in developed method [14].
Working standard solutions of lapatinib (concentration ranges from 2 to 60 μg/ml) containing gemcitabine hydrochloride (5 μg/ml) as an IS were prepared using mobile phase as a solvent. Twenty microliters of each working solution was injected into the HPLC system to obtain the chromatogram. Area under curve (AUC) values for analyte and IS were measured (Table 1). The calibration curve was constructed by plotting ratio of AUC (of analyte to an IS) versus analyte standard concentration. The linear dynamic was found to be within the range 1-60 μg/ml. The regression of the curve was computed by linear regression fitting and its mathematical expression was y=0.025949x+0.054673 (where y is the ratio of peak areas of the analyte to that of an IS and x is the concentration of lapatinib) with a correlation coefficient (R2) of 0.996408. This regression equation was used to estimate the amount of lapatinib in tablet.
| Concentration (μg/ml) | Ratio of AUC of Lapatinib to gemcitabine HCl* | |
|---|---|---|
| Lapatinib | Gemcitabine HCl (IS) | |
| 2 | 5 | 0.1166 |
| 4 | 5 | 0.1566 |
| 6 | 5 | 0.1827 |
| 8 | 5 | 0.2141 |
| 10 | 5 | 0.3299 |
| 20 | 5 | 0.5546 |
| 30 | 5 | 0.8984 |
| 40 | 5 | 1.1649 |
| 50 | 5 | 1.3613 |
| 60 | 5 | 1.5358 |
*Average of three determinations, IS=Internal standard, AUC=Area under curve
Table 1: Calibration values for lapatinib
A standard solution containing 20 μg/ml of lapatinib and 5 μg/ml of gemcitabine hydrochloride was used to determine the precision of the developed method. The prepared solution was injected into the HPLC system in six replicates under the same chromatographic conditions. The chromatograms were recorded for the intra and inter-day variations (Table 2). The percentage of relative standard deviation (RSD) for the calculated values was 0.223, which were very much within the allowable limit of 2% [15], which indicates that the developed method shows precision. In the measurement of a range of tyrosine kinase inhibitors in human plasma using turbulent flow LC-MS/MS [10,11] and UPLC-MS/MS [12] methods, the percentage of RSD varied from 3.9 to 8.1 for lapatinib estimation.
| Day | Concentration of lapatinib (μg/ml)* | Mean ratio of AUC of drug to IS* | %RSD | Mean % RSD |
|---|---|---|---|---|
| Day 1 | 20 | 0.5684 | 0.289 | 0.223 |
| Day 2 | 20 | 0.5436 | 0.128 | |
| Day 3 | 20 | 0.5646 | 0.253 |
*All the values are the averages of three determinations, IS=Internal standard, AUC=Area under curve, RSD=Relative standard deviation
Table 2: Precision readings of the proposed method
The solutions of lapatinib containing 10 μg/ml were subjected to the proposed HPLC method of analysis to find out the recovery by the proposed method. The recovery studies were carried out by adding known amount of lapatinib to the preanalysed sample and subjected them to the proposed HPLC method of analysis. The mean percentage of recovery was found to be 100.89% (Table 3).
| Concentration of lapatinib in sample solution (μg/ml) | Amount added (μg/ml) | Total amount (μg/ml) | Amount found (μg/ml)* | % Recovery | Mean % recovery |
|---|---|---|---|---|---|
| 10 | 5 | 15 | 14.775 | 104.53 | 100.89 |
| 10 | 10 | 20 | 19.913 | 95.90 | |
| 10 | 15 | 25 | 25.088 | 102.24 |
*All the values are the averages of three determinations
Table 3: Recovery studies of the proposed high-performance liquid chromatography method
For determination of sensitivity of the proposed method, LOD and LOQ were calculated. The lowest detectable concentration of the analyte by the method is LOD whereas the minimum quantifiable concentration is LOQ. LOD and LOQ for lapatinib were calculated according to the International Conference on Harmonization guidelines by using S (standard deviation of the response) and σ (slope of the calibration curve) and the results indicate that the proposed method is sensitive to detect and quantify. The system suitability parameters were: Theoretical plates per meter (10,136); height equivalent to theoretical plates (0.0098); tailing factor (2.0). The limit of detection (LOD=3.3×σ/S) and limit of quantitation (LOQ=10×σ/S) were established at a signal-to-noise ratio 1:3 and were found to be 0.265 and 0.884 μg/ml, respectively.
The ruggedness of the proposed method was evaluated by applying the developed procedure for assay of lapatinib using the same instrument by two different analysts under the same optimized conditions at different days. The obtained results were found to be reproducible, since there was no significant difference between analysts. Thus, the proposed methods could be considered rugged.
Two tablets (Tykerb-250 mg) were powdered and used for estimation. An IS was added prior to the tablet powder treatment step, so that any losses of the analyse during sample preparation can be paralleled by losses of the IS. Accordingly, the standard will correct for these losses. An accurately weighed portion of powder equivalent to 25 mg of lapatinib was dissolved in 25 ml of the mixture of acetonitrile and water (50:50 v/v) and filtered through 0.4 μm membrane filter. From the filtrate, 0.1 ml was pipette in to 10 ml graduated test tube and made up to volume with the mobile phase. Twenty microliters of the sample was injected in to the column. The extracts of the formulations containing lapatinib showed no significant peaks except that of lapatinib. This indicates that the excipients in the solid dosage form did not interfere the estimation and hence, the proposed method was found to be specific. The drug content in the tablet was quantified using the regression equation was found to be 99.98%.
In conclusion, the values of recovery studies indicate that the method was accurate and low percent RSD indicates the reproducibility of assay of lapatinib in bulk drug and dosage forms. The method was rapid as the run time was only 8 min and method was also economical as the mobile phase was a mixture of acetonitrile and water.
References
- Julia L, Michelle G, Mark P, Joyce S. Lapatinib: New opportunities for management of breast cancer. Breast Cancer (London) 2010;2:79-91.
- Medina PJ, Goodin S. Lapatinib: A dual inhibitor of human epidermal growth factor receptor tyrosine kinases. ClinTher 2008;30:1426-47.
- Petrov KG, Zhang YM, Carter M, Cockerill GS, Dickerson S, Gauthier CA, et al. Optimization and SAR for dual ErbB-1/ErbB-2 tyrosine kinase inhibition in the 6-furanylquinazoline series. Bioorg Med ChemLett 2006;16:4686-91.
- Gomez HL, Doval DC, Chavez MA, Ang PC, Aziz Z, Nag S, et al. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol 2008;26:2999-3005.
- Rezai K, Urien S, Isambert N, Roche H, Dieras V, Berille J, et al. Pharmacokinetic evaluation of the vinorelbine-lapatinib combination in the treatment of breast cancer patients. Cancer ChemotherPharmacol 2011;68:1529-36.
- Haribabu B, BalaMurali Krishna K, Rama Krishnaveni P. Development and validation of HPLC method for the estimation of lapatinib in bulk drugs and pharmaceutical formulations. Int J Res Rev Pharm ApplSci 2011;1:207-14.
- Bai F, Freeman BB 3rd, Fraga CH, Fouladi M, Stewart CF. Determination of lapatinib (GW572016) in human plasma by liquid chromatography electrospray tandem mass spectrometry (LC-ESI-MS MS). J Chromatogr B AnalytTechnol Biomed Life Sci 2006;831:169-75.
- Haouala A, Zanolari B, Rochat B, Montemurro M, Zaman K, Duchosal MA, et al. Therapeutic Drug Monitoring of the new targeted anticancer agents imatinib, nilotinib, dasatinib, sunitinib, sorafenib and lapatinib by LC tandem mass spectrometry. J Chromatogr B AnalytTechnol Biomed Life Sci 2009;877:1982-96.
- Sandra R, Gillian McM, Martin C, Robert OC. Development and application of novel analytical methods for molecularly targeted cancer therapeutics. J Chromatogr B 2009;877:3982-90.
- Götze L, Hegele A, Metzelder SK, Renz H, Nockher WA. Development and clinical application of a LC-MS/MS method for simultaneous determination of various tyrosine kinase inhibitors in human plasma. ClinChimActa 2012;413:143-9.
- Couchman L, Birch M, Ireland R, Corrigan A, Wickramasinghe S, Josephs D, et al. An automated method for the measurement of a range of tyrosine kinase inhibitors in human plasma or serum using turbulent flow liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 2012;403:1685-95.
- Bouchet S, Chauzit E, Ducint D, Castaing N, Canal-Raffin M, Moore N, et al. Simultaneous determination of nine tyrosine kinase inhibitors by 96-well solid-phase extraction and ultra performance LC/ MS-MS. ClinChimActa 2011;412:1060-7.
- Snyder LR, Kirkland JJ, Glajch JL. editors. Practical HPLC Method Development. 2nded. New York, USA: John Wiley and Sons; 1997. p. 165.
- ICH. Validation of Analytical Procedures: Text and Methodology. Geneva: International Conference on Harmonization; 2005.
- Isabel T, Marc DL, Erik VB. Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. Trends Anal Chem 2004;23:535-52.