- *Corresponding Author:
- K. R. Iyer
Department of Pharmaceutical Chemistry, Bombay College of Pharmacy (Autonomous), Mumbai-400098, India
E-mail: krishna.iyer@bcp.edu.in
| Date of Received | 14 October 2021 |
| Date of Revision | 28 April 2022 |
| Date of Acceptance | 21 November 2022 |
| Indian J Pharm Sci 2022;84(6):1343-1357 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The 21st century has witnessed 3 major pandemics caused by coronaviruses namely, severe acute respiratory syndrome, Middle East respiratory syndrome and coronavirus disease-19. Though previously considered non-pathogenic in humans, these viruses have found a way to infect the human population. The viruses have traversed various intermediate host pools from their natural reservoirs before spilling out in humans. From severe acute respiratory syndrome in 2003 to Middle East respiratory syndrome in 2012 to coronavirus disease-19 in 2019, clinical research, safety and efficacy studies performed during each outbreak have led to new developments in the pharmacotherapeutics of human coronavirus infections. Severe acute respiratory syndrome was initially identified as a disease-causing pneumonia and hence was treated by antibiotics followed by corticosteroids for acute respiratory distress syndrome. After detecting the viral origin of the disease, the use of antivirals like ribavirin, ritonavir/lopinavir, Type I Interferons and their combinations started to gain momentum. Middle East respiratory syndrome led to repositioning of therapies employed in severe acute respiratory syndrome along with introduction of new agents like mycophenolic acid, cyclosporine and remdesivir. The emergence of novel coronavirus causing coronavirus disease-19 has led to repurposing of molecules like chloroquine, remdesivir and favipiravir for containing the virus. In addition, the effectiveness of plasma therapy and antibody treatment has been investigated through small scale observational studies.
Keywords
Pharmacotherapeutics, coronavirus, severe acute respiratory syndrome, Middle East respiratory syndrome, coronavirus disease-19
Coronavirus (CoV) belong to the subfamily Coronavirinae in the family Coronaviridae of the order Nidovirales[1]. These enveloped viruses contain unusually large single-stranded positive Ribonucleic Acid (RNA) as their genome (27 to 32 kb)[2,3]. Out of the four genera in this subfamily, alpha-CoVs and beta-CoVs infect mammals only (Table 1). In humans, 229E, OC43, NL63 and HKU1 strains of Human CoV (HCoV) are prevalent causing upper respiratory tract and gastrointestinal tract infections in immunocompetent individuals. The infections may vary from mild conditions like common cold to severe manifestations like bronchitis and pneumonia[4].
| Type of Coronavirus | Virus | Host |
|---|---|---|
| Alpha | 229E | Human |
| NL63 | Human | |
| TGEV | Pig | |
| PRCoV | Pig | |
| FIPV | Cat | |
| Beta | OC43 | Human |
| HKU1 | Human | |
| SARS-HCoV | Human | |
| MHV | Mouse | |
| BCoV | Cow | |
| MERS-CoV | Human | |
| Gamma | IBV | Chicken |
| Turkey coronavirus | Turkey | |
| Delta | BuCoV HKU11 | Bird |
| ThCoV HKU12 | Bird | |
| MunCoV HKU13 | Bird | |
| NHCoV HKU19 | Bird | |
| PorCoV HKU15 | Pig |
Table 1: Classification of Coronaviruses and their Host Species
In 1965, the first occurrence of HCoV was detected in the nasal discharge of a patient[4]. Before the beginning of the 21st century, CoVs were abundantly circulating and vastly studied in veterinary medicine because of their reduced impact on human health. However, the advent of the 21st century witnessed two highly pathogenic incidences of HCoVs namely, Severe Acute Respiratory Syndrome (SARS) in 2003 and Middle East Respiratory Syndrome (MERS) in 2012. Both these strains led to worldwide epidemic situations with notably severe morbidities and mortalities associated with their spread[5,6]. Though causative agents of SARS and MERS dwell mainly in zoonotic reservoirs, occasional spill-over via intermediate hosts has occurred in susceptible human populations[4]. HCoVs are likely to have originated in bats and rodents, which are considered as the natural hosts. Human transmission of some viruses has been proven through intermediate hosts like camels, cows, civets and pigs[1].
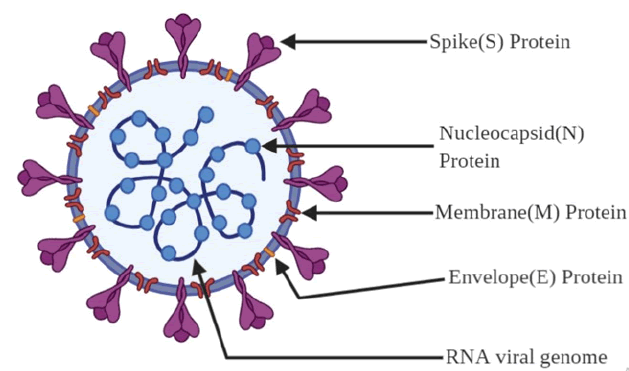
CoVs are composed of different viral proteins (fig. 1), the first one being Spike (S), a type I transmembrane protein found on the virus surface, which gives the virus a distinctive ‘corona’ or crown like appearance (hence the name); a Membrane (M) protein, a protein which contains triple-spanning transmembrane region with its N terminal ending in exterior region and long C terminal ending in interior; a small membrane Envelope protein (E), a highly hydrophobic enigmatic protein which plays a role in virion assembly and finally, a Nucleocapsid (N) protein which undergoes multimerization and packages the viral genome into a ribonucleoprotein complex and is crucial for the virus assembly[2,6,7]. It is estimated that the mutation rate in CoV is higher compared to other singlestranded RNA (ssRNA) viruses and its average substitution rate is about ~10-4 substitutions per year per site. These substitutions are the reason for wider host domain of CoVs and their transmission among various species[4].
Coronavirus Disease-19 (COVID-19), the recently emerged CoV disease caused by SARS-CoV-2 is phylogenetically related to SARS-like bat CoV. Though not proven yet, SARS-CoV-2 may have enhanced human-to-human transmission rate as compared to other HCoVs[8]. In this review, we discuss the origin and epidemiology with descriptive information on already used and potential therapeutic approaches to treat SARS (2003), MERS (2012) and COVID-19 infections.
In November 2002, atypical pneumonia outbreaks were reported in southern China, starting with fever and mild respiratory symptoms and progressing to pneumonia in most cases. The median incubation period was found to be 4-5 d with maximum being 10 d. By February 2003, the disease had spread outside China to South-East Asia, North America and Europe[9]. On March 13, 2003, World Health Organization (WHO) declared a global alert with respect to the disease and termed it SARS. As of July 2003, 8439 cases were reported with 812 fatalities. The human chain of transmission was broken in July owing to strict isolation and tracking of infected patients which led to the receding of the first CoV pandemic. At the end of the outbreak, WHO had recorded a global case fatality ratio of 11 %[10].
In the case of SARS-CoV, it was found that masked palm civets (Paguma larvata), which were sold in the live animal markets of Guangdong province were the intermediate hosts. On the other hand, wild horseshoe bats (Rhinolophidae family) which were also found in the live animal markets and served in restaurants of Guangdong province, showed presence of SARSCoV virus and thus were suggestive of a bat origin of the SARS-CoV. Evolutionary relationship between CoVs and bats showed how the virus initially spread to bats from where it diverged to civets and eventually to humans[11,12].
Initial treatment protocols for SARS:
Since SARS was unknown before 2002 and it arrived in a rather explosive manner with clustered outbreaks around the globe, it is comprehensible that there was no time to plan and conduct prospective randomized clinical trials on any pharmacotherapeutic approaches to ensure the safety and efficacy before employment for treatment[13]. To deal with initially prevalent conditions, treatment began with broad spectrum antibiotics if the case was classified as community acquired pneumonia as per guidelines of American Thoracic Society. Further, different antibiotics such as cefotaxime, levofloxacin and clarithromycin were employed for treatment. Since SARS does not respond to antibiotics, patients who were showing recovery signs with antibiotics were excluded from having SARS infection[14]. Antivirals like oseltamivir were used if patients showed possible influenza infection. Later, a combination therapy of ribavirin and low dose corticosteroids like prednisolone and hydrocortisone were used as treatment methods. Pulses of methylprednisolone were also given in response to persistence or recurrence of fever and lung opacity[15].
Antiviral regimen for SARS:
Ribavirin, a guanosine analogue, is known to have a broad spectrum of antiviral activity[16]. Though its mechanism of action is still obscure, it has been suggested to be an inhibitor of viral inosine monophosphate dehydrogenase[17]. The end state is complete disruption of RNA or DNA genome of viruses[18]. Ribavirin has been associated with hepatitis C virus, respiratory syncytial virus and various influenza virus therapies[16]. The initial experiments in Vero (African green monkey kidney) cells suggested that ribavirin played no role in inhibition of SARS-CoV replication[19,20]. However, other investigations demonstrated anti SARS-CoV activity of Ribavirin (concentration of 50 mg/ml) in foetal rhesus kidney 4 cells, although the activity tended to decrease after an incubation of 48 h[21,22]. There was no concrete clinical data to prove the effectiveness or ineffectiveness of ribavirin in SARS pharmacotherapy. Also, the observation of adverse events like dose-dependent haemolytic anaemia leading to longer hospital stay due to ribavirin therapy demotivated its use[23,24].
Upon evident information on ribavirin claiming that high drug concentration is required to inhibit the virus, further studies led to evaluation of other antivirals like lopinavir/ritonavir. The clinical findings suggested that patients showed mild action against the disease course and relatively fewer side effects compared to the control group[22]. Improved clinical outcomes were reported with the inclusion of lopinavir/ritonavir to a standard treatment protocol as an initial treatment for SARS. Certain preliminary and cohort studies show that inclusion of lopinavir/ritonavir along with other therapeutics lead to reduction in mortality rates and intubation as compared to control groups[25,26]. It is recommended to evaluate the efficacy of this treatment through randomised controlled trials during future epidemics[25].
As stated earlier, ribavirin is ineffective in Vero cells while Interferons (IFN) inhibit viral replication. Upon studying their combination in five different cell lines it was concluded that their combination was a potential therapy for SARS, owing to viral inhibitory action by drastically reduced concentrations compared to single treatment. It was suggested that this combination could potentially help in suppressing SARS-CoV replication in the early phase and thus avert subsequent immunopathological damages, reduce virus shedding and thus reduce the risk of transmission[23].
IFN therapy for SARS:
The body, on exposure to any agent capable of causing infections, expresses innate defence mechanisms like Type I IFN (IFN-α/β) to control the replication of the agent[27]. Further in vitro studies inferred that Type I IFNs can be investigated for use against SARS-CoV[28-30]. A study with the aim of assessing the antiviral potential of recombinant IFNs α, β and γ was carried out against clinical isolates from Frankfurt and Hong Kong. Based on the results, it was concluded that IFNs inhibited the replication of SARS-CoV and IFN-β was more selective and more efficacious on Vero cells as compared to the other two. Though IFN-α inhibited SARS-CoV replication, its selectivity index was found to be 50-90 times lower than that of IFN-β. In Colorectal Adenocarcinoma (Caco2) cells, IFN-β was 5 to 10 times more effective[31]. A concentration of 1000 U/ ml of IFN-β was found to inhibit the replication of the virus[32]. It was observed that when IFNs are used in combination they showed better antiviral effects. In another in vitro study, it was observed that IFN- γ synergized with other IFNs (IFNs-α and IFNs-β) and showed antiviral effects against both RNA and DNA viruses[33]. IFN-α, a registered drug for Hepatitis C viral infection has also been shown to inhibit SARS replication in vitro when tested in macaques. IFN-α was pegylated to improve its pharmacokinetic properties and the results obtained showed pegylated IFN-α to have an inhibitory action in the macaque model of infection[34]. Thus, it was inferred that type I IFNs could be a potential treatment for CoV infections and randomized clinical trials are required to further establish the clinical outcomes.
Corticosteroids for treatment of SARS:
According to non-randomized, retrospective preliminary studies of corticosteroid treatment in SARS virus, some benefits were seen in patients but optimal dosage and timing for administration was unexplored. It was demonstrated that upon early administration of corticosteroids like hydrocortisone, there was a delay in the clearance of viral load and prolongation of viraemia. This maybe a result of the immunosuppressive effects of corticosteroids in the human body[35]. Pulsed methylprednisolone has therefore been evaluated for treatment in SARS. The patients in the treatment group (pulsed methylprednisolone) had less oxygen requirement, a better radiographic outcome and less chance of requiring rescue corticosteroid therapy as compared to control (non-pulsed treatment). There were no other critical differences observed in terms of intubation, mortality rates and intensive care unit admission in the two groups[36].
A study was performed to study the effect of corticosteroids and its adverse events associated with therapy. Results obtained were not affirmative to say that the adverse events were associated with the therapy or with the comorbidities and age factor[37]. The use of corticosteroids in therapy is still controversial as there is no substantial evidence for the same. Though employment of corticosteroid therapy alone for SARS outweighs its risks as compared to its benefits, their combination with IFN-alfacon 1 has shown better oxygen saturation levels and superior treatment of pneumonia during preliminary experimentation[38].
Other potential therapeutic approaches for SARS:
During the search for finding a treatment method, it was seen that patients cured of SARS showed presence of neutralizing bodies in their sera. This led to further studies in this direction and results showed that humoral immunity and vaccines are attainable. A study was performed on the use of recombinant fusion protein (Receptor Binding Domain (RBD)- Fc) to generate immune response in rabbits. The antibodies generated against the RBD were highly effective in blocking the RBD-receptor and neutralizing the CoVs. A robust antibody response was obtained in the rabbits and the antisera extracted could completely block the SARS infection[39].
Data available on use of convalescent plasma therapy for various viral infections caused by Lassa, Ebola, Junin and Machupo virus had suggested clinical benefits[40-43]. Thus, it was postulated that antibodies from SARS patients may be used to suppress SARS. Plasma therapy was effective only in the early phase (before 14 d of onset of symptoms) for higher discharge rate of patients. Late phase administration (after 14 d of onset) of plasma led to higher mortality rates and longer hospital stay. Plasma infusion showed no adverse reactions. However, it was seen that plasma therapy had better outcomes when patients were PCR positive and seronegative indicating that the timing of therapy was of importance[44].
A DNA vaccine as a new potential treatment option for SARs-CoV is under study. The DNA encoded by Spike (S) glycoprotein of the SARS-CoV is being used to induce immune response in mice[39]. Results have shown a robust immune response mediated by CD4 cells, CD8 cells and neutralising antibodies. The study has showed an appreciable reduction in viral replication in the lungs of mice[45].
A study on antiviral activities of ribavirin, 6-azauridine, pyrazofurin, Mycophenolic Acid (MPA) and glycyrrhizin were performed on two clinical isolates of CoVs (FFM-1 and FFM-2) obtained from patients with SARS. The results showed the glycyrrhizin was the most potent inhibitor of SARSCoV and had the highest selectivity index of 67 as compared to the other entities tested[20]. However, no extensive randomized clinical trials to prove the safety and efficacy of therapeutic approaches have yet been conducted.
MERS 2012:
A decade after the first pandemic of the 21st century, another viral outbreak occurred which re-challenged therapeutic and public health interventions. A virus with very close resemblance to SARS-CoV was discovered[46]. In June 2012, a patient from Saudi Arabia was identified with SARS. Upon isolation and detection of the causative, it was found to be a novel CoV (nCoV)[47]. The virus was then named MERS-CoV because the infections were restricted to the Middle East and North Africa region with few clusters in the United States, Europe and South East Asia[48]. According to WHO data, 2494 (2102 from Saudia Arabia) cases of MERS have been detected across 27 countries around the globe and 858 patients have succumbed to it since September 2012. The case fatality ratio as of November 2019 was 34.4 %. The incubation period for symptomatic MERS is about 2-14 d.
After screening of various bats which were suspected to be the source of MERS-CoV, a bat was identified in South Africa with a very close phylogenetic relationship to MERS-CoV, though there is no concrete proof[47]. Dromedary camels have been identified as the intermediate hosts for MERS-CoV[48,49]. Data suggests that the virus has been circulating in the camels since decades before spilling over in the human population[47,50].
Initially a few clusters of MERS-CoV infections reported possible human-to-human transmission[51] which were later labelled limited to family contacts or due to nosocomial infections[48]. Unlike SARS, the MERS-CoV infections led to progressive lower respiratory tract symptoms and lymphopenia within a week after mild symptoms were seen, thus explaining a higher mortality rate.
Initial treatment protocol for MERS:
Patients found positive with MERS-CoV showed upper and lower respiratory tract infections. In many cases severe pneumonia with acute respiratory distress syndrome were also observed. Initial treatment began with broad spectrum antibiotics which were later followed by antiviral therapy. The antiviral regime and their implications were postulated from the previous experience of SARS pharmacotherapy[46]. The first line treatments possibly recommended for MERS were convalescent plasma, monoclonal antibodies and immunoglobulins in addition to standard anti-CoV antivirals[52]. It was found that repurposing of already available clinical agents was a much more effective approach[46,52,53].
Repurposing of antiviral drugs and IFNs for MERS:
There are currently no clinically proven antiviral drugs for the treatment of MERS-CoV infection. Antiviral studies reported to date have mostly been in vitro studies. A number of antivirals and IFNs were used as therapy options for SARS 2002-2003. An array of cohort and case studies discussed their effectiveness and ineffectiveness. Upon screening of the list of possible treatments, about 33 drugs were termed active against MERS-CoV[54].
Ribavirin, a nucleoside analogue, gets activated by the host kinases to the nucleotide (active substrate). A high concentration of ribavirin was required to inhibit the MERS-CoV replication, which was difficult to achieve in vivo[55,56]. In vitro studies examining the combination of ribavirin and IFN-α2b showed that their inhibitory concentrations dropped to ranges which could be achievable in humans. When used either prophylactically or in the early phase of treatment, clinical outcomes in patients were improved. This suggested reduced secondary transmission through reduced virus shedding[57].
A retrospective cohort study also indicated improvement in survival benefits, however, the small size of the study proved to be a limitation[58]. Recent observational studies in critically ill patients contradicted the preclinical data. There was no association derived between reduced mortality rates or faster RNA clearance upon administration of ribavirin/IFN-α combination[59]. Another retrospective cohort showed that the combination of ribavirin and IFN-α2a/IFN-β2a is ineffective for the treatment of MERS[60]. Randomized controlled trials would be helpful to determine if this combination is clinically efficacious or not. A study was performed in common marmoset models with severe disease resembling MERS in humans. The results showed that lopinavir/ritonavir improved the clinical, radiological and pathological features in the MERSCoV infected marmosets and the treated animals had lowest viral loads[61].
Assessment of IFNs for SARS therapy indicated that Type I IFNs (α/β) can be effective therapeutic options for SARS[31]. MERS-CoV was observed to be 50-100 times more sensitive to pegylated IFN-α compared to SARS-CoV[62]. In vitro studies performed on infected cells showed that the virus causing lysis in the target cell were effectively inhibited by IFNs of uninfected cells proving their utility in prophylaxis or early phase after exposure[63].
In 2018, a study protocol was laid for the first randomized controlled trial investigating the combination of ritonavir/lopinavir plus IFN-β1b in laboratory confirmed MERS[64]. As an update to the trial, a statistical analysis plan has been published recently in advance of trial completion[65].
Nitazoxanide is another broad spectrum antiviral which was found to be effective against MERS-CoV by inhibiting the expression of viral N protein[66]. In the screening performed on National Institutes of Health clinical collection library 2, nitazoxanide was the top compound which showed significant anti-CoV activity[67,68]. In vitro studies revealed that tizoxanide, the active metabolite of nitazoxanide, inhibited the replication of CoVs[66].
New treatment approaches for MERS:
Cyclosporine-A was found to inhibit SARS-CoV and HCoV 229E in vitro, by blocking functional interactions between viral proteins and cellular cyclophilins[62,69,70]. In vitro study also showed that this drug showed inhibitory action against MERSCyclosporine-A was found to inhibit SARS-CoV and HCoV 229E in vitro, by blocking functional interactions between viral proteins and cellular cyclophilins[62,69,70]. In vitro study also showed that this drug showed inhibitory action against MERSCoV replication. The study was performed on Vero cells and mock-infected Huh7 cells[62]. A study employing human lung tissue cells showed that the combination of IFN-α and cyclosporine-A led to considerable abatement in virus replication in the ex vivo culture compared to single treatment by either of them. The increased levels of IFN stimulated genes may reduce the potential immunosuppressive effect of cyclosporine-A[71]. No large scale clinical trials have been performed to prove cyclosporine’s efficacy against MERS-CoV.
MPA like ribavirin, inhibits cellular inosine monophosphate dehydrogenase and thus shows antiviral potential against a number of viruses including influenza A[72]. Although MPA showed no effect on SARS-CoV, a screening study suggested that of all the drugs being screened, MPA exhibited the inhibitory action on MERS-CoV at concentration achieved by standard clinical oral dose[73]. Because data suggested that ribavirin did not inhibit MERSCoV replication, MPA was considered a potential drug. In vitro studies on various IFNs and MPA showed that MERS-CoV infections can be treated with MPA (IC50=2.87 μM). The study stated that the combination of IFN-β and MPA can be a potential treatment and should be studied for MERS-infected patients[74].
A comparative study was performed to determine the therapeutic efficacy of remdesivir and combination lopinavir/ritonavir and IFN-β on MERS-CoV. Assessment performed on the mouse model showed that remdesivir was active both prophylactically and therapeutically. Remdesivir improved lung function and reduced the viral loads and severe lung pathology whereas the combination lopinavir/ritonavir and IFN-β was unable to reduce viral replication or pulmonary pathology[75]. Remdesivir was tested for treatment of MERS-CoV in a non-human primate model of rhesus macaque. Prophylactic remdesivir treatment inhibited viral replication in lung tissues thus preventing clinical disease. Clinical benefits of remdesivir therapy were seen with reduction in clinical signs, inhibition of viral replication and reduction in lung lesions. Thus, it was suggested that Remdesivir was a highly effective therapeutic against MERS-CoV and could be used prophylactically, in early phase of mild symptoms or to improve recovery in severe cases[76].
Camostat and Heptad Repeat 2 peptide were reported as MERS-CoV fusion inhibitors. In vitro studies on Calu-3 cells derived from human bronchial submucosal gland suggested that Camostat inhibited the entry of viruses in these cells. But the same results were not observed in immature lung tissue[77]. Viral replication and cell-cell fusion was inhibited by HR2P[78,79]. Another study on Vero-TMPRSS2 cells showed that Camostat is capable of reducing the MERS-CoV entry in healthy cells by 15-folds[77].
Antibodies for treatment of MERS:
Passive immunotherapy through monoclonal and polyclonal antibodies serves as a superior therapeutic approach for highly pathogenic existing and newly emerging viruses[80]. According to a study report, the spike (S) protein of MERS-CoV[81] interacts with CD26 (also known as DPPIV) receptor for triggering an infection[82]. In the human body, CD26 surface protein can be found in T lymphocytes, bronchial mucosa and the brush border of proximal tubules. Laboratory developed anti-CD26 monoclonal antibodies have been shown to neutralize the MERSCoV infection by inhibiting interactions between spike protein of virus and CD26 surface cells[83].
Another study targeted to find outcomes in a human transgenic mouse model using humanized Monoclonal Antibodies (hMS-1) against MERSCoV. The results of pre in vitro studies performed suggested that they have potent ability to neutralize the MERS-CoV infection. After 3 d of hMS-1 therapy, the viral titres from the mouse lungs were significantly lower compared to the control [84].
A potent MERS-CoV neutralizing monoclonal antibody was extracted from memory B cells of an infected individual. The antibody named LCA60 interfered with the binding of MERS-CoV to the cellular receptor CD26. The study was conducted on a mouse model infected with MERS-CoV where the LCA60 was administered intranasally. Reduction of 100-1000 fold in the lung viral titres were achieved which showed that the antibody showed high efficacy both in prophylaxis and therapy[85].
Human polyclonal antibodies for MERS-CoV obtained from Transchromosomic (Tc) bovines prove to be potent in vitro and in mouse models. The study revealed that the antibodies produced by Tc bovine in response to spike protein nanoparticle vaccine produced antibodies which showed promising results in mice model by rapidly reducing the viral titres in lungs below the limit of detection[86].
COVID-19:
The end of 2019 witnessed some pneumonia cases of unknown etiology in Wuhan, Hubei province, China[87]. Upon genetic sequencing, the causative was found to be a nCoV with 88 % resemblance to two bat-derived SARS-like CoVs and hence it was named nCoV or SARS-CoV-2[88,89]. The SARSCoV- 2 genome has been found to be 99 % identical to CoVs from pangolins. This indicates that pangolins may act as intermediate hosts for transmission but there has been no concrete proof[90]. The onset of fever, cough and dyspnea are regarded as the most common clinical manifestations for COVID-19[91,92]. On 30th January 2020, WHO declared COVID-19 as a public health emergency of international concern[93]. The disease was declared as a pandemic by the WHO on 11th March 2020[94]. The incubation period for COVID-19 ranges from 2-14 d with the median being 5.2 d[95-97]. In contrast to MERS and SARS where transmission was reported significantly through nosocomial infections[98], SAR-CoV-2 transmission has been shown through intimate contact with infected patients and asymptomatic carriers[99]. A study showed that few asymptomatic cases were identified in close contacts and they were possible asymptomatic carriers. These carriers were confirmed positive for COVID-19 but none presented any obvious symptoms with nucleic acid screening[100]. At the time of writing this article, more than 19 million cases have been reported worldwide in about 217 countries and the number of deaths have crossed the 0.7 million mark[101]. Although there have been many advancements in the public health and therapeutics sector since the first pandemic of 2003, the world is still struggling to stop the spread of the virus. Scientists all over the world are striving to develop effective and safe treatment approaches as well as vaccines to curb the growing threat of SARSCoV- 2 to humankind.
Repurposing of drugs for treatment of COVID-19:
Repurposing of drugs plays an important role in tackling the COVID-19 outbreak. In vitro study on United States Food and Drug Administration (US FDA) approved antiviral drugs including ribavirin, penciclovir, nitazoxanide, nafamostat, Chloroquine (CQ), favipiravir and remdesivir was performed on clinical isolates of SARS-CoV-2. Results revealed that low-micromolar concentration of remdesivir and CQ potently blocked the virus infection with high selectivity index. Favipiravir also showed inhibitory action against the virus infection recommending in vivo evaluation for further efficacy[102]. A single centered retrospective, observational study was performed using two different drug regimens. Regimen I (azithromycin, prednisolone, naproxen, and lopinavir/ritonavir) and regimen II (meropenem, levofloxacin, vancomycin, hydroxychloroquine (HCQ) and oseltamivir) study results showed that regimen I was more effective in improving clinical outcomes and shortening the patient's hospital stay[103].
CQ and HCQ are thought to block the viral entry through receptor glycosylation, proteolysis, endosomal acidification and other immunomodulatory mechanisms[104,105]. Initial study reports stated that CQ could inhibit the replication of various HCoVs[106–109]. An observational study in China employed CQ for treatment of COVID-19 patients. Preliminary reports suggest that the molecule helped in rapid decline in fever, quicker recovery time and improved Lung CTs[110]. An in vitro study determining the antiviral activity of HCQ against CQ on SARS-CoV-2 infected cells showed that HCQ was more potent than CQ. Further, physiology based pharmacokinetic modelling was used to find the most effective dosage regimen[111]. Among 1376 COVID-19 patients involved in an observational cohort study in New York City, none were significantly benefited or harmed through administration of HCQ for treatment[112]. A small size non-randomized trial suggested that the combination of HCQ and azithromycin decreased pulmonary viral loads in patients infected with SARS-CoV-2[113]. However, a retrospective study on HCQ and azithromycin revealed they did not show any effect on the survival outcomes or the need of mechanical ventilation, whether they were given individually or in combination[114]. Efficacy of HCQ as postexposure treatment was investigated by a randomized, double-blind placebo-controlled trial on 821 asymptomatic patients. The outcome obtained from the trial was not as expected. No significant benefit was obtained by HCQ as post exposure prophylaxis for COVID-19[115]. At the time of writing this article, 247 clinical trials were being evaluated to investigate the use of HCQ and 84 of CQ for COVID-19, with 107 and 33 respectively, in Phase 3.
Remdesivir shows broad antiviral spectrum against paramyxoviruses, pneumoviruses, and CoVs being a monophosphoramidate prodrug of an adenosine analogue. In vitro studies of remdesivir showed that this drug inhibited all human and animal CoVs including SARS-CoV-2[116,117]. A randomised, double-blind, placebo-controlled, multicentre trial on COVID-19 patients suggested that intravenous administration of remdesivir did not improve the time required to attain clinical improvement in comparison to placebo although remdesivir showed clinically meaningful changes in various other parameters[118]. In a randomized clinical trial funded by National Institute of Allergy and Infectious Diseases that enrolled COVID-19 infected candidates with lower respiratory tract problems, remdesivir shortened the time to recovery effectively as compared to placebo[119]. Remdesivir was approved by US FDA to be used against COVID-19 in May 2020. The European medicines agency granted approval to remdesivir in case of COVID-19 patients requiring supplemental oxygen in June 2020[120]. At the time of writing this article, 44 clinical trials were evaluating the use of remdesivir for COVID-19, with 19 of them in Phase 3 trials and 3 completed.
Favipiravir is an inhibitor of RNA-dependent RNA polymerase in viral cells. It has been shown to be effective against various viruses including influenza, H1N1, avian viruses and many others[121]. In vitro studies revealed that favipiravir showed activity against SARS-CoV-2 but required higher drug concentration in comparison to remdesivir or CQ[102]. An open-label controlled study was performed on COVID-19 patients, in China. Those treated with favipiravir showed faster viral clearance and improved chest images than patients treated with lopinavir/ritonavir[122]. At the time of writing this article, 32 clinical trials were evaluating the use of favipiravir for COVID-19 with 14 of them in Phase 3.
The outcomes from a randomized study evaluating lopinavir/ritonavir as a therapy for hospitalized severe COVID-19 cases stated that when added to standard supportive care; it was not associated with improved clinical results, reduced pulmonary viral loads or reduced mortality[123,124]. A multicentre randomised open-label phase 2 trial on triple combination of an injectable IFN-β-1b, oral protease inhibitor (lopinavir-ritonavir), and an oral nucleoside analogue (ribavirin) was performed on COVID-19 patients. Results showed that the combination was effective in suppressing the shedding of SARSCoV- 2 when given within 7 d of symptom onset in comparison to lopinavir-ritonavir alone[125]. More clinical trials studying the efficacy of ritonavir/lopinavir and their combinations seem necessary.
Nirmatrelvir, in combination with ritonavir, showed relatively fewer drug-related adverse reactions in addition to substantial antiviral activity against CoV. In vitro studies of nirmatrelvir carried out in human alveolar and bronchial epithelial cells suggested that nirmatrelvir inhibited SARS-CoV-2 viral replication. Animal studies in SARS-CoV-2 modelled mice reveal that nirmatrelvir significantly reduces viral load in the lungs of the mice. Results from the phase 2/3 combined clinical trial of nirmatrelvir plus ritonavir showed that the combination drug reduces the risk of development of severe disease among symptomatic patients by reducing the viral load. Nirmatelvir/ ritonavir is one of the first orally available therapies for COVID-19. This combination gained the emergency approval authorization by the FDA for use in individuals 12 y and older in December 2021[126-129].
A systemic screening was performed to determine the inhibitory action of several drugs by inhibiting the S glycoprotein induced cell fusion. Drugs included in study were cardiac glycosides (ouabain and digoxin), kinases inhibitors (e.g. imatinib mesylate) and anti-HIV drugs (e.g. nelfinavir mesylate) of which nelfinavir mesylate drastically inhibited Sn? and So?mediated cell fusion at micromolar concentrations[130].
SARS-CoV-2 is reported to have a significant sensitivity to IFN-α in vitro. IFN are suggested to decrease the viral titres by several folds. Also, studies demonstrate that SARS-CoV-2 is more sensitive to IFNs-I as compared to SARS-CoV. A study also states that IFN-I can be used as a prophylactic agent against COVID-19[131]. Few preprints showed that Type I and type III IFN show some activity against SARSCoV- 2 in vitro in Vero cells[132]. An uncontrolled, exploratory study was performed on hospitalised COVID-19 patients using IFN-α2b, arbidol or a combination as treatment regimen. It was observed that IFN-α2b whether given with or without arbidol caused reduction in viral shedding and reduction in virally induced inflammation by lowering the levels of CRP and IL-6, suggesting that interferon should be further investigated as therapy for COVID-19[133]. At the time of writing this article, 84 clinical trials were listed to evaluate IFN for COVID-19 treatment, of which, 17 were in Phase 3.
Antibody treatment for COVID-19:
A single canter study in Italy found that COVID-19 patients with Acute Respiratory Distress Syndrome (ARDS) characterized by hyper inflammatory syndrome showed improvement upon administration of intravenous tocilizumab (hMS-1 and Interleukin 6 inhibitor)[134]. A retrospective, observational cohort study on patients with severe COVID-19 pneumonia suggested that tocilizumab reduces the risk of invasive mechanical ventilation or death in severe patients whether administered intravenous or subcutaneous[135]. A preliminary study in China in 6 patients (2 of which were confirmed SARSCoV positive) concluded that convalescent plasma therapy improved clinical outcomes and radiological abnormalities. The two positive patients showed elimination of virus and the other two showed increased circulating antibodies[136]. In another case, 5 patients characterized as critically ill with COVID-19 and ARDS were given convalescent plasma therapy containing neutralizing antibodies. Patients showed great response, their viral load declined within a few days of treatment and they no longer needed mechanical ventilation support[137]. The US FDA has approved the use of convalescent plasma for life threatening COVID-19 infections. Large scale multicenter randomized trials are required to confirm the safety and efficacy of plasma therapy for SARS-CoV-2. Currently 150 clinical trials on plasma therapy for COVID-19 are underway with 25 of them in Phase 3.
Vaccines for COVID-19:
Vaccine candidates for SARS and MERS demonstrated risk of direct worsening pulmonary conditions and antibody dependent exacerbation of lung disease. Therefore, testing of COVID-19 vaccines in suitable animal models and significant safety monitoring in human phase trials is challenging[138]. The S-protein of SARS-CoV-2 serves as the major target for vaccine development owing to the studies on neutralizing antibodies and their interaction with the protein[139]. An animal study on macaques for vaccine development showed a single shot vaccine inducing a robust neutralizing antibodies response and complete protection against SARS-CoV-2 infection[140].
According to the latest report by the WHO, 153 vaccine candidates are currently under clinical development phase and 196 are in the preclinical stage. Four vaccines candidates AstraZeneca-SK Bio, Serum Institute of India, Janssen and Moderna have received approval for emergency use. Pfizer/ BioNTech is the only vaccine candidate that has received the full US FDA approval for the individuals 16 y and older. Table 2 lists all the vaccine candidates in Phase 4 trials currently.
| S. No | COVID-19 Vaccine developer/manufacturer | Vaccine platform | Type of candidate vaccine | Clinical Trial Phase |
|---|---|---|---|---|
| 1 | Sinovac Research and Development Co., Ltd | Inactivated virus | CoronaVac; inactivated SARS-CoV-2 vaccine (Vero cell) | Phase 4-NCT04756830 |
| 2 | Sinopharm; China National Biotec Group Co; Wuhan Institute of Biological Products | Inactivated virus | Inactivated SARS-CoV-2 vaccine (Vero cell) | Phase 4-NCT05065892 |
| 3 | Sinopharm; China National Biotec Group Co; Wuhan Institute of Biological Products; Beijing Institute of Biological Products | Inactivated virus | Inactivated SARS-CoV-2 vaccine (Vero cell), vaccine name BBIBP-CorV | Phase 4-NCT05075083 |
| 4 | AstraZeneca+University of Oxford | Viral vector (non-replicating) | ChAdOx1-S-(AZD1222) | Phase 4-NCT04760132 |
| 5 | CanSino Biological Inc./Beijing Institute of Biotechnology | Viral vector (non-replicating) | Recombinant novel coronavirus vaccine (Adenovirus type 5 vector) | Phase 4-NCT04892459 |
| 6 | Janssen Pharmaceutical | Viral vector (non-replicating) | Ad26.COV2.S | Phase 4-EUCTR2021-002327-38-NL |
| 7 | Moderna+National Institute of Allergy and Infectious Diseases (NIAID) | RNA based vaccine | mRNA-1273 | Phase 4-NCT04760132 |
| 8 | Pfizer/BioNTech+Fosun Pharma | RNA based vaccine | BNT162b2 (3 LNP-mRNAs) | Phase 4-NCT04760132 |
| 9 | Medigen Vaccine Biologics+Dynavax+National Institute of Allergy and | Protein subunit | MVC-COV1901 (Spike-2P protein+adjuvant CpG 1018) | Phase 4-NCT05079633 |
Table 2: The Top Nine Vaccine Candidates as per who Draft Landscape
According to the WHO latest report, 26 candidate vaccines are undergoing clinical evaluation while 139 are in the preclinical stage[141]. Listed in Table 2 are the top 9 vaccine candidates in development at the time of writing this overview[142,143].
Summary
Though the SARS 2002, MERS 2012 and COVID-19 causatives are not entirely identical, similar pharmacotherapeutic approaches have been evaluated for use during the emergence of these pandemics (Table 3). Introduction and employment of newer agents helped in improving the sensitivity and providing a wider range of therapy for mild to severe infection types. COVID-19 has proved to be the most explosive HCoV outbreak with enhanced human-to-human transmission rate. To combat the spread, immense efforts have been put in to evaluate previously employed approaches for repositioning of drugs along with continuous attempts to introduce effective and clinically proven pharmaceutical interventions, most importantly led by vaccines.
| Virus | Treatment | Level of study | Effective | Reference |
|---|---|---|---|---|
| SARS-CoV | Antibiotics (cefotaxime, levofloxacin and clarithromycin) | Observationalstudy | No | [14] |
| Ribavirin | Observational study | No | [23,24] | |
| Lopinavir/Ritonavir | Preliminary and Cohort study | Yes | [25,26] | |
| Interferons | In vitro and preclinical evaluation | Yes | [28,29,30,31,32] | |
| Corticosteroids | Non-randomized preliminary study | Yes | [35] | |
| MERS-CoV | Antibiotics | Observational study | No | [46] |
| Ribavirin+IFNs | Retrospective cohort study | Yes | [57,58] | |
| Cyclosporine | In vitro | Yes | [62] | |
| Nitazoxanide | In vitro | Yes | [66] | |
| Mycophenolic acid | In vitro | Yes | [73,74] | |
| Remdesivir | Preclinical evaluation | Yes | [75,76] | |
| Monoclonal and polyclonal antibodies | In vitro and preclinical evaluation | Yes | [80,84,86] | |
| COVID-19 | Chloroquine/Hydroxychloroquine | In vitro and Observational cohort study | Yes | [110,111] |
| Remdesivir | In vitro and Randomized controlled study | Yes | [116,118,119] | |
| Favipiravir | In vitro and Controlled cohort study | Yes | [102,122] | |
| Lopinavir/Ritonavir | Randomized study | Yes | [123,124] | |
| Triple regime (Ribavirin+IFN+LPV/RTV) | Randomized multicentre study | Yes | [125] | |
| IFNs | Observational nosocomial study | Yes | [129] | |
| Tocilizumab | Retrospective observational cohort | Yes | [131] | |
| Plasma therapy | Preliminary study | Yes | [132,133] |
Table 3. Summary of Treatments for Coronavirus Outbreaks Based on In Vitro and Randomized Observational Studies
Conflict of interests:
The authors declared no conflict of interests.
References
- Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 2019;17(3):181-92.
[Crossref] [Google Scholar] [PubMed]
- Weiss SR, Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev 2005;69(4):635-64.
[Crossref] [Google Scholar] [PubMed]
- Lai MM, Cavanagh D. The molecular biology of coronaviruses. Adv Virus Res 1997;48:1-100.
- Su S, Wong G, Shi W, Liu J, Lai AC, Zhou J, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol 2016;24(6):490-502.
[Crossref] [Google Scholar] [PubMed]
- Paules CI, Marston HD, Fauci AS. Coronavirus infections—more than just the common cold. JAMA 2020;323(8):707-8.
[Crossref] [Google Scholar] [PubMed]
- Ruch TR, Machamer CE. The coronavirus E protein: Assembly and beyond. Viruses 2012;4(3):363-82.
[Crossref] [Google Scholar] [PubMed]
- Fan H, Ooi A, Tan YW, Wang S, Fang S, Liu DX, et al. The nucléocapside protein of coronavirus infectious bronchitis virus: Crystal structure of its N-terminal domain and multimerization properties. Structure 2005;13(12):1859-68.
[Crossref] [Google Scholar] [PubMed]
- Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: Emergence, transmission, and characteristics of human coronaviruses. J Adv Res 2020;24:91-8.
[Crossref] [Google Scholar] [PubMed]
- Zhong NS, Zheng BJ, Li YM, Poon LL, Xie ZH, Chan KH, et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, Peoples Republic of China, in February, 2003. Lancet 2003;362(9393):1353-8.
[Crossref] [Google Scholar] [PubMed]
- Severe Acute Respiratory Syndrome (SARS). World Health Organization; 2020.
- Ge XY, Li JL, Yang XL, Chmura AA, Zhu G, Epstein JH, et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 2013;503(7477):535-8.
[Crossref] [Google Scholar] [PubMed]
- Lau SK, Li KS, Huang Y, Shek CT, Tse H, Wang M, et al. Ecoepidemiology and complete genome comparison of different strains of severe acute respiratory syndrome-related Rhinolophus bat coronavirus in China reveal bats as a reservoir for acute, self-limiting infection that allows recombination events. J Virol 2010;84(6):2808-19.
[Crossref] [Google Scholar] [PubMed]
- Zhaori G. Antiviral treatment of SARS: Can we draw any conclusions? CMAJ 2003;169(11):1165-6.
[Google Scholar] [PubMed]
- Tsang K, Zhong NS. SARS: Pharmacotherapy. Respirology 2003;8:S25-30.
[Crossref] [Google Scholar] [PubMed]
- Sung JJ, Wu A, Joynt GM, Yuen KY, Lee N, Chan PK, et alSevere acute respiratory syndrome: Report of treatment and out come after a major out break. Thorax 2004;59(5):414-20.
[Crossref] [Google Scholar] [PubMed]
- Nyström K, Waldenström J, Tang KW, Lagging M. Ribavirin: Pharmacology, multiple modes of action and possible future perspectives. Future Virol 2019;14(3):153-60.
- Koren G, King S, Knowles S, Phillips E. Ribavirin in the treatment of SARS: A new trick for an old drug? CMAJ 2003;168(10):1289-92.
[Google Scholar] [PubMed]
- Cameron CE, Castro C. The mechanism of action of ribavirin: Lethal mutagenesis of RNA virus genomes mediated by the viral RNA-dependent RNA polymerase. Curr Opin Infect Dis 2001;14(6):757-64.
[Crossref] [Google Scholar] [PubMed]
- Ströher U, DiCaro A, Li Y, Strong JE, Aoki F, Plummer F, et al. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon-α. J Infect Dis 2004;189(7):1164-7.
[Crossref] [Google Scholar] [PubMed]
- Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr H. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003;361(9374):2045-6.
[Crossref] [Google Scholar] [PubMed]
- Chen F, Chan KH, Jiang Y, Kao RY, Lu HT, Fan KW, et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol 2004;31(1):69-75.
[Crossref] [Google Scholar] [PubMed]
- Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, et al. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax 2004;59(3):252-6.
[Crossref] [Google Scholar] [PubMed]
- Morgenstern B, Michaelis M, Baer PC, Doerr HW, Cinatl Jr J. Ribavirin and interferon-β synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem Biophys Res Commun 2005;326(4):905-8.
[Crossref] [Google Scholar] [PubMed]
- Chiou HE, Liu CL, Buttrey MJ, Kuo HP, Liu HW, Kuo HT, et al. Adverse effects of ribavirin and outcome in severe acute respiratory syndrome: Experience in two medical centers. Chest 2005;128(1):263-72.
[Crossref] [Google Scholar] [PubMed]
- Chan KS, Lai ST, Chu CM, Tsui E, Tam CY, Wong MM, et al. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: A multicentre retrospective matched cohort study. Hong Kong Medical J 2003.
[Google Scholar] [PubMed]
- Fujii T, Nakamura T, Iwamoto A. Current concepts in SARS treatment. J Infect Chemother 2004;10(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol 2002;14(4):432-6.
[Crossref] [Google Scholar] [PubMed]
- Dahl H, Linde A, Strannegård Ö. In vitro inhibition of SARS virus replication by human interferons. Scandinavian J Infect Dis 2004;36(11-12):829-31.
[Crossref] [Google Scholar] [PubMed]
- Barnard DL, Day CW, Bailey K, Heiner M, Montgomery R, Lauridsen L, et al. Evaluation of immunomodulators, interferons and known in vitro SARS-coV inhibitors for inhibition of SARS-coV replication in BALB/c mice. Antivir Chem Chemother 2006;17(5):275-84.
[Crossref] [Google Scholar] [PubMed]
- Kumaki Y, Ennis J, Rahbar R, Turner JD, Wandersee MK, Smith AJ, et al. Single-dose intranasal administration with mDEF201 (adenovirus vectored mouse interferon-alpha) confers protection from mortality in a lethal SARS-CoV BALB/c mouse model. Antivir Res 2011;89(1):75-82.
[Crossref] [Google Scholar] [PubMed]
- Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Treatment of SARS with human interferons. Lancet 2003;362(9380):293-4.
[Crossref] [Google Scholar] [PubMed]
- Hensley LE, Fritz EA, Jahrling PB, Karp C, Huggins JW, Geisbert TW. Interferon-β 1a and SARS coronavirus replication. Emerg Infect Dis 2004;10(2):317.
[Crossref] [Google Scholar] [PubMed]
- Sainz Jr B, Mossel EC, Peters CJ, Garry RF. Interferon-beta and interferon-gamma synergistically inhibit the replication of severe acute respiratory syndrome-associated coronavirus (SARS-CoV). Virology 2004;329(1):11-7.
[Crossref] [Google Scholar] [PubMed]
- Haagmans BL, Kuiken T, Martina BE, Fouchier RA, Rimmelzwaan GF, Van Amerongen G, et al. Pegylated interferon-α protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med 2004;10(3):290-3.
[Crossref] [Google Scholar] [PubMed]
- Lee N, Chan KA, Hui DS, Ng EK, Wu A, Chiu RW, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol 2004;31(4):304-9.
[Crossref] [Google Scholar] [PubMed]
- Ho JC, Ooi GC, Mok TY, Chan JW, Hung I, Lam B, et al. High–dose pulse versus nonpulse corticosteroid regimens in severe acute respiratory syndrome. Am J Respir Crit Care Med 2003;168(12):1449-56.
[Crossref] [Google Scholar] [PubMed]
- Auyeung TW, Lee JS, Lai WK, Choi CH, Lee HK, Lee JS, et al. The use of corticosteroid as treatment in SARS was associated with adverse out comes: A retrospective cohort study. J Infect 2005;51(2):98-102.
[Crossref] [Google Scholar] [PubMed]
- Loutfy MR, Blatt LM, Siminovitch KA, Ward S, Wolff B, Lho H, et al. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: A preliminary study. JAMA 2003;290(24):3222-8.
[Crossref] [Google Scholar] [PubMed]
- He Y, Zhou Y, Liu S, Kou Z, Li W, Farzan M, Jiang S. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: Implication for developing subunit vaccine. Biochem Biophys Res Commun 2004 Nov 12;324(2):773-81.
[Crossref] [Google Scholar] [PubMed]
- Stinebaugh BJ, Schloeder FX, Johnson KM, Mackenzie RB, Entwisle G, De Alba E. Bolivian hemorrhagic fever: A report of four cases. Am J Med 1966;40(2):217-30.
[Crossref] [Google Scholar] [PubMed]
- Ruggiero HA, Milani HA, Barri A, Val A, Maglio F, Astarloa L, et al. Treatment of Argentine hemorrhagic fever with convalescent's plasma. 4433 cases. Presse Med 1986;15(45):2239-42.
[Google Scholar] [PubMed]
- Frame JD, Verbrugge GP, Gill RG, Pinneo L. The use of Lassa fever convalescent plasma in Nigeria. Trans R Soc Trop Med Hyg 1984;78(3):319-24.
[Crossref] [Google Scholar] [PubMed]
- Mupapa K, Massamba M, Kibadi K, Kuvula K, Bwaka A, Kipasa M, et al. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. J Infect Dis 1999;179(1):S18-23.
[Crossref] [Google Scholar] [PubMed]
- Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis 2005;24(1):44-6.
[Crossref] [Google Scholar] [PubMed]
- Yang ZY, Kong WP, Huang Y, Roberts A, Murphy BR, Subbarao K, et al. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature 2004;428(6982):561-4.
- Sharif-Yakan A, Kanj SS. Emergence of MERS-CoV in the Middle East: Origins, transmission, treatment, and perspectives. PLoS Pathog 2014;10(12):e1004457.
[Crossref] [Google Scholar] [PubMed]
- Mohd HA, Al-Tawfiq JA, Memish ZA. Middle East respiratory syndrome coronavirus (MERS-CoV) origin and animal reservoir. Virol J 2016;13(1):1-7.
- Ramadan N, Shaib H. Middle East respiratory syndrome coronavirus (MERS-CoV): A review. Germs 2019;9(1):35-42.
[Crossref] [Google Scholar] [PubMed]
- Al-Tawfiq JA. Middle East respiratory syndrome-coronavirus infection: An overview. J Infect Public Health 2013;6(5):319-22.
- Chan JF, Lau SK, Woo PC. The emerging novel Middle East respiratory syndrome coronavirus: The “knowns” and “unknowns”. J Formos Med Assoc 2013;112(7):372-81.
[Crossref] [Google Scholar] [PubMed]
- Tahir M, Gajraj R, Bardhan M, Mohammed H, Dyke L, Charlemagne P, et al. Evidence of person-to-person transmission within a family cluster of novel coronavirus infections, United Kingdom, February 2013. Euro Surveill 2013;18(11):2807.
[Crossref] [Google Scholar] [PubMed]
- MERS-CoV: Clinical decision making support for treatment. Gov.UK; 2020.
- Mustafa S, Balkhy H, Gabere MN. Current treatment options and the role of peptides as potential therapeutic components for Middle East Respiratory Syndrome (MERS): A review. J Infect Public Health 2018;11(1):9-17.
[Crossref] [Google Scholar] [PubMed]
- Dyall J, Coleman CM, Venkataraman T, Holbrook MR, Kindrachuk J, Johnson RF, et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother 2014;58(8):4885-93.
[Crossref] [Google Scholar] [PubMed]
- Chong YP, Song JY, Seo YB, Choi JP, Shin HS. Antiviral treatment guidelines for middle east respiratory syndrome. Infect Chemother 2015;47(3):212-22.
[Crossref] [Google Scholar] [PubMed]
- Momattin H, Al-Ali AY, Al-Tawfiq JA. A Systematic review of therapeutic agents for the treatment of the Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Travel Med Infect Dise 2019;30:9-18.
[Crossref] [Google Scholar] [PubMed]
- Falzarano D, De Wit E, Martellaro C, Callison J, Munster VJ, Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci Rep 2013;3(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Omrani AS, Saad MM, Baig K, Bahloul A, Abdul-Matin M, Alaidaroos AY, et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: A retrospective cohort study. Lancet Infect Dis 2014;14(11):1090-5.
[Crossref] [Google Scholar] [PubMed]
- Arabi YM, Shalhoub S, Mandourah Y, Al-Hameed F, Al-Omari A, Al Qasim E, et al. Ribavirin and interferon therapy for critically ill patients with middle east respiratory syndrome: A multicenter observational study. Clin Infect Dis 2020;70(9):1837-44.
[Crossref] [Google Scholar] [PubMed]
- Shalhoub S, Farahat F, Al-Jiffri A, Simhairi R, Shamma O, Siddiqi N, et al. IFN-α2a or IFN-β1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: A retrospective study. J Antimicrob Chemother 2015;70(7):2129-32.
[Crossref] [Google Scholar] [PubMed]
- Chan JF, Yao Y, Yeung ML, Deng W, Bao L, Jia L, et al. Treatment with lopinavir/ritonavir or interferon-β1b improves out come of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis 2015;212(12):1904-13.
[Crossref] [Google Scholar] [PubMed]
- de Wilde AH, Raj VS, Oudshoorn D, Bestebroer TM, van Nieuwkoop S, Limpens RW, et al. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-α treatment. J General Virol 2013;94(Pt 8):1749.
[Crossref] [Google Scholar] [PubMed]
- Haagmans BL, Osterhaus AD. Coronaviruses and their therapy. Antivir Res 2006;71(2-3):397-403.
[Crossref] [Google Scholar] [PubMed]
- Arabi YM, Alothman A, Balkhy HH, Al-Dawood A, AlJohani S, Al Harbi S, et al. Treatment of Middle East respiratory syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): Study protocol for a randomized controlled trial. Trials 2018;19(1):81.
[Crossref] [Google Scholar] [PubMed]
- Arabi YM, Asiri AY, Assiri AM, Aziz Jokhdar HA, Alothman A, Balkhy HH, et al. Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-β1b (MIRACLE trial): Statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials 2020;21(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Rossignol JF. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J Infect Public Health. 2016;9(3):227-30.
[Crossref] [Google Scholar] [PubMed]
- Rossignol JF. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J Infect Public Health 2016 May 1;9(3):227-30.
[Crossref] [Google Scholar] [PubMed]
- Mo Y, Fisher D. A review of treatment modalities for Middle East respiratory syndrome. J Antimicrob Chemother 2016;71(12):3340-50.
[Crossref] [Google Scholar] [PubMed]
- de Wilde AH, Zevenhoven-Dobbe JC, van der Meer Y, Thiel V, Narayanan K, Makino S, et al. Cyclosporin A inhibits the replication of diverse coronaviruses. J Gen Virol 2011;92(11):2542.
[Crossref] [Google Scholar] [PubMed]
- Pfefferle S, Schöpf J, Kögl M, Friedel CC, Müller MA, Carbajo-Lozoya J, et al. The SARS-coronavirus-host interactome: Identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog 2011;7(10):e1002331.
[Crossref] [Google Scholar] [PubMed]
- Li HS, Kuok DI, Cheung MC, Ng MM, Ng KC, Hui KP, et al. Effect of interferon alpha and cyclosporine treatment separately and in combination on Middle East Respiratory Syndrome Coronavirus (MERS-CoV) replication in a human in vitro and ex vivo culture model. Antiviral Res 2018;155:89-96.
[Crossref] [Google Scholar] [PubMed]
- Markland W, McQuaid TJ, Jain J, Kwong AD. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: A comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob Agents Chemother 2000;44(4):859-66.
[Google Scholar] [PubMed]
- Chan JF, Chan KH, Kao RY, To KK, Zheng BJ, Li CP, et al. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect 2013;67(6):606-16.
[Crossref] [Google Scholar] [PubMed]
- Hart BJ, Dyall J, Postnikova E, Zhou H, Kindrachuk J, Johnson RF, et al. Interferon-β and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J Gen Virol 2014;95(3):571.
[Crossref] [Google Scholar] [PubMed]
- Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun 2020;11(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- De Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci 2020;117(12):6771-6.
[Crossref] [Google Scholar] [PubMed]
- Shirato K, Kawase M, Matsuyama S. Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J Virol 2013;87(23):12552-61.
[Crossref] [Google Scholar] [PubMed]
- Momattin H, Al-Ali AY, Al-Tawfiq JA. A Systematic review of therapeutic agents for the treatment of the Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Travel Med Infect Dis 2019;30:9-18.
[Crossref] [Google Scholar] [PubMed]
- Lu L, Liu Q, Zhu Y, Chan KH, Qin L, Li Y, et al. Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor. Nat Commun 2014;5(1):3067.
[Crossref] [Google Scholar] [PubMed]
- Luke TC, Casadevall A, Watowich SJ, Hoffman SL, Beigel JH, Burgess TH. Hark back: Passive immunotherapy for influenza and other serious infections. Crit Care Med 2010;38:e66-73.
[Crossref] [Google Scholar] [PubMed]
- Mou H, Raj VS, Van Kuppeveld FJ, Rottier PJ, Haagmans BL, Bosch BJ. The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J Virol 2013;87(16):9379-83.
[Crossref] [Google Scholar] [PubMed]
- Raj VS, Mou H, Smits SL, Dekkers DH, Müller MA, Dijkman R, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013;495(7440):251-4.
[Crossref] [Google Scholar] [PubMed]
- Ohnuma K, Haagmans BL, Hatano R, Raj VS, Mou H, Iwata S, et al. Inhibition of Middle East respiratory syndrome coronavirus infection by anti-CD26 monoclonal antibody. J Virol 2013;87(24):13892-9.
[Crossref] [Google Scholar] [PubMed]
- Qiu H, Sun S, Xiao H, Feng J, Guo Y, Tai W, et al. Single-dose treatment with a humanized neutralizing antibody affords full protection of a human transgenic mouse model from lethal Middle East respiratory syndrome (MERS)-coronavirus infection. Antiviral Res 2016;132:141-8.
[Crossref] [Google Scholar] [PubMed]
- Corti D, Zhao J, Pedotti M, Simonelli L, Agnihothram S, Fett C, et al. Prophylactic and postexposure efficacy of a potent human monoclonal antibody against MERS coronavirus. Proc Natl Acad Sci 2015;112(33):10473-8.
[Crossref] [Google Scholar] [PubMed]
- Luke T, Wu H, Zhao J, Channappanavar R, Coleman CM, Jiao JA, et al. Human polyclonal immunoglobulin G from transchromosomic bovines inhibits MERS-CoV in vivo. Sci Transl Med 2016;8(326):326ra21.
[Crossref] [Google Scholar] [PubMed]
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl Med 2020.
[Crossref] [Google Scholar] [PubMed]
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020;395(10224):565-74.
[Crossref] [Google Scholar] [PubMed]
- Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. J Virol 2020;94(7):e00127-20.
[Crossref] [Google Scholar] [PubMed]
- Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses 2020;12(4):372.
[Crossref] [Google Scholar] [PubMed]
- Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) out side of Wuhan, China: Retrospective case series. BMJ 2020;368.
- Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). J Gen Int Med 2020;35(5):1545-9.
[Crossref] [Google Scholar] [PubMed]
- WHO Director-General's statement on IHR Emergency Committee on Novel Coronavirus (2019-nCoV). World Health Organization; 2020.
- WHO Director-General's opening remarks at the media briefing on COVID-19-11 March 2020. World Health Organization; 2020.
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med 2020;382(13):119-1207.
- Zhai P, Ding Y, Wu X, Long J, Zhong Y, Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents 2020;55(5):105955.
[Crossref] [Google Scholar] [PubMed]
- Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: Estimation and application. Ann Int Med 2020;172(9):577-82.
[Crossref] [Google Scholar] [PubMed]
- Chowell G, Abdirizak F, Lee S, Lee J, Jung E, Nishiura H, et al. Transmission characteristics of MERS and SARS in the healthcare setting: A comparative study. BMC Med 2015;13(1):1-2.
[Crossref] [Google Scholar] [PubMed]
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382(18):1708-20.
- Hu Z, Song C, Xu C, Jin G, Chen Y, Xu X, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci 2020;63(5):706-11.
[Crossref] [Google Scholar] [PubMed]
- Coronavirus disease (COVID-19) pandemic. World Health Organization; 2020.
- Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30(3):269-71.
- Vahedi E, Ghanei M, Ghazvini A, Azadi H, Izadi M, Panahi Y, et al. The clinical value of two combination regimens in the management of patients suffering from Covid-19 pneumonia: A single centered, retrospective, observational study. Daru 2020;28(2):507-16.
[Crossref] [Google Scholar] [PubMed]
- Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review. JAMA. 2020;323(18):1824-36.
- Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 2020;6(1):1-4.
- Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: An old drug against today's diseases. Lancet Infect Dis 2003;3(11):722-7.
[Crossref] [Google Scholar] [PubMed]
- Kono M, Tatsumi K, Imai AM, Saito K, Kuriyama T, Shirasawa H. Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: Involvement of p38 MAPK and ERK. Antiviral Res 2008;77(2):150-2.
[Crossref] [Google Scholar] [PubMed]
- De Wilde AH, Jochmans D, Posthuma CC, Zevenhoven-Dobbe JC, Van Nieuwkoop S, Bestebroer TM, et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother 2014 Aug;58(8):4875-84.
[Crossref] [Google Scholar] [PubMed]
- Shen L, Yang Y, Ye F, Liu G, Desforges M, Talbot PJ, et al. Safe and sensitive antiviral screening platform based on recombinant human coronavirus OC43 expressing the luciferase reporter gene. Antimicrob Agents Chemother 2016;60(9):5492-503.
[Crossref] [Google Scholar] [PubMed]
- Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 2020;14(1):72-3.
[Crossref] [Google Scholar] [PubMed]
- Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020;71(15):732-9.
[Crossref] [Google Scholar] [PubMed]
- Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 2020;382(25):2411-8.
- Gautret P, Lagier JC, Parola P, Meddeb L, Mailhe M, Doudier B, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949.
[Crossref] [Google Scholar] [PubMed]
- Magagnoli J, Narendran S, Pereira F, Cummings TH, Hardin JW, Sutton SS, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. Med 2020;1(1):114-27.
[Crossref] [Google Scholar] [PubMed]
- Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med 2020;383(6):517-25.
[Crossref] [Google Scholar] [PubMed]
- Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 2017 Jun 28;9(396):eaal3653.
[Crossref] [Google Scholar] [PubMed]
- Brown AJ, Won JJ, Graham RL, Dinnon III KH, Sims AC, Feng JY, et al. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res 2019;169:104541.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020 May 16;395(10236):1569-78.
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med 2020;383(19):1813-36.
[Crossref] [Google Scholar] [PubMed]
- Wise J. Covid-19: Remdesivir is recommended for authorisation by European Medicines Agency. BMJ 2020;369:m2610.
[Crossref] [Google Scholar] [PubMed]
- Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 2013;100(2):446-54.
[Crossref] [Google Scholar] [PubMed]
- Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, et al. Experimental treatment with favipiravir for COVID-19: An open-label control study. Engineering 2020;6(10):1192-8.
[Crossref] [Google Scholar] [PubMed]
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. New Engl J Med 2020.
[Crossref] [Google Scholar] [PubMed]
- Stower H. Lopinavir–ritonavir in severe COVID-19. Nat Med 2020;26(4):465.
- Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial. Lancet 2020;395(10238):1695-704.
[Crossref] [Google Scholar] [PubMed]
- Owen DR, Allerton CM, Anderson AS, Aschenbrenner L, Avery M, Berritt S, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 2021;374(6575):1586-93.
[Crossref] [Google Scholar] [PubMed]
- Leist SR, Dinnon III KH, Schäfer A, Longping VT, Okuda K, Hou YJ, et al. A mouse-adapted SARS-CoV-2 induces acute lung injury and mortality in standard laboratory mice. Cell. 2020 Nov 12;183(4):1070-85.
[Crossref] [Google Scholar] [PubMed]
- Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med 2022;386(15):1397-408.
- Coronavirus (COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19. US FDA; 2021.
- Musarrat F, Chouljenko V, Dahal A, Nabi R, Chouljenko T, Jois SD, et al. The anti?HIV drug nelfinavir mesylate (Viracept) is a potent inhibitor of cell fusion caused by the SARSCoV?2 spike (S) glycoprotein warranting further evaluation as an antiviral against COVID?19 infections. J Med Virol 2020 Oct;92(10):2087-95.
[Crossref] [Google Scholar] [PubMed]
- Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res 2020;178:104791.
[Crossref] [Google Scholar] [PubMed]
- Felgenhauer U, Schoen A, Gad HH, Hartmann R, Schaubmar AR, Failing K, et al. Inhibition of SARS–CoV-2 by type I and type III interferons. J Biol Chem 2020;295(41):13958-64.
[Crossref] [Google Scholar] [PubMed]
- Zhou Q, Chen V, Shannon CP, Wei XS, Xiang X, Wang X, et al. Interferon-α2b Treatment for COVID-19. Front Immunol 2020;11:1061.
[Crossref] [Google Scholar] [PubMed]
- Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev 2020;19(7):102568.
[Crossref] [Google Scholar] [PubMed]
- Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19: A retrospective cohort study. Lancet Rheumatol 2020;2(8):e474-84.
[Crossref] [Google Scholar] [PubMed]
- Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, et al. Treatment with convalescent plasma for COVID?19 patients in Wuhan, China. J Med Virol 2020;92(10):1890-901.
[Crossref] [Google Scholar] [PubMed]
- Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 2020;323(16):1582-9.
[Crossref] [Google Scholar] [PubMed]
- Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 vaccines at pandemic speed. N Engl J Med 2020;382(21):1969-73.
[Crossref] [Google Scholar] [PubMed]
- Wu SC. Progress and concept for COVID?19 vaccine development. Biotechnol J 2020.
[Crossref] [Google Scholar] [PubMed]
- Mercado NB, Zahn R, Wegmann F, Loos C, Chandrashekar A, Yu J, et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020;586(7830):583-8.
[Crossref] [Google Scholar] [PubMed]
- COVID-19 vaccine tracker and landscape. World Health Organization; 2020.
- DRAFT landscape of COVID-19 candidate vaccines. World Health Organization; 2022.
- Overview of COVID-19 Vaccines. Centers for Disease Control and Prevention; 2022.