- *Corresponding Author:
- Evan Prince
School of Bio Sciences and Technology, Vellore Institute of Technology, Vellore, Tamil Nadu 632014, India
E-mail: eps674@gmail.com
| Date of Received | 09 November 2021 |
| Date of Revision | 20 April 2023 |
| Date of Acceptance | 11 September 2023 |
| Indian J Pharm Sci 2023;85(5):1291-1301 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Inflammation can heal and revitalize. However, in some conditions, the individual develops a chronic phase of inflammation that could last for entire life. Damaged regulation of apoptotic cell death may play a role in the pathogenesis of autoimmune disorders, acquired immunodeficiency syndrome, cancer, and central nervous system degenerative diseases. Though different medicinal approaches are promising, they are not only expensive but more often result in side effects. As a result, there are a constantly rising need for novel, reliable, and more beneficial initiatives to control and regulate inflammation and apoptosis protein response. In this study, we have targeted tumor necrosis factor receptor-1, FAS, tumor necrosis factor-related apoptosis inducing ligand-receptor 2, B-cell lymphoma 2, caspase-9 and caspase-3 proteins for apoptosis and nuclear factor kappa B, inducible nitric oxide synthase, and pregnane X receptor protein for inflammation. The swissADME was used to retrieve the molecular properties of Andrographis paniculata phytochemical compounds. To assess the anti-apoptosis and anti-inflammatory activity, the online server, the prediction of activity spectra for substances prediction, was used. AutoDock Vina software was used for docking the ligands with proteins of interest, and in the Discovery Studio software, the protein-ligand interaction was assessed and visualized. Anthranilic acid, N-methyl-, butyl ester, and Ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- has a potential binding with high hydrogen bonds with the targeted protein of apoptosis and inflammation and in addition, 2-(acetoxymethyl)-3-(methoxycarbonyl)biphenylene also had a good affinity with inflammation protein. Herbal pharmacological regulators of inflammation and apoptosis open up new opportunities for managing and treating various disease conditions.
Keywords
Andrographis paniculata, apoptosis, inflammation, SwissADME, molecular docking, AutoDock Vina
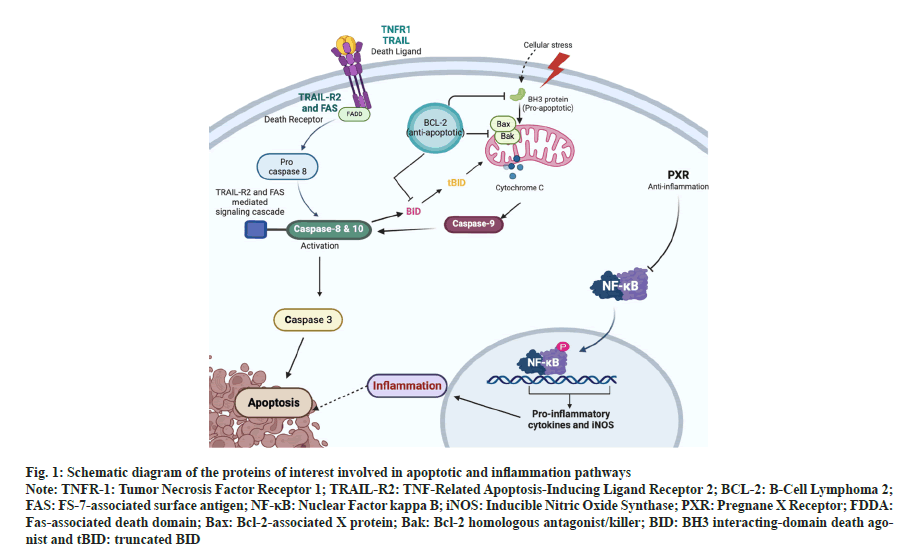
Apoptosis is a homeostatic mechanism that keeps tissue cell populations in check during growth and maturation. Apoptosis is also a protective mechanism in immune responses when cells get injured by disease or toxic substances[1]. For example, to limit an immune response, inducing the apoptosis of T and B cells at a particular time is necessary because a prolonged reaction would be harmful. Also, apoptosis is required for the immune system to be functionally mature because it excludes redundant T and B cells[2]. The two different pathways involved are extrinsic and intrinsic apoptosis pathways. In the extrinsic pathway, the "death ligand" comes in contact with the death receptors on the cell surface, and the conformational changes will initiate apoptotic cell death. Tumor Necrosis Factor receptor (TNF-R1), TNF-Related Apoptosis-Inducing Ligand (TRAIL) and Death Receptors (DR4 and DR5), and Fas receptor are the members of the TNF Receptor Superfamily (TNFRSF)[3]. Intrinsic apoptosis occurs through mitochondria that are intervened by Mitochondrial Outer Membrane Permeabilization (MOMP). As a result, apoptosomes form, caspase-9 is activated, and effector caspases are initiated[4]. B Cell Lymphoma 2 (BCL-2) is a protein family that includes both the pro-apoptotic and antiapoptotic members, which are essential for regulating the process initiation and execution of apoptotic cell death[5]. Caspase-3 is the primary enzyme in executing apoptosis, with which the intrinsic and extrinsic apoptotic pathways (fig. 1) assemble[6].
Fig. 1: Schematic diagram of the proteins of interest involved in apoptotic and inflammation pathways
Note: TNFR-1: Tumor Necrosis Factor Receptor 1; TRAIL-R2: TNF-Related Apoptosis-Inducing Ligand Receptor 2; BCL-2: B-Cell Lymphoma 2; FAS: FS-7-associated surface antigen; NF-κB: Nuclear Factor kappa B; iNOS: Inducible Nitric Oxide Synthase; PXR: Pregnane X Receptor; FDDA: Fas-associated death domain; Bax: Bcl-2-associated X protein; Bak: Bcl-2 homologous antagonist/killer; BID: BH3 interacting-domain death agonist and tBID: truncated BID
Inflammation is the sequence of changes that occur when an injury occurs in tissues that are not severe enough to destroy its structural composition and energy all at once. It underlies a broad range of physiopathology mechanisms[7,8]. The Nuclear Factor Kappa B (NF-κB) pathway regulates the pro-inflammatory production of cytokines, leukocyte formation and survival of cells, all of which significantly contribute to the response to inflammation[9]. One of the significant impacts of an inflammation reaction is the expression of inducible Nitric Oxide Synthase (iNOS)[10]. The iNOS, TNF-alpha (TNF-α), Phospholipase A2 (PLA2), Cyclooxygenase-2 (COX-2), and Heat Shock Proteins (HSPs) are all controlled by NF-κB (fig. 1), which regulates the activation of these genes that are involved in immune function, inflammation, and stress responses[11]. The Pregnane X Receptor (PXR) is a member of the nuclear receptor superfamily responsible for detecting non-resident harmful chemicals and increasing the expression of various metabolic enzymes[12]. When the ligand activates the PXR genes, it suppresses the expression of NF- κB, whereas NF-κB activation also suppresses the expression of PXR genes[13]. As it has a protective response, activation of PXR inhibits the other biochemical pathways, including the inflammatory response[14].
Andrographis paniculata (Acanthaceae), often known as "kalmegh," is a well-known herbal plant that has been used in India, Bangladesh, Hong Kong, China, Indonesia, Philippines, Thailand, Malaysia, Pakistan and Southeast Asia for centuries to treat various diseases[15]. Andrographis paniculata is one of the most commonly used medicinal plants in Unani and Ayurvedic medicine to treat snake bites, jaundice, diarrhea, dysmenorrhoea, gonorrhea, leucorrhoea, diabetes, fever, bug bites, and malaria[16,17]. The pharmacological properties are antioxidant, antidiabetic, anti-inflammatory, anticancer, immunomodulatory, antihypertensive, hepatoprotective, antibacterial, and antimalarial[18]. The phytochemical components of the ethanol extract of Andrographis paniculata were identified by Gas Chromatography-Mass Spectroscopy (GC- MS) analysis to perform molecular docking with inflammation and apoptosis receptors.
The present study aimed to assess the phytochemical constituents of Andrographis paniculata and investigate their potential as regulators of inflammation and apoptosis proteins. To achieve this, molecular docking was employed to analyze the ligands' hydrogen bond interactions and binding energy with the target proteins, thereby providing insights into their therapeutic efficacy.
Materials and Methods
Ligand compound selection and preparation:
The primary phytochemical compounds of ethanol extract of Andrographis paniculata were identified by GC-MS analysis. The main compounds of phytochemical screening are 2-(acetoxymethyl)- 3-(methoxycarbonyl) biphenylene (C17H14O4); 5-methyl-2-phenylindolizine (C15H13N); anthranilic acid, N-methyl-, butyl ester (C12H17NO2); bicyclo[3.1.1]heptane, 2,6,6-trimethyl-, (1alpha,2beta,5alpha)- (C10H18); ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- (C10H12O4); gamma-sitosterol (C29H50O); hexadecanoic acid, methyl ester (C17H34O2); n-hexadecanoic acid (C16H32O2 ); oleic acid (C18H34O2); phytol (C20H40O); and stigmasterol (C29H48O) )[19]. From the PubChem database (https://pubchem.ncbi.nlm.nih.gov/), the Canonical SMILES of the ligands are retrieved and used to generate the 3D ligand structure in a Protein Data Bank (PDB) format from the CORINA molecular network (https://www.mn-am.com/ online_demos/corina_demo). The PDB format of the ligand is converted to PDBQT format with the help of AutoDock tools to make them suitable for docking in AutoDock vina.
Evaluation of ligands Absorption, Distribution, Metabolism and Excretion (ADME) property and Prediction of Activity Spectra for Substances (PASS) prediction:
The potential therapeutic values of the selected ligand compounds are evaluated for pharmacokinetics properties, drug-likeness, bioavailability, and pharmaceutical chemistry properties with the help of the online server SwissADME (http://www.swissadme.ch/). The Canonical SMILES used for SwissADME and ligands that didn’t violate Lipinski’s rule were chosen for further study. The bioactive score of the ligand compounds was evaluated using the online tool called PASS online (http://www.way2drug.com/passonline/) that estimates nearly 4000 different types of biological activity, including pharmaceutical therapeutic influences. The result obtained for each ligand as Probable activity (Pa) and Probable inactivity (Pi) evaluating their anti- inflammatory and antiapoptotic activity.
Preparation of protein for molecular docking:
The targeted protein 3D structure of apoptotic and inflammatory signalling pathway (Table 1) was obtained in PDB format from Research Collaboratory for Structural Bioinformatics (RCSB) PDB (https://www.rcsb.org/).
| Receptor | PDB ID |
|---|---|
| TNFR-1 | 1EXT |
| TRAIL-R2 | 3X3F |
| Fas receptor | 3TJE |
| Caspase 3 | 1GFW |
| Caspase 9 | 3V3K |
| BCL-2 | 2W3L |
| NF-κB | 1NFK |
| iNOS | 4NOS |
| PXR | 1ILG |
Table 1: Apoptosis and Inflammatory Targeted Proteins with Pdb- Ids
Protein-ligand docking and visualization
The 3D structure of the proteins was converted from PDB format to PDBQT format to make them suitable for AutoDock studies using the automated docking tool called AutoDock Vina. The ligand and the receptor are docked, and the obtained output was visualized by BIOVIA Discovery Studio software, and the hydrogen bonds and binding energy were recorded from the result.
Results and Discussion
Table 2 shows the ADME analysis of the active phytochemical compounds of Andrographis paniculata. Generally, a desirable drug molecule should adhere to Lipinski's rule of five for the physicochemical property. According to Lipinski's rule of five, molecular weight should be <500 g/ mol, log P<5, Hydrogen Bond Donors (HBD)<5, Hydrogen Bond Acceptor (HBA)<10, Polar Surface Area (PSA)≤140 Å, and Rotatable Bonds (RB)<10[20]. Here, all compounds adhered to the Lipinski rule, and most exhibited a high Gastrointestinal (GI) absorption rate. In this study, we evaluated a set of compounds. We found 7 of them followed the Lipinski rule with only 1 violation in MLogP>4.15, where the value is slightly higher than the recommended limit. However, based on previous literature[21], this level of violation is considered acceptable for drug-likeness as long as other criteria are met. Therefore, these 7 compounds are potentially suitable for further study to develop drug candidates. The following compounds had high GI absorption- 2-(acetoxymethyl)-3-(methoxycarbonyl) biphenylene; 5-methyl-2-phenylindolizine; anthranilic acid, N-methyl-, butyl ester; ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)-; hexadecanoic acid, methyl ester; n-hexadecanoic acid; and oleic acid. The following compounds had low GI absorption- bicyclo[3.1.1]heptane, 2,6,6-trimethyl-, (1alpha,2beta,5alpha)-; gamma-sitosterol; phytol; and stigmasterol.
| S No | Compound name | MW | HBD | HBA | Log P | MR | RB | Lipinski | TPSA (A?) | GIA |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2-(acetoxymethyl)-3-(methoxycarbonyl)biphenylene | 282.29 g/mol | 0 | 4 | 3.04 | 78.02 | 5 | Yes, 0 V | 52.60 | High |
| 2 | 5-methyl-2-phenylindolizine | 207.27 g/mol | 0 | 0 | 2.88 | 67.8 | 1 | Yes, 0 V | 4.41 | High |
| 3 | anthranilic acid, N-methyl-, butyl ester | 207.27 g/mol | 1 | 2 | 2.43 | 61.45 | 6 | Yes, 0 V | 38.33 | High |
| 4 | Bicyclo[3.1.1]heptane, 2,6,6-trimethyl-, (1alpha,2beta,5alpha)- | 138.25 g/mol | 0 | 0 | 2.68 | 45.7 | 0 | Yes, 1 V MLOGP >4.15 | 0.00 | Low |
| 5 | Ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- | 196.20 g/mol | 1 | 4 | 1.98 | 51.64 | 3 | Yes, 0 V | 55.76 | high |
| 6 | gamma-sitosterol | 414.71 g/mol | 1 | 1 | 4.79 | 133.23 | 6 | Yes, 1 V MLOGP >4.15 | 20.23 | Low |
| 7 | Hexadecanoic acid, methyl ester | 270.45 g/mol | 0 | 2 | 4.41 | 85.12 | 15 | Yes, 1 V MLOGP >4.15 | 26.30 | high |
| 8 | n-hexadecanoic acid | 256.42 g/mol | 1 | 2 | 3.85 | 80.8 | 14 | Yes, 1 V MLOGP >4.15 | 37.30 | High |
| 9 | oleic acid | 282.46 g/mol | 1 | 2 | 4.27 | 89.94 | 15 | Yes, 1 V MLOGP >4.15 | 37.30 | High |
| 10 | Phytol | 296.53 g/mol | 1 | 1 | 4.71 | 98.94 | 13 | Yes, 1 V MLOGP >4.15 | 20.23 | Low |
| 11 | Stigmasterol | 412.69 g/mol | 1 | 1 | 4.96 | 132.75 | 5 | Yes, 1 V MLOGP >4.15 | 20.23 | Low |
Table 2: Swiss Adme Properties of Phytochemical Compounds of Andrographis Paniculata
Table 3 shows the computational analysis of the PASS algorithm predicts anti-inflammatory and anti-apoptosis activity for compounds. In the Pass analysis of Andrographis paniculata compounds, almost all the compounds have shown apoptosis antagonist and anti-inflammatory pa and pi values. However, Bicyclo[3.1.1]heptane, 2,6,6-trimethyl-, (1alpha,2beta,5alpha)- didn't show any anti-apoptosis and anti-inflammatory activity. Also, stigmasterol doesn't have anti-apoptosis activity. Consequently, the inclusion of these two compounds did not meet the criteria for further verification in the present investigation. The highest anti-apoptosis activity was shown by oleic acid (0.471) and the lowest by phytol (0.183). Similarly, the highest anti-inflammatory activity was shown by oleic acid (0.685) and the lowest 5-methyl-2-phenylindolizine (0.369).
| S no | Compound name | Apoptosis antagonist (pa) | Apoptosis antagonist (pi) | Anti-inflammatory (pa) | Anti-inflammatory (pi) |
|---|---|---|---|---|---|
| 1 | 2-(acetoxymethyl)-3-(methoxycarbonyl)biphenylene | 0.261 | 0.057 | 0.524 | 0.05 |
| 2 | 5-methyl-2-phenylindolizine | 0.229 | 0.084 | 0.369 | 0.112 |
| 3 | Anthranilic acid, N-methyl-, butyl ester | 0.244 | 0.072 | 0.502 | 0.056 |
| 4 | Bicyclo[3.1.1]heptane, 2,6,6-trimethyl-, (1alpha,2beta,5alpha)- | - | - | - | - |
| 5 | Ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- | 0.287 | 0.038 | 0.497 | 0.058 |
| 6 | Gamma-sitosterol | 0.2 | 0.121 | 0.467 | 0.067 |
| 7 | Hexadecanoic acid, methyl ester | 0.336 | 0.016 | 0.51 | 0.054 |
| 8 | n-hexadecanoic acid | 0.417 | 0.006 | 0.515 | 0.052 |
| 9 | Oleic acid | 0.471 | 0.004 | 0.685 | 0.003 |
| 10 | Phytol | 0.183 | 0.155 | 0.458 | 0.07 |
| 11 | Stigmasterol | - | - | 0.542 | 0.045 |
Table 3: Pass Analysis on the Anti-Apoptosis and Anti-Inflammatory Activity of Andrographis Paniculata Compounds
Autodock vina:
Docking ligands with receptors were chosen based on the antiapoptotic and anti-inflammatory activity. Therefore, nine ligands docked with apoptotic receptors and ten ligands with inflammatory receptors. In an AutoDock tool, receptors and ligands have docked, and results are analyzed based on the binding affinity (kcal/mol) and hydrogen bonds. The lower the binding energy and higher the hydrogen bonds show, the strong connection between the docked complex. From the result, more than four hydrogen bonds were considered to interpret the docked compounds. Table 4 and Table 5 show the binding energy and hydrogen bonds of Andrographis paniculata compounds docked with apoptotic and inflammatory proteins, respectively.
| S.no | Compound name | TNFR-1 | Caspase 3 | Caspase 9 | TRAIL-R2 | BCL-2 | FAS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| kcal/mol | Bonds | kcal/mol | Bonds | kcal/mol | Bonds | kcal/mol | Bonds | kcal/mol | Bonds | kcal/mol | Bonds | ||
| 1 | 2-(acetoxymethyl)-3-(methoxycarbonyl)biphenylene | -7.3 | 4 | -6.5 | 3 | -5.9 | 6 | -6.3 | 4 | -6.5 | 4 | -5.9 | 3 |
| 2 | 5-methyl-2-phenylindolizine | -6.8 | 3 | -7.7 | 0 | -6.5 | 0 | -6.5 | 0 | -6.5 | 1 | -5.9 | 2 |
| 3 | anthranilic acid, N-methyl-, butyl ester | -4.9 | 7 | -5 | 2 | -4.8 | 5 | -4.3 | 5 | -5.2 | 6 | -4.9 | 5 |
| 4 | Ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- | -5.7 | 6 | -5.1 | 4 | -4.7 | 5 | -4.9 | 6 | -5.3 | 5 | -5.2 | 5 |
| 5 | gamma-sitosterol | -5.6 | 1 | -7.2 | 2 | -6.3 | 1 | -7.2 | 2 | -7.3 | 3 | -6.7 | 1 |
| 6 | Hexadecanoic acid, methyl ester | -4.3 | 5 | -4.6 | 3 | -4.4 | 4 | -3.7 | 2 | -3.9 | 3 | -4.1 | 4 |
| 7 | n-hexadecanoic acid | -4.7 | 5 | -4.7 | 4 | -3.9 | 4 | -4 | 3 | -4 | 4 | -4.2 | 5 |
| 8 | oleic acid | -4.5 | 4 | -4.8 | 4 | -4.2 | 4 | -4.4 | 5 | -4.4 | 4 | -4.7 | 4 |
| 9 | Phytol | -5.4 | 3 | -5 | 2 | -4.5 | 2 | -4.1 | 2 | -4.6 | 1 | -4.9 | 3 |
Table 4: Binding Energy and Hydrogen Bonds of Andrographis Paniculata’s Compounds Docked with Apoptotic Proteins
| S no | Compound name | NF-κB | iNOS | PXR | |||
|---|---|---|---|---|---|---|---|
| kcal/mol | Bonds | kcal/mol | Bonds | kcal/mol | Bonds | ||
| 1 | 2-(acetoxymethyl)-3-(methoxycarbonyl)biphenylene | -7.6 | 6 | -4.2 | 4 | -7.4 | 5 |
| 2 | 5-methyl-2-phenylindolizine | -7.4 | 1 | -3.9 | 1 | -8.7 | 1 |
| 3 | anthranilic acid, N-methyl-, butyl ester | -6.5 | 6 | -2.8 | 3 | -5.9 | 4 |
| 4 | Ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- | -6.9 | 6 | -3.2 | 3 | -5.7 | 4 |
| 5 | gamma-sitosterol | -5.9 | 1 | -4.2 | 1 | -4.6 | 1 |
| 6 | Hexadecanoic acid, methyl ester | -5.5 | 5 | -2.5 | 2 | -5.8 | 2 |
| 7 | n-hexadecanoic acid | -5.7 | 3 | -2.5 | 3 | -5.6 | 2 |
| 8 | oleic acid | -6.6 | 5 | -2.9 | 3 | -5.7 | 2 |
| 9 | Phytol | -6.1 | 3 | -2.4 | 2 | -6.2 | 2 |
| 10 | Stigmasterol | -4.3 | 3 | -4.8 | 1 | -3.6 | 1 |
Table 5: Binding Energy and Hydrogen Bonds of Andrographis Paniculata’s Compounds Docked with Inflammatory Proteins
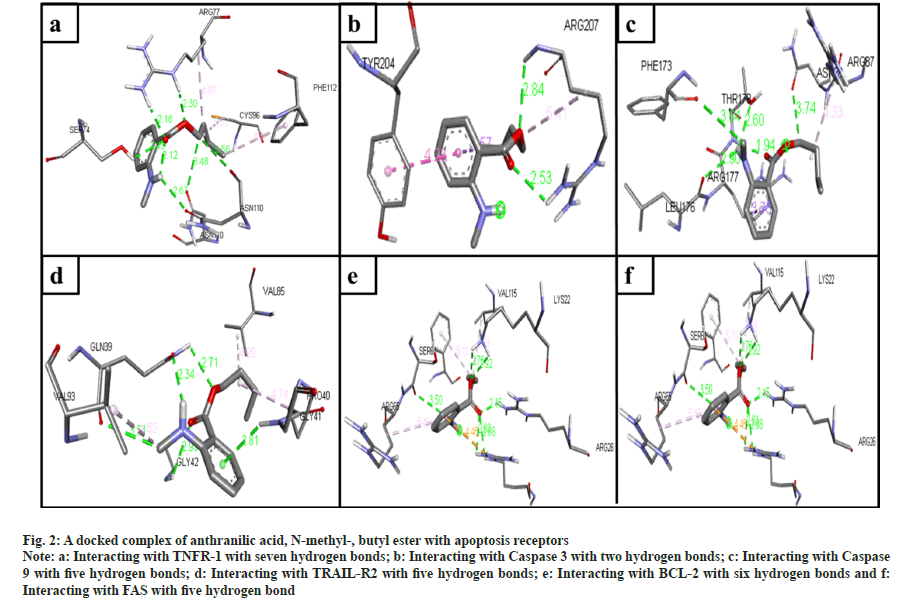
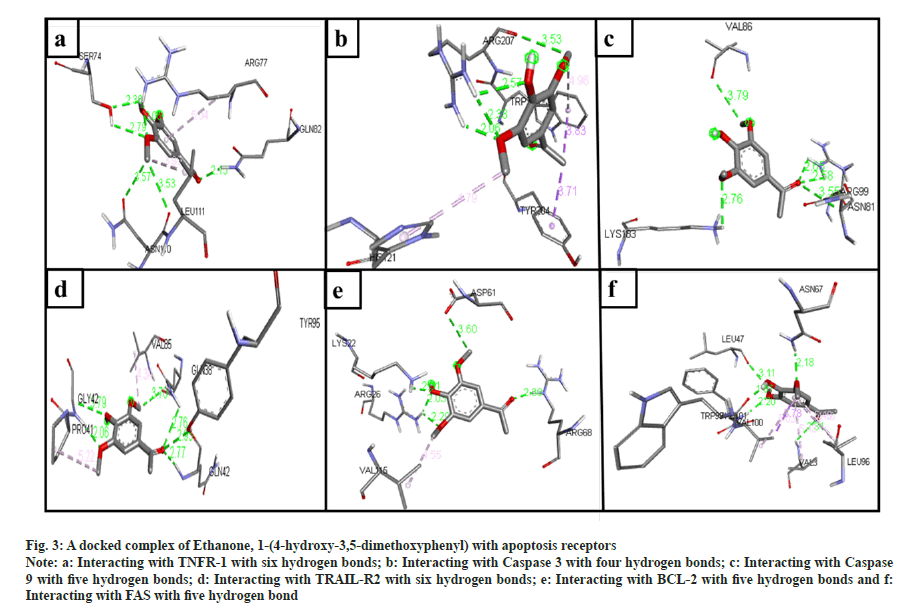
The phytochemical compounds docked with the TNFR-1 protein in an active site. The highest hydrogen bond observed in anthranilic acid, N-methyl-, butyl ester (7 bonds) (fig. 2a); ethanone, 1-(4-hydroxy-3,5- dimethoxyphenyl)- (6 bonds) (fig. 3a); Hexadecanoic acid, methyl ester (5 bonds); n-hexadecanoic acid (5 bonds); oleic acid (4 bonds) and 2-(acetoxymethyl)- 3-(methoxycarbonyl)biphenylene (4 bonds) with the binding energy -4.9, -5.7, -4.3, -4.7, -4.5 and -7.3 respectively.
Fig. 2: A docked complex of anthranilic acid, N-methyl-, butyl ester with apoptosis receptors
Note: a: Interacting with TNFR-1 with seven hydrogen bonds; b: Interacting with Caspase 3 with two hydrogen bonds; c: Interacting with Caspase 9 with five hydrogen bonds; d: Interacting with TRAIL-R2 with five hydrogen bonds; e: Interacting with BCL-2 with six hydrogen bonds and f: Interacting with FAS with five hydrogen bond
The ligands docked with caspase 3 apoptotic protein, and the maximum hydrogen bonds were seen in three compounds. The following compounds are ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- (4 bonds) (fig. 3b); n-hexadecanoic acid (4 bonds); and oleic acid (4 bonds) with the binding energy -5.1, -4.7, and -4.8, respectively. The anthranilic acid, N-methyl-, butyl ester (2 bonds, with least binding energy -5.0) (fig. 2b). However, even though 5-methyl- 2-phenylindolizine has the lowest binding affinity energy of -7.7, it has zero hydrogen bonds.
The compounds docked with caspase 9 apoptotic protein. The maximum hydrogen bonds observed compounds 2-(acetoxymethyl)-3-(methoxycarbonyl) biphenylene (6 bonds); anthranilic acid, N-methyl-, butyl ester (5 bonds) (fig. 2c); ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- (5 bonds) (fig. 3c); hexadecanoic acid, methyl ester (4 bonds); n-hexadecanoic acid (4 bonds); and oleic acid (4 bonds) with the binding energy -5.9, -4.8, -4.7, -4.4, -3.9, and -4.2, respectively. However, 5-methyl- 2-phenylindolizine has the lowest binding affinity energy of -6.5 and has zero hydrogen bonds.
Fig. 3: A docked complex of Ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl) with apoptosis receptors
Note: a: Interacting with TNFR-1 with six hydrogen bonds; b: Interacting with Caspase 3 with four hydrogen bonds; c: Interacting with Caspase 9 with five hydrogen bonds; d: Interacting with TRAIL-R2 with six hydrogen bonds; e: Interacting with BCL-2 with five hydrogen bonds and f: Interacting with FAS with five hydrogen bond
The docked ligands with the TRAIL-R2 also have shown a good affinity between them. Ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- (6 bonds) (fig. 3d) has the maximum hydrogen bonds with a binding energy of -4.9. Anthranilic acid, N-methyl-, butyl ester (5 bonds) (fig. 2d), and oleic acid (5 bonds) with the binding energy -4.3 and -4.4, respectively. On the other hand, 5-methyl-2-phenylindolizine has zero hydrogen bonds with the second least binding energy of -6.5.
The ligand docked with the BCL-2 apoptotic protein, the anthranilic acid, N-methyl-, butyl ester has the maximum hydrogen bonds of 6 with the binding energy -5.2 (fig. 2e). The other following compounds are ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- (5 bonds) (fig. 3e); 2-(acetoxymethyl)-3- (methoxycarbonyl)biphenylene (4 bonds); n-hexadecanoic acid (4 bonds); and oleic acid (4 bonds) with the binding energy -5.3, -6.5, -4.0, and -4.4, respectively.
The compounds docked with FAS apoptotic protein. The maximum number of hydrogen bonds are in anthranilic acid, N-methyl-, butyl ester (5 bonds) (fig. 2f); ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- (5 bonds) (fig. 3f); n-hexadecanoic acid (5 bonds); hexadecanoic acid, methyl ester (4 bonds); and oleic acid (4 bonds) with the binding energy -4.9, -5.2, -4.2, -4.1 and -4.7, respectively.
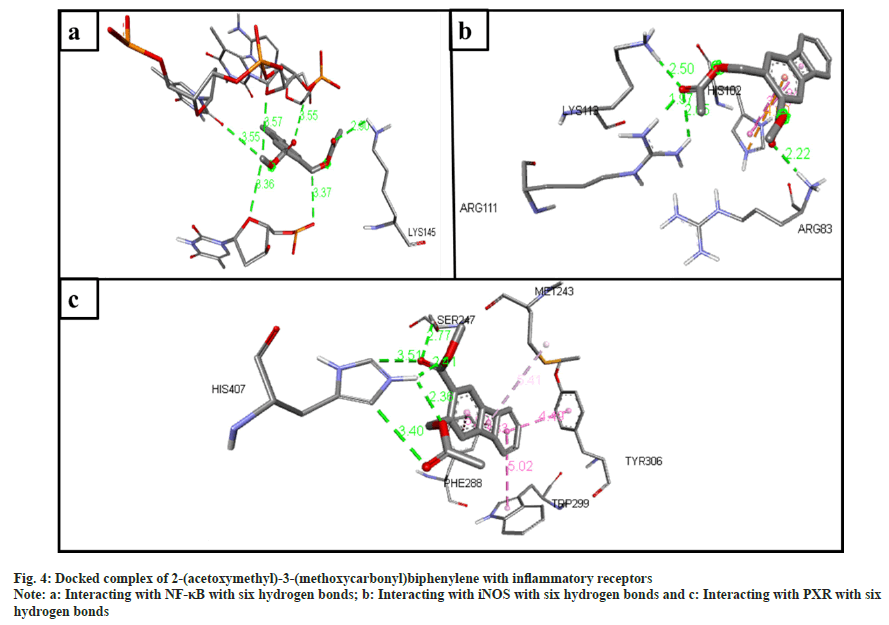
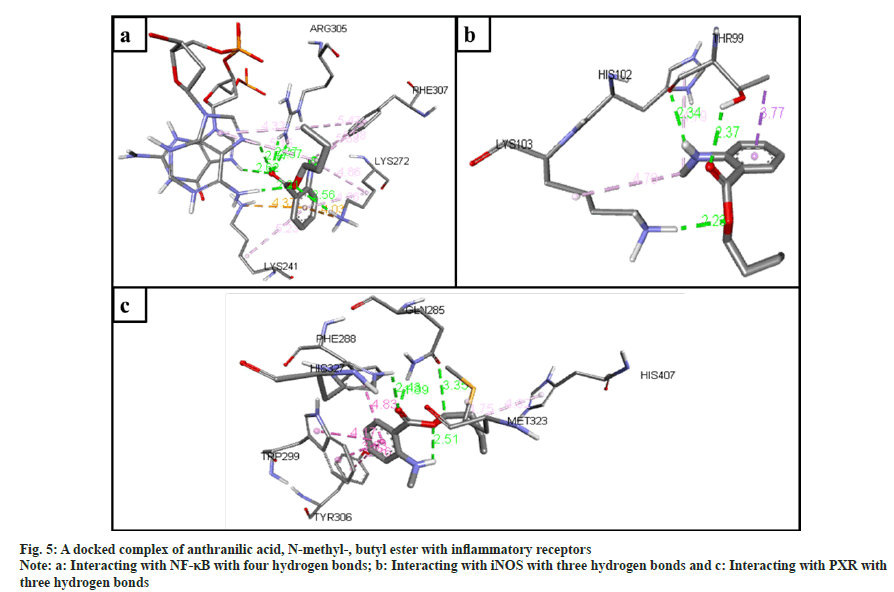
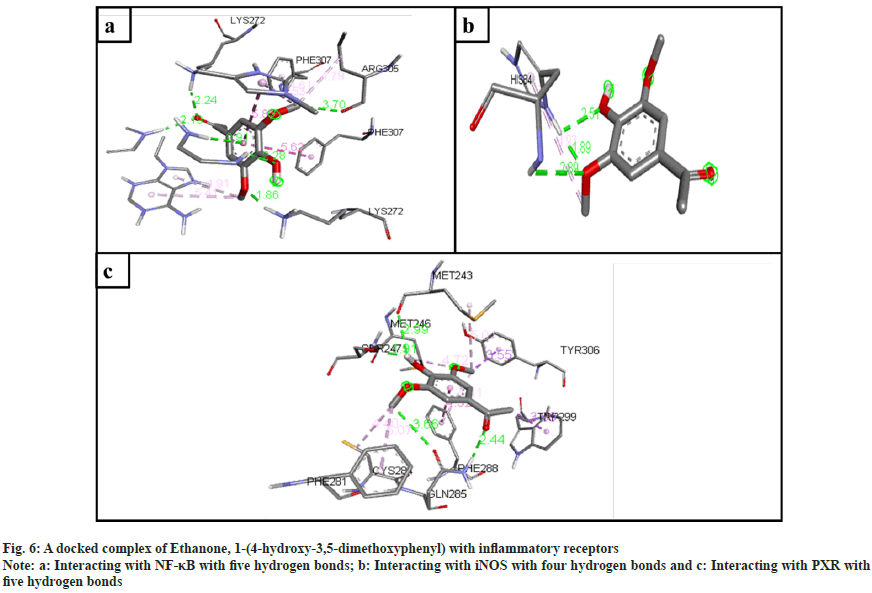
The inflammatory protein NF-κB docked with the ligands has shown good binding interactions. The following compounds are 2-(acetoxymethyl)- 3-(methoxycarbonyl)biphenylene (6 bonds) (fig. 4a); anthranilic acid, N-methyl-, butyl ester (6 bonds) (fig. 5a); and ethanone, 1-(4-hydroxy-3,5- dimethoxyphenyl)- (6 bonds) (fig. 6a) with the binding energy -7.6, -6.5 and -6.9, respectively. The other compounds that interact well with protein are hexadecanoic acid, methyl ester (5 bonds, -5.5 kcal/ mol), and oleic acid (5 bonds, -6.6 kcal/mol).
The inflammatory protein iNOS docked with the ligands hasn't shown good interaction. Here only 2-(acetoxymethyl)-3-(methoxycarbonyl) biphenylene has a minimum of 4 hydrogen bonds with the binding energy -4.2 (fig. 4b); anthranilic acid, N-methyl-, butyl ester (3 bonds, -2.8 kcal/ mol) (fig. 5b); and ethanone, 1-(4-hydroxy-3,5- dimethoxyphenyl)- (3 bonds, -3.2 kcal/mol) (fig. 6b).
The ligand compounds docked with the PXR inflammatory protein. The maximum number of hydrogen bonds observed in 2-(acetoxymethyl)-3- (methoxycarbonyl)biphenylene (5 bonds, -7.4 kcal/ mol) (fig. 4c); anthranilic acid, N-methyl-, butyl ester (4 bonds, -5.9 kcal/mol) (fig. 5c); and ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- (5 bonds, -5.7 kcal/mol) (fig. 6c).
Despite rapid and significant advances in medical science, the search for safe and effective herbs for all diseases continues. Herbal medicines are gaining popularity due to their accessibility and inexpensive cost[22]. According to the World Health Organization report, about 80 % of people are estimated to depend on medicinal plants for their health-beneficial needs[23]. Andrographis paniculata is a widely consumed herb for various diseases in Ayurvedic, Siddha and Unani medicine and home remedies. It is an herbaceous plant, and its parts are used to treat toxicities, cardiovascular diseases, leprosy, malaria, jaundice, and snakebite. These beneficial activities were due to the presence of components such as flavonoids, polyphenols, xanthones, and fixed oils[24]. Here, we have taken steps to scrutinize the beneficial phytochemicals of Andrographis paniculata for more effects on various diseases. For that, phytochemicals collected from the literature review were primarily analyzed for PASS prediction, which revealed that most of the compound of Andrographis paniculata shows antiapoptotic and anti-inflammatory effect.
Further analysis of ADME results in all the compounds follows the Lipinksi rules of five. According to various research, inflammation, and apoptosis are the main factors and most common pathways reported in most diseases[1,25]. Thus, this study aimed to inhibit the major proteins involved in apoptosis and inflammatory pathways. Inhibitory effects were analyzed by identifications of compounds having better interactions with targets involved in apoptosis and inflammatory pathways.
Molecular docking was performed to aid the selective phytochemical of Andrographis paniculata binding with screened receptors. The presence of a binding interaction with a hydrogen bond indicates that the binding is effective and that target inhibition is conceivable[26]. In our study, the ligands with four or more hydrogen bonds with targets are considered to have an effective inhibitory effect. The compounds anthranilic acid, N-methyl-, butyl ester, and ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- were found to have better interactions with major receptors in the apoptotic pathway with a minimum of 4 and a maximum of 7 hydrogen bonds with binding energy less than -4.3. The compounds 2-(acetoxymethyl)- 3-(methoxycarbonyl)biphenylene; anthranilic acid, N-methyl, butyl ester; and ethanone, 1-(4-hydroxy- 3,5-dimethoxyphenyl)- found to have better interactions with major receptors in the inflammatory pathway with a minimum of 3 and maximum of 6 hydrogen bonds with binding energy less than -2.8.
In our study, 2-(acetoxymethyl)-3-(methoxycarbonyl) biphenylene; anthranilic acid, N-methyl-, butyl ester; and ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- having efficient interactions with apoptotic and inflammatory receptors, thus, it would be the potential drug as personalized medicine for apoptosis and inflammatory mediated diseases.
A highly effective compound should achieve its target in the body in appropriate quantities and remain bioactive for an adequate time for the expected pharmacological activity. Recently, many research works have focused on finding potential phytochemical compounds that control inflammation and apoptosis caused by disease. This study used various in silico methods such as swissADME, PASS prediction, and AutoDock vina software to recognize the potential phytoactive compounds. Anthranilic acid, N-methyl-, butyl ester; ethanone, 1-(4-hydroxy- 3,5-dimethoxyphenyl)-; and 2-(acetoxymethyl)-3- (methoxycarbonyl)biphenylene are three compounds has the higher interaction with the binding site of all the targeted proteins, implying that they could be used as effective antiapoptotic and anti-inflammatory compounds by controlling the targeted proteins and minimizing apoptosis and inflammation caused during disease condition. A further in vivo study can be done to understand the exact molecular mechanism of these potential activities of phytochemical compounds of Andrographis paniculata in the future.
Acknowledgements:
The authors are indebted to the Vellore Institute of Technology (VIT) for providing the necessary assistance.
Conflict of interests:
The authors declare that they have no competing interest.
References
- Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol 2007;35(4):495-516.
[Crossref] [Google Scholar] [PubMed]
- Xu X, Lai Y, Hua ZC. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci Rep 2019;39(1):BSR20180992.
[Crossref] [Google Scholar] [PubMed]
- Xu G, Shi Y. Apoptosis signaling pathways and lymphocyte homeostasis. Cell Res 2007;17(9):759-71.
[Crossref] [Google Scholar] [PubMed]
- Brentnall M, Rodriguez-Menocal L, de Guevara RL, Cepero E, Boise LH. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol 2013;14:1-9.
[Crossref] [Google Scholar] [PubMed]
- Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol 2019;20(3):175-93.
[Crossref] [Google Scholar] [PubMed]
- Lossi L, Castagna C, Merighi A. Caspase-3 mediated cell death in the normal development of the mammalian cerebellum. Int J Mol Sci 2018;19(12):3999.
[Crossref] [Google Scholar] [PubMed]
- Punchard NA, Whelan CJ, Adcock I. The journal of inflammation. J Inflamm 2004;1(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Medzhitov R. Origin and physiological roles of inflammation. Nature 2008;454(7203):428-35.
[Crossref] [Google Scholar] [PubMed]
- Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol 2009;1(6):a001651.
[Crossref] [Google Scholar] [PubMed]
- Suschek CV, Schnorr O, Kolb-Bachofen V. The role of iNOS in chronic inflammatory processes in vivo: Is it damage-promoting, protective, or active at all. Curr Mol Med 2004;4(7):763-75.
[Crossref] [Google Scholar] [PubMed]
- Natarajan K, Abraham P, Kota R, Isaac B. NF-κB-iNOS-COX2-TNF α inflammatory signaling pathway plays an important role in methotrexate induced small intestinal injury in rats. Food Chem Toxicol 2018;118:766-83.
[Crossref] [Google Scholar] [PubMed]
- Dou W, Zhang J, Li H, Kortagere S, Sun K, Ding L, et al. Plant flavonol isorhamnetin attenuates chemically induced inflammatory bowel disease via a PXR-dependent pathway. J Nutr Biochem 2014;25(9):923-33.
[Crossref] [Google Scholar] [PubMed]
- Qiu Z, Cervantes JL, Cicek BB, Mukherjee S, Venkatesh M, Maher LA, et al. Pregnane X receptor regulates pathogen-induced inflammation and host defense against an intracellular bacterial infection through toll-like receptor 4. Sci Rep 2016;6(1):31936-12.
[Crossref] [Google Scholar] [PubMed]
- Sun M, Cui W, Woody SK, Staudinger JL. Pregnane X receptor modulates the inflammatory response in primary cultures of hepatocytes. Drug Metab Dispos 2015;43(3):335-43.
[Crossref] [Google Scholar] [PubMed]
- Kumar RA, Sridevi K, Kumar NV, Nanduri S, Rajagopal S. Anticancer and immunostimulatory compounds from Andrographis paniculata. J Ethnopharmacol 2004;92(2-3):291-5.
[Crossref] [Google Scholar] [PubMed]
- Hossain MS, Urbi Z, Sule A, Rahman KM. Andrographis paniculata (Burm. f.) Wall. ex Nees: A review of ethnobotany, phytochemistry, and pharmacology. Sci World J 2014;2014:274905.
[Crossref] [Google Scholar] [PubMed]
- Okhuarobo A, Falodun JE, Erharuyi O, Imieje V, Falodun A, Langer P. Harnessing the medicinal properties of Andrographis paniculata for diseases and beyond: A review of its phytochemistry and pharmacology. Asian Pac J Trop Dis 2014;4(3):213-22.
[Crossref] [Google Scholar] [PubMed]
- Worasuttayangkurn L, Nakareangrit W, Kwangjai J, Sritangos P, Pholphana N, Watcharasit P, et al. Acute oral toxicity evaluation of Andrographis paniculata-standardized first true leaf ethanolic extract. Toxicol Rep 2019;6:426-30.
- Purushothaman A, Sufiya P, Meenatchi P, Sundaram R, Saravanan N. Antigenotoxic and antimutagenic effects of Andrographis paniculata, a traditional medicinal herb against genotoxicity of cyclophosphamide: An in vitro study on human peripheral lymphocytes. Prev Nutr Food Sci 2020;25(3):246-53.
- Chagas CM, Moss S, Alisaraie L. Drug metabolites and their effects on the development of adverse reactions: Revisiting Lipinski’s rule of five. Int J Pharm 2018;549(1-2):133-49.
[Crossref] [Google Scholar] [PubMed]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 1997;23(1-3):3-25.
[Crossref] [Google Scholar] [PubMed]
- Ajayi AF, Akhigbe RE, Adewumi OM, Okeleji LO, Mujaidu KB, Olaleye SB. Effect of ethanolic extract of Cryptolepis sanguinolenta stem on in vivo and in vitro glucose absorption and transport: Mechanism of its antidiabetic activity. Indian J Endocrinol Metab 2012;16(Suppl1):S91-6.
[Crossref] [Google Scholar] [PubMed]
- WHO global report on traditional and complementary medicine 2019. 2019
- Mehta S, Sharma AK, Singh RK. Pharmacological activities and molecular mechanisms of pure and crude extract of Andrographis paniculata: An update. Phytomed Plus 2021;1(4):100085.
- Yang Y, Jiang G, Zhang P, Fan J. Programmed cell death and its role in inflammation. Mil Med Res 2015;2(1):1-2.
[Crossref] [Google Scholar] [PubMed]
- Meng XY, Zhang HX, Mezei M, Cui M. Molecular docking: A powerful approach for structure-based drug discovery. Curr Comput Aided Drug Des 2011;7(2):146-57.
[Crossref] [Google Scholar] [PubMed]