- *Corresponding Author:
- Yongjun Deng
Shanghai Pudong New Area Guangming Hospital of Traditional Chinese Medicine, Pudong, Shanghai 201300, P. R. China
E-mail: dengyjspnagh@protonmail.com
| Date of Received | 26 May 2021 |
| Date of Revision | 14 July 2022 |
| Date of Acceptance | 04 April 2023 |
| Indian J Pharm Sci 2023;85(2):456-462 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
We aimed to investigate the mechanism of action of Yupingfeng capsule for combined allergic rhinitis and asthma syndrome via the toll-like receptor 4/nuclear factor-kappa B pathway. Rats were divided into normal, model, experimental and control groups. Combined allergic rhinitis and asthma syndrome model was established through sensitization with ovalbumin and aluminium hydroxide and intranasal instillation and atomization of ovalbumin. Then experimental and control groups were given Yupingfeng capsules and dexamethasone at 2 mg/kg by gavage once a day for 14 consecutive days, while normal and model groups were intraperitoneally injected with an equal dose of normal saline. Their nasal symptoms were scored and cell apoptosis in lung tissues was observed by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling staining. Serum levels of interleukin-5 and interleukin-13 were detected by enzyme-linked immunosorbent assay and toll-like receptor 4 and nuclear factor-kappa B expressions in lung and nasal mucosa tissues were measured by Western blotting. The score of nasal symptoms, levels of interleukin-5 and interleukin-13, cell apoptosis rate in lung tissues and expressions of toll-like receptor 4 and nuclear factor-kappa B were significantly lower in experimental and control groups than in model group (p<0.05). Yupingfeng capsule can obviously suppress the release of inflammatory factors and the apoptosis of cells as well as relieve the symptoms of combined allergic rhinitis and asthma syndrome rats probably by inhibiting the toll-like receptor 4/nuclear factor-kappa B pathway.
Keywords
Yupingfeng capsule, allergic rhinitis, asthma syndrome, toll-like receptor 4, nuclear factor-kappa B pathway
Combined Allergic Rhinitis and Asthma Syndrome (CARAS) is an allergic inflammatory disease common in the department of respiratory medicine, with nasal obstruction, watery nasal discharge, sneezing, recurrent cough and other allergic symptoms of the upper and lower respiratory tracts as the clinical manifestations[1]. The pathogenesis of CARAS has not been completely elaborated yet and traditional Western medicine treatments focus on inhibiting inflammation and allergy, and can only relieve symptoms temporarily. The easy recurrence of the disease and apparent side effects of traditional therapeutic drugs seriously affect the normal life of patients[2]. Traditional Chinese Medicine (TCM) and pharmacy have prominent advantages and significant efficacy. Clinical doctors of TCM have accumulated numerous therapeutic methods and prescriptions such as warming lung and relieving stuffiness, reinforcing qi and dispelling wind, Xuanbi Huayin decoction and Yupingfeng capsule throughout thousands of years and satisfactory clinical efficacy has been obtained[3]. However, the action pathways and onset methods of TCM have not been clarified, thus hindering its recognition and popularizing prospect. Yupingfeng capsule is made of active components including Astragalus membranaceus, Dendranthema indicum, Platycarya strobilacea Sieb. et Zucc, Flos Magnoliae, Prunella vulgaris L., Saposhnikovia divaricate Root, Glycyrrhiza and Ligusticum chuanxiong Hort, which possesses the efficacy of relieving stuffiness and pain as well as dispelling wind and evil. Yupingfeng capsule can inhibit inflammation, antagonize allergic media and regulate immunity favorably[4]. Wen et al.[5] demonstrated that Yupingfeng capsule had definite efficacy in treating moderate-to-severe allergic persistent rhinitis, but there has been no report about its application in CARAS. Toll- Like Receptor 4 (TLR4)/Nuclear Factor-kappa B (NF-κB) pathway is a vital signaling pathway of inflammatory stress in organisms. Jin et al.[6] demonstrated that repressing the TLR4/NF-κB pathway can remarkably ameliorate inflammatory responses in rats with allergic rhinitis. In this study, rat models of CARAS were established to explore the action mechanism of Yupingfeng capsule in CARAS through the TLR4/NF-κB pathway, aiming to provide new ideas for clinicians in treating CARAS.
Materials and Methods
Experimental animals:
A total of 100 Specific Pathogen Free (SPF) grade male Sprague-Dawley (SD) rats (license no: SCXK (Zhejiang) 2019-0005) aged (7-8) w old and weighing (200-220) g were purchased from Westlake Institute for Advanced Study. They were adaptively fed with standard diets in separate cages (4 cages) at room temperature and had free access to water for 1 w according to the laboratory animal administration methods of our hospital, followed by tests.
Main reagents and apparatus:
Yupingfeng capsules (Jiangsu Jibeier Pharmaceutical Co., Ltd., NMPN: Z10980026) and dexamethasone tablets (Guangdong Huanan Pharmaceutical Group Co., Ltd., 0.75 mg×100 s, NMPN: A44024469) were applied.
Enzyme-Linked Immunosorbent Assay (ELISA) kits were bought from Selleck Chemicals (United States of America (USA)), protein concentration assay kits and enhanced chemiluminescence kits were purchased from Tianjin Baihaoxin Biotechnology Co., Ltd., and antibodies against TLR4 and NF-κB were provided by Santa Cruz (USA). Anesthetic agent, paraformaldehyde and xylene were bought from Pall Corporation (USA), Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End Labeling (TUNEL) staining kit was offered by Sakura (USA) and Western blotting kit was purchased from Abcam (USA).
Air Tech super-clean bench was manufactured by Beijing Liuyi Instrument Plant, tissue embedding machine (model: LEICAEG1150) was provided by Leica (Germany) and Nikon Ti-U/Ti-s inverted fluorescence microscope (model: H-7650) was bought from Mitsubishi Heavy Industries, Ltd. 5810R high-speed centrifuge was offered by Shimadzu Corporation (Japan) and E-Gel Imager was manufactured by Beckman Coulter (USA).
Modeling and grouping:
The 100 SPF grade male SD rats aged (7-8) w old were randomly divided into 4 groups; normal group (n=25), model group (n=25), Qingdouling mixture intervention group (experimental group, n=25) and positive control dexamethasone intervention group (control group, n=25). The CARAS rat models were prepared in model group, experimental group and control group according to a previous literature[7]. Firstly, the rats were sensitized with normal saline containing 0.3 mg Ovalbumin (OVA) and 30 mg Aluminium hydroxide (Al (OH)3) via intraperitoneal injection at 1 ml/kg/h for 14 d. On the 15th d, the rats were subjected to intranasal instillation of 50 μg of 5 % OVA and atomization of 1 % OVA for 10-15 min until asthma symptoms occurred. The rats in normal group were intraperitoneally injected with an equal volume of normal saline and then given intranasal instillation and atomization. After the last atomization, nasal secretions and numbers of nose scratching and sneezing of the rats were observed within 30 min. Moreover, the amount of nasal secretions, number of sneezing and degree of nasal itching of the rats were recorded. The scoring methods were as follows[8]; the nasal secretion (nasal discharge) was rated 1 point (the nasal discharge flows to the anterior nares), 2 points (the nasal discharge flows out of the anterior nares) and 3 points (the nasal discharge covers the face or cirrus). The sneezing was scored 1 point for less than 4 times of sneezing, 2 points for 5-10 times of sneezing and 3 points for more than 10 times of sneezing. In terms of nasal itching, mild nose scratching was given 1 point, frequent nose scratching or repeated scratching of face was rated 2 points and constant nose scratching was scored 3 points. The score was calculated using superposition method, and total score ≥5 points indicated successful animal modeling.
Following successful modeling, the rats in experimental group and control group were given Yupingfeng capsules and dexamethasone at 2 mg/ kg by gavage once a day for 14 consecutive days, while those in normal group and model group were intraperitoneally injected with an equal dose of normal saline once a day for 14 consecutive days. All rats were raised with standard diets and had free access to food and water.
Scoring of nasal symptoms:
After 14 d of continuous administration, the nasal symptoms of each group of rats were scored according to the following standards[9]; mild symptoms such as nose scratching (1-4 times) or sneezing (1-4 times) within 30 min, a small amount of nasal discharge in nasal cavity or nasal congestion were recorded as 1 point. Moderate symptoms such as nose scratching (5-9 times) or sneezing (5-9 times) within 30 min, a large amount of nasal discharge in nasal cavity or nasal redness were recorded as 2 points. Severe symptoms such as nose scratching (≥10 times) or sneezing (≥10 times) within 30 min, nasal discharge attached in nasal cavity or nasal redness and bleeding were rated 3 points.
Collection of serum samples and biochemical detection for Eosinophil (EOS) counting:
Venous blood was drawn from the tail of rats after 7 consecutive days of administration, which was centrifuged to obtain the serum and the serum EOS count in each group was detected using an automatic biochemical tester and analyzer.
Detection of cell apoptosis in lung tissues by TUNEL staining:
About 10 % lung tissues of rats were taken out of a refrigerator, fixed, frozen and sliced into sections, followed by soaking in xylene and gradient alcohol. After air drying, the sections were immersed in 3 % hydrogen peroxide-methanol, washed, soaked in 0.1 % Triton X-100 and 0.1 % buffer solution sequentially and washed again. Subsequently, TUNEL reaction solution was added for reaction in a dark wet box and the sections were rinsed, transparentized, mounted and observed under a fluorescence microscope.
Measurement of serum Interleukin (IL)-5 and IL-13 levels by ELISA:
The levels of serum IL-5 and IL-13 in rats were measured by ELISA kits strictly according to the requirements of kits and analyzed using a multifunctional micro plate reader. Moreover, the expressions of inflammatory factors in each group were subjected to statistical analysis.
Detection of nuclear translocation of NF-κB in lung tissues by immunofluorescence assay:
The rat lung tissues (10 %) was taken out of the refrigerator, routinely made into sections and deparaffinized. After washing, the sections were sealed in 3 % hydrogen peroxide and heated by microwave for 10 min. Then fluorescent dye-labeled primary antibodies were added for 1 h of incubation in the dark and the sections were counterstained with 4',6-Diamidino-2-Phenylindole (DAPI), mounted in neutral resin and observed under the fluorescence microscope. Following nuclear translocation of NF- κB, 6 fields of vision dominated by nuclear staining were randomly selected to calculate the mean fluorescence intensity of samples[10].
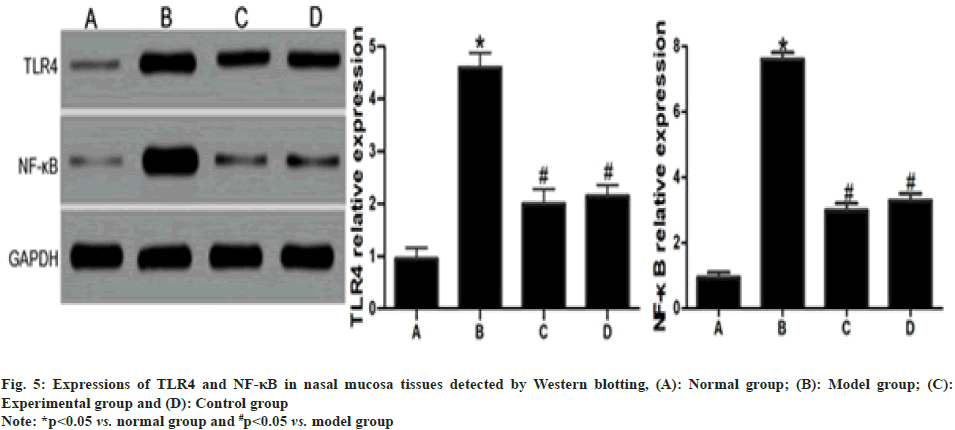
Detection of TLR4 and NF-κB expressions in lung and nasal mucosa tissues by Western blot:
The samples to be detected were fetched, accurately weighed, lysed and centrifuged at 13 000 r/min and 4° for 10 min to acquire the supernatant. After measuring concentration, the supernatant underwent electrophoresis, membrane transfer and blocking in egg white. Next, primary antibodies (1:100) were added in drops, incubated at 4° overnight and added with secondary antibodies (1:100), followed by flushing with Phosphate Buffer Saline (PBS) and color development. Finally, the grayscale value of each band was read using the gel imager.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 16.0 software was employed for data analysis. Measurement data conforming to normal distribution were expressed by mean±standard deviation. Oneway analysis of variance was adopted for comparison among multiple groups, independent t-test was used for pairwise comparison and p<0.05 suggested that the difference was statistically significant.
Results and Discussion
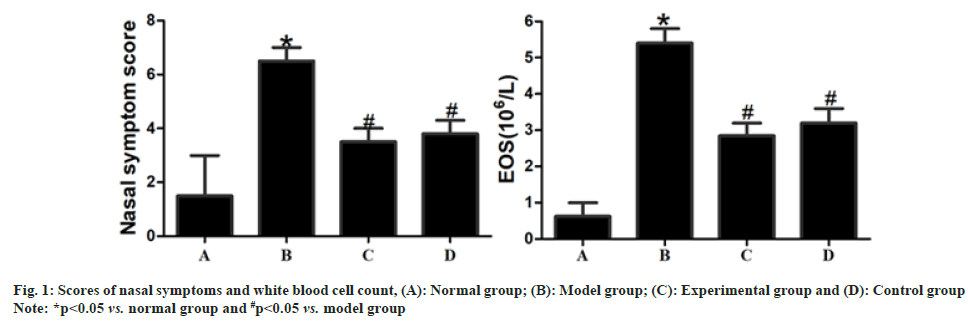
Compared with those in normal group, the score of nasal symptoms and white blood cell count were significantly increased in model group (p<0.05). After drug intervention, the score of nasal symptoms and white blood cell count were significantly lower in experimental group and control group than those in model group (p<0.05), but they displayed no statistically significant differences between experimental group and control group (p>0.05) as shown in fig. 1.
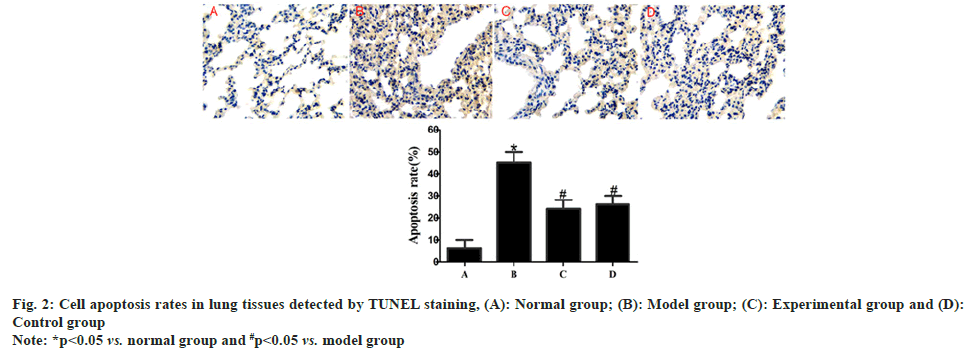
Compared with those in normal group, rats in model group exhibited a significantly raised cell apoptosis rate in lung tissues (p<0.05). After drug intervention, the cell apoptosis rate in lung tissues was significantly lower in experimental group and control group than that in model group (p<0.05), but there was no statistically significant difference in such an index between experimental group and control group (p>0.05) as shown in fig. 2.
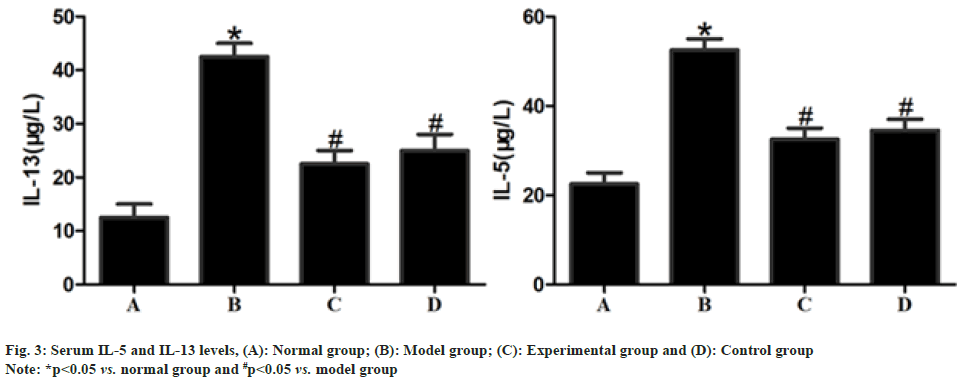
The serum levels of IL-5 and IL-13 were significantly higher in model group than those in normal group (p<0.05) but significantly lower in experimental group and control group than those in model group after drug intervention (p<0.05) and displayed no statistically significant differences between experimental group and control group (p>0.05) as shown in fig. 3.
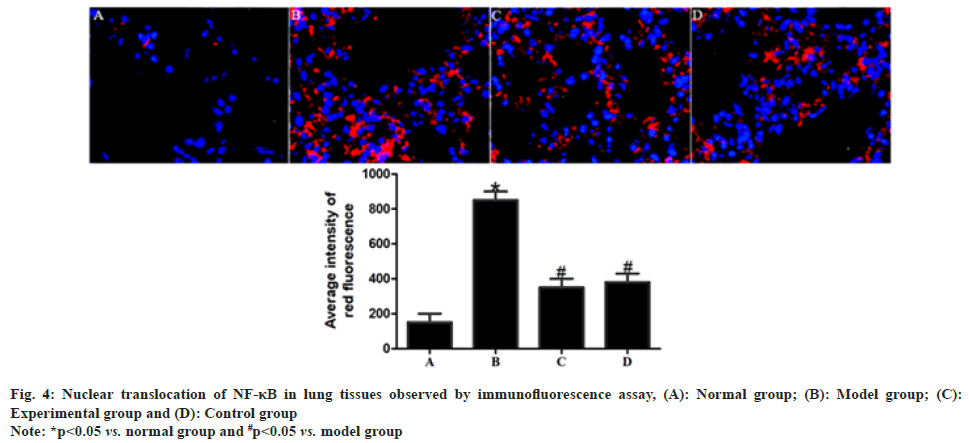
In comparison with normal group, model group had significantly enhanced red fluorescence intensity in rat lung tissues (p<0.05). After drug intervention, the red fluorescence intensity in rat lung tissues was significantly weakened in experimental group and control group compared with that in model group (p<0.05), while no statistically significant difference was found in the red fluorescence intensity between experimental group and control group (p>0.05) as shown in fig. 4.
Compared with those in normal group, the expressions of TLR4 and NF-κB in rat nasal mucosa tissues rose significantly in model group (p<0.05). Such expressions were significantly lower in experimental group and control group than those in model group (p<0.05) but displayed no statistically significant differences between experimental group and control group (p>0.05) as shown in fig. 5.
CARAS refers to allergic rhinitis and bronchial asthma caused by allergic stress, which is an inflammatory disease of the respiratory system mostly prevalent in children and adolescents. There are no effective radical Western medical methods for its treatment and its recurrence rate is high, bringing a huge economic burden and severely affecting the quality of life of patients and the healthy growth of adolescents[11]. For this reason, discovering effective drugs to prevent and control the deterioration of the disease is of great significance in clinic.
There is much experience about CARAS in TCM. Clinical TCM physicians offer individualized treatment for patients from the perspective of syndrome differentiation. In addition to oral decoction for the onset of the disease, various treatment approaches, including acupoints application, auricular therapy, acupuncturemoxibustion and nasal fumigation and foot bath therapy, are employed concurrently, which result in few side effects and achieve stable efficacy, displaying unique advantages[12]. Peng et al.[13] proved that aminophylline compound pastes could relieve the long-term symptoms of CARAS patients. Yang and Chen demonstrated that the symptoms of CARAS children treated with Astragalus granules were prominently relieved, with clearly improved immunity[14]. Yupingfeng capsules contain such active TCM components as Astragalus membranaceus, Dendranthema indicum, infructescence of Platycarya strobilacea Sieb. et Zucc, Flos Magnoliae, Prunella vulgaris L., Saposhnikovia divaricate Root, Glycyrrhiza and Ligusticum chuanxiong Hort. Pharmacological studies have manifested that Yupingfeng capsules have strong anti-inflammatory, anti-allergic, antiviral and immune-regulating effects[15]. Chen et al.[16] confirmed that Yupingfeng capsules had a significant efficacy in the treatment of allergic rhinitis, with few adverse reactions. Additionally, Gan et al.[17] reported that Yupingfeng capsules applied in treating children with upper respiratory tract infection and rhinitis significantly alleviated the clinical symptoms of these children. The above findings suggest that the compound preparation is effective in the treatment of CARAS to some extent. In this study, the score of nasal symptoms and serum EOS count were obviously reduced in experimental group after establishing the CARAS rat model, proving the therapeutic effect of the drug on the disease.
Inflammatory stress is a basic pathological process of CARAS. Research showed that allergic rhinitis and bronchial asthma are both caused by the predominance of T lymphocyte subgroup T Helper Cell 2 (Th2) immunity[18]. IL-5 and IL-13 secreted by Th2 cells are the main pro-inflammatory molecules in nasal mucosa and airway tissues, which induce the recruitment of EOS and further lead to the apoptosis of a large number of cells in nasal mucosa, airway and lung tissues, thereby inducing structural remodeling and worsening CARAS. Research by Hao et al.[19] manifested that the increase in IL-5 level in the peripheral blood of CARAS children promoted the release of large amounts of toxic proteins from EOS and activated many inflammatory factors to expand inflammatory signals, expanding the inflammatory stress cascade and inducing more obvious CARAS symptoms in children. Tian et al.[20] showed that the serum IL-13 level of CARAS patients directly affected the airway structural cells, stimulated the activation of fibroblasts, enhanced the inflammatory response of the airway and participated in the structural remodeling of the airway in patients. The results of this study uncovered that the serum levels of IL-5 and IL-13 and the apoptosis rate of cells in lung tissues evidently declined in experimental group and control group, further confirming that the drug has good anti-inflammatory and anti-apoptotic effects.
Exploring the pathway by which drugs act is conducive to the clarification of the pathogenesis of diseases and the improvement of the efficacy of drugs. The TLR4/NF-κB pathway is a vital immuneregulation inflammatory pathway in the body and its role in rhinitis, asthma and other respiratory diseases has drawn widespread attention[21]. Qiao et al.[22] reported that repressing the expression of TLR4/NF- κB signal in asthmatic mice dramatically alleviate the damage to lung tissues in mice and their symptoms. Moreover, Sun et al.[23] uncovered that in patients with allergic rhinitis, the accumulation of massive inflammatory factors activated TLR4 molecules and NF-κB and triggered nuclear translocation, thus stimulating inflammatory signals to enter the nucleus and the blockage of such a pathway overtly relieved the damage to the nasal mucosa of patients. In this study, the expressions of TLR4 and NF-κB in rat nasal mucosa tissues were signally lowered in experimental group and control group, proving the action sites of the drug on CARAS.
In conclusion, Yupingfeng capsules can obviously repress the release of inflammatory factors, suppress the apoptosis of cells and relieve the symptoms of CARAS rats probably by inhibiting the TLR4/NF- κB pathway. However, further studies should be conducted to explore the stability of Yupingfeng capsules in treating CARAS and whether they also affect the progression of the disease through other pathways.
Funding:
This study was supported by Subject Construction Project of Health and Family Planning Commission of Pudong New Area (No. PWZzb2017-20).
Conflict of interest:
The authors declared no conflict of interest.
References
- Tao B, Ruan G, Wang D, Li Y, Wang Z, Yin G. Imbalance of peripheral Th17 and regulatory T cells in children with allergic rhinitis and bronchial asthma. Iran J Allergy Asthma Immunol 2015;14(3):273-9.
[Google Scholar] [PubMed]
- Ferreira LK, Ferreira LA, Monteiro TM, Bezerra GC, Bernardo LR, Piuvezam MR. Combined allergic rhinitis and asthma syndrome (CARAS). Int Immunopharmacol 2019;74:105718.
[Crossref] [Google Scholar] [PubMed]
- Luo Q, Zhang C, Yang L, Zhang A, Guo X, Xue C, et al. Potential effectiveness of Chinese herbal medicine Yu ping feng san for adult allergic rhinitis: A systematic review and meta-analysis of randomized controlled trials. BMC Complement Altern Med 2017;17(1):1-20.
[Crossref] [Google Scholar] [PubMed]
- Ying W, Qian Y, Kun Z. Drugs supply and pharmaceutical care management practices at a designated hospital during the COVID-19 epidemic. Res Soc Admin Pharm 2021;17(1):1978-83.
- Wen J, Zhu J, Li J, Yuan G, Xiang F. Experimental study of Yupingfeng granule on allergic rhinitis in rat and guinea pig. Chin Tradit Patent Med 2011;33:934-7.
- Jin Y, Zhu Z, Wu L, Xuan L. Effect of acupoints application on TLR-NF-κB signal pathway in nasal mucosa of allergic rhinitis model rats. J Tradit Chin Med 2018;59:1054-7.
- Wang X, Liu C, Wu L, Zhu S. Potent ameliorating effect of hypoxia-inducible factor 1α (HIF-1α) antagonist YC-1 on combined allergic rhinitis and asthma syndrome (CARAS) in rats. Eur J Pharmacol 2016;788:343-50.
[Crossref] [Google Scholar] [PubMed]
- Tang H, Li T, Han X, Sun J. TLR4 antagonist ameliorates combined allergic rhinitis and asthma syndrome (CARAS) by reducing inflammatory monocytes infiltration in mice model. Int Immunopharmacol 2019;73:254-60.
[Crossref] [Google Scholar] [PubMed]
- Zvezdin BS. Allergic asthma and rhinitis comorbidity. Vojnosanit Pregl 2015;72(11):1024-31.
- Barros GC, Ferreira LK, Ferreira LA, Monteiro TM, Alves AF, de Alencar Pereira R, et al. 4-Carvomenthenol ameliorates the murine combined allergic rhinitis and asthma syndrome by inhibiting IL-13 and mucus production via p38MAPK/NF-κB signaling pathway axis. Int Immunopharmacol 2020;88:106938.
[Crossref] [Google Scholar] [PubMed]
- Krzych-Fałta E, Furmańczyk K, Lisiecka-Biełanowicz M, Sybilski A, Tomaszewska A, Raciborski F, et al. The effect of selected risk factors, including the mode of delivery, on the development of allergic rhinitis and bronchial asthma. Postępy Dermatol Alergol 2018;35(3):267-73.
[Crossref] [Google Scholar] [PubMed]
- Zheng J, Shao SJ, Wang PY, Qin XY, Wang QB, Zhang XG, et al. “SHAO's five-needle method" as the main treatment for allergic rhinitis and asthma syndrome: A multi-center randomized controlled trial. Zhongguo Zhen Jiu 2020;40(5):483-7.
[Crossref] [Google Scholar] [PubMed]
- Peng B, Liu L, Wang H, Wu W, Zhang C, Zhou X, et al. Influence on IFN-γ/IL-4 of kechuanting paste therapy on patients with combined allergic rhinitis and asthma syndrome. Liaoning J Tradit Chin Med 2018;45:343-5.
- Yang L, Chen SY. Effect of Astragalus granule on serum Th1/Th2 cytokine levels in children with allergic rhinitis and asthma syndrome. Shandong Med J 2016;56:108.
- Li S, Fang Y, Liu Q, Li C. The immunoregulation and clinical effect of Yupingfeng capsule combined with seretide on patients with cough variant asthma. Int J Tradit Chin Med 2016;38(6):512-4.
- Chen J, Wei FR, Nie LY, Qiao FY. Randomized, double-blind, parallel controlled multicentre clinical trial of Yupingfeng capsules in the treatment of allergic rhinitis. J Pharm Res 2013;32:546-7.
- Gan HY. Analysis of the curative effect of Yupingfeng capsule combined with desloratadine citrate disodium tablets on allergic rhinitis. China Foreign Med Treat 2018;37:114-6.
- Ai J, Xie Z, Qing X, Li W, Liu H, Wang T, et al. Clinical effect of endoscopic vidian neurectomy on bronchial asthma outcomes in patients with coexisting refractory allergic rhinitis and asthma. Am J Rhinol Allergy 2018;32(3):139-46.
[Crossref] [Google Scholar] [PubMed]
- Hao X, Yan Z, Zhang L, Tian Y, Sun L. Effect of spleen aminopeptide combined with glucocorticoid on the expression levels of Th1/Th2 cytokines in peripheral blood of children with allergic rhinitis and asthma syndrome. Mater Child Health Care China 2018;33:2007-10.
- Tian Y, Hao X, Liu L, Zhang L, Wang Y, Liu X. Glucocorticoid combined with spleen aminopeptide in the treatment of allergic rhinitis-asthma syndrome in children. Lab Med Clin 2018;15:1477-80.
- Wang J, Cui Z, Liu L, Zhang S, Zhang Y, Zhang Y, et al. miR-146a mimic attenuates murine allergic rhinitis by down-regulating TLR4/TRAF6/NF-κB pathway. Immunotherapy 2019;11(13):1095-105.
[Crossref] [Google Scholar] [PubMed]
- Qiao JY, Song L, Zhang YL, Luan B. HMGB1/TLR4/NF-κB signaling pathway and role of vitamin D in asthmatic mice. Zhongguo Dang Dai Er Ke Za Zhi 2017;19(1):95-103.
[Google Scholar] [PubMed]
- Sun YD, Liu Q, Xiang XH, Yang CG, He XF, Zhong LK, et al. Mechanism of Hezhong Zhiqiu granules on TLR-NF-κB pathway regulated of allergic rhinitis. Otorhinolaryngol Res Tradit Chin Med 2019;7:22-6.