- *Corresponding Author:
- P. Rajalakshmi
Centre for Advanced Research in Indian System of Medicine (CARISM), SASTRA University, Thanjavur-613 401, India
E-mail: rajalakshmi@carism.sastra.edu
| Date of Submission | 17 August 2016 |
| Date of Revision | 03 April 2017 |
| Date of Acceptance | 12 October 2017 |
| Indian J Pharm Sci 2017;79(6): 987-993 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In Siddha system of medicine, herbs, metals, minerals, hydrochemicals, arsenics and animal products are predominantly used for medicine preparations. Annabethi is a common hydrochemical drug, which is popularly known as green vitriol (chemical name is ferric sulphate). Naturally Annabethi is collected in hills and also synthesized, which is green in colour with crystal form, nauseous astringent taste and has solubility in water. It is commercially prepared by mixing iron wire with sulphuric acid and evaporating the solution to crystallization. Annabethi chenthuram is commonly used in Siddha system of medicine for treating anemia, fever and dysentery. Even though some research studies have been conducted on Ayurvedic kasisa bhasma (similar to Annabethi chenthuram in Siddha), there is no scientific work carried out on the Annabethi chenthuram. Hence, the present research work was focused on the in-house preparation and characterization of Annabethi chenthuram. Annabethi chenthuram was prepared, physico-chemical parameters analysed and characterized using Fourier-transform infrared spectroscopy, X-ray diffraction, X-ray fluorescence, scanning electron microscope and particle size analyzer. Physico-chemical parameters such as loss on drying (1.00 %), total ash (65.94 %), water soluble ash (1.864 %) and acid insoluble ash (39.02 %) were determined. Fourier-transform infrared spectra revealed an increase of iron content (peaks at 617 and 430) when compared to the raw drug. X-ray diffraction pattern confirmed the presence of α-Fe2O3 and absence of β-Fe2O3 in Annabethi chenthuram. X-ray fluorescence analytical data showed that the medicine contained iron (66.46 %) and iron oxide (93.58 %). These results provided scientific evidence for the conversion of iron sulphate into an edible and bioavailable form in Annabethi chenthuram.
Keywords
Annabethi chenthuram, physico-chemical standards, FTIR, XRD, XRF, SEM
Siddha system of medicine is a unique system providing better cure for all chronic ailments like carcinoma, syphilis, respiratory diseases, infectious diseases, diabetes, dermatological cases and autoimmune disorders. The Siddha system includes not only medicine and alchemy but also yoga and philosophy. In Siddha system, 220 types of metals, mineral, arsenics and hydrochemicals are used for preparing medicines [1]. Annabethi contained the hydrochemical, ferric sulphate with the chemical formula of Fe2SO4.7H2O [2]. In English, Annabethi is called as green vitriol or green copper or salts of steel. It is called in Tamil Annabethi, Sanskrit Kaaseesa, Telugu Annabhedi, Malayalam Turus, Kannada kaasisa, Hindi Hara tutia, Gujarathi Hara-kasis, Punjabi and Kashmiri San-i-sabz, Urdu Hirakashish, Latin Ferric sulphate, French Sulphate ferreux, German Schwefelsaures Eisenoxydul and Arabian Zaje-Asfara [3]. It is an antihaemorrhagic drug without any odour that reacts with acid, soluble in water and insoluble in alcohol [3]. According to Siddha system of medicine, Annabethi is classified based on colour into four types, black, yellow, green and white [1,3]. It is one of the 120 Uparasam (hydrochemicals) mentioned in Siddha Materia Medica (Gunapadam: Thathu, Seevavaguppu) [1]. It reacts with air and becomes white in colour. It is a very common Siddha mineral drug available in herbal drug shops of Tamilnadu and used for iron based herbo-metallic preparations like Annabethi chenthuram, Vedi Annabethi chenthuram [1], Annapavazha chenthuram and Kadukkai mathirai [4]. There are several processes mentioned in Siddha text for preparing this drug. One important method mentioned in ancient Siddha literature is dissolving the iron rods in sulphuric acid by the aid of heat and it must be used in medicine only after purification.
Annabethi is used for the treatment of anaemia, indigestion and eye diseases. It also used to treat venereal diseases, amenorrhea (absence of menstruation), leucorrhoea (white discharge from uterus), whooping cough, malarial fever, worm infestation and swelling. Externally used for herpes zoster, venereal ulcer, fistula, uterus prolapse and rectal prolapse [1]. It is considered in high esteem as a haematinic in cases of anaemia [4]. It is also given as a substitute for other preparations of iron. In certain cases of jaundice and anaemia, it is considered as a specific drug. It improves the colour and vigour of the body by enriching the blood. Due to its astringent properties, it is given in combination with other drugs in cases of dysentery, diarrhoea and haemorrhage [5]. For reducing the body tiredness or drowsiness detoxified Annabethi (130 mg) is provided with 1 ounce of Andrographis paniculata decoction or same quantity of Trachyspermum ammi extract for 3 times per day [1,3]. For amenorrhoea and chronic constipation, one part of purified Annabethi is administered with 2 parts of Musamparam (Aloe vera latex) and ground with sufficient quantity of honey [6]. Therapeutically, it acts as a nutrient, astringent, emmenagogue (stimulates menstruation), deodorant, antihelminthic and antiperiodic (antagonizes the poison of periodic disorders like ague) [1,7].
‘Chenthuram’ is a category of medicine with reddish colour and powder form and it retain potency for 75 y [4]. Formulary of Siddha medicine shows the usage of Annabethi chenthuram for treating fever, dysentery, amenorrhea and jaundice [7], but major therapeutic use is anaemia. Annabethi chenthuram should be taken with honey in a dose of 65 to 130 mg [8]. According to Narayanaswami [9], ghee also can be used as a vehicle. Previously, Nisha Kumari et al. [10] explained that it has antimicrobial activity and this medicine is proved to be non-toxic and also reduces SGPT level in animal model [2,11]. A human clinical trial revealed that this medicine is very much effective for sickle cell anaemia [12].
Even though some research studies have been conducted on Ayurvedic kasisa bhasma (Similar to Annabethi chenthuram in Siddha), there is no scientific work carried out on the Annabethi chenthuram. Hence, the present research was focused on the inhouse preparation and characterization of Annabethi chenthuram.
Materials and Methods
Preparation of Annabethi chenthuram
Annabethi (iron sulphate) was procured from local raw drug shop of Thanjavur, Tamilnadu. The Citrus limon fruit was bought from the vegetable market of Thanjavur. The drug was detoxified as per the methods mentioned in authentic Siddha text book [13]. Fresh juice of lemon was used for preparation. The common procedure of Annabethi chenthuram preparation involves detoxification, incineration, trituration and verification. For detoxification, Annabethi (1 kg) was dissolved in 1 l of water and filtered through muslin cloth in order to remove the dust and other impurities. To the filtrate, 10 drops of sulphuric acid was added and this mixture was kept under sunlight until evaporated completely and formation of solids. Use of any one of herbal ingredients (lime juice, sour rice water, Mollugo lotoides plant juice) is recommended for the preparation of Annabethi chenthuram as per Siddha literature [1] and in this study we have used lemon juice. Detoxified Annabethi (500 g) was taken and mixed with 500 ml of lemon juice. This mixture was kept under shaded condition for 12 h and kept under sunlight till it fully dried. After drying, chenthuram was incinerated with 50 cow dung cakes for one time by traditional method (Pandri putam, Figure 1).
Physicochemical analysis
Physiochemical standards were analysed in triplicate following the standard procedures [14,15]. The determination of the total ash content of raw Annabethi, detoxified Annabethi and Annabethi chenthuram was done by following the method of Joshi and Aeri [16]. Powdered drug (1 g) was added to a pre-weighed silica crucible and heated in the muffle furnace at 400° for about 3 h. Then the crucible was safely placed in a desiccator and allowed to cool to room temperature and the weight was finally measured. Percent weight of the total ash was calculated using the formula: weight of the ash/weight of the drug×100. Percent acid insoluble ash was calculated using the formula: weight of the residue/weight of the powder×100, where the weight of the residue is the net weight of ash. The water soluble extractives of the samples were analysed according to the same methodology. Dry powder (1 g) was taken in a beaker and 50 ml of water was added in and shaken well manually. The beaker was kept aside for 24 h and thereafter 10 ml of the solution was taken and kept in hot air oven at 105°. Finally the percent weight of the extract is calculated using the same formula.
The loss on drying (LOD) was estimated by taking 1 g of powdered drug in a pre-weighed dish and placed in the hot air oven at a temperature of 105° and the LOD was calculated by using the formula: weight of the dish before heating–weight of the dish after heating/weight of the sample×100.
Instrumental analysis
Raw and final drugs were analysed between 4000- 400 cm–1 in the Fourier-transform infrared (FTIR) spectrometer (Spectrum100, Perkin Elmer, USA). The raw Annabethi and Annabethi chenthuram were examined from 10-60° (2θ) with a step size of 0.01° using an X-ray diffractometer (XRD, D8 Focus, Bruker, Germany), by irradiating with Cu– Kα radiation. The surface morphology of the drugs was studied qualitatively using a cold field emission scanning electron microscope (SEM, JSM6701F, Jeol, Japan). The sample was mounted on a brass stub and sputter coated with platinum and introduced into the specimen chamber and imaging was carried out at an acceleration voltage of 3 kV. The elemental composition of Annabethi chenthuram was determined using a X-ray fluorescence (XRF) spectrometer (S8 Tiger, Bruker AXS, Germany) using a 4 kW rhodium anode X-ray tube. Determination of average particle size of raw Annabethi and Annabethi chenthuram were carried out by the Microtrac particle size analyser that uses three precisely placed red laser diodes to accurately characterize particles. The patented Tri- Laser system provides accurate, reliable and repeatable particle size analysis for a diverse range of applications by utilizing the proven theory of Mie compensation for spherical particles and the proprietary principle of Modified Mie calculations for non-spherical particles. The S3500 measures particle size from 0.02 to 2800 microns (Microtrac Blue wave, S3500, USA).
Results and Discussion
Iron deficiency is the most common type of anaemia in India, which is due to decrease in the haemoglobin (Hb) level. Decrease in the Hb intensity may disrupt the normal lifespan of red blood cells synthesized in the bone marrow and liver [17]. Siddha literature denotes that the cause for anaemia are excessive intake of salty, sour food items, toxic foods, liver disorders, bleeding piles, blood vomiting, polymenorrhagia and dysentery [5]. In Siddha system, Annabethi chenthuram is prescribed as excellent medicine for anaemia. Even though this drug is commonly used for long time, till date no scientific analytical works have been done to reveal the chemical composition of this drug. Hence, in this present work, we have determined the physico-chemical standards and surface characteristics of Annabethi chenthuram for the first time.
Central Council for Research in Ayurveda and Siddha (CCRAS), Department of AYUSH, New Delhi given some analytical specifications for chenthuram [18]. The physicochemical properties of raw, detoxified and final drug of Annabethi were investigated and the results are given in Table 1. The colour and taste characteristics were changed during detoxification and final drug preparation when compared to raw Annabethi, whereas no change was observed in odour.
| Name of the test | Raw Annabethi | Detoxified Annabethi | Annabethichenthuram |
|---|---|---|---|
| Colour | Green | Whitish yellow | Brown coloured powder |
| Odour | Odourless | Odourless | Odourless |
| Taste | Astringent taste | Sour with astringent taste | No characteristic taste |
| Loss on drying (LOD, %) | 25.91 | 17.20 | 1.00 |
| Total ash (%) | 31.57 | 33.58 | 65.94 |
| Water soluble ash (%) | 0.518 | 0.958 | 1.864 |
| Acid insoluble ash (%) | 11.40 | 15.64 | 39.02 |
Table 1: Physicochemical Properties of raw, Detoxified and Final Drug of Annabethi
LOD test indicates the amount of volatile matter (i.e. moisture drying off from the drug). The LOD of raw, detoxified and final drug of Annabethi was 25.91, 17.20 and 1.00 % (Table 1), respectively, which shows the presence of high level of water content in the raw Annabethi.
But during detoxification and the final drug preparation, the moisture content was significantly reduced. The LOD level of final drug is equal to the standard limit of Pharmacopoeial standards for Ayurveda [19]. Total ash content was high (65.94 %) in final product of Annabethi, which indicates that this drug was prepared from non-volatile inorganic material.
The remaining portion (34.06 %) of chenthuram composed of combustible organic matter (sulphur), which might be evaporated under high temperature. Acid-insoluble ash was high in chenthuram compared to raw and detoxified annabethi because of the inorganic material and this is in agreement with the previous findings on insoluble nature of inorganic matters in acid medium [20]. Previously Yadav and Debnath [21] carried out analysis in the traditionally prepared Kasisa Bhasma and found 86.85-92.94 % of total ash content.
FTIR spectra of raw Annabethi showed the presence of huge OH stretch around 3200 cm–1 (Figure 2). Peak at 1623 cm–1 showed the presence of carbonyl groups. Peaks at 1147 and 1099 cm–1 showed the presence of SO4. Peaks at 617 and 430 cm–1 showed the presence of Fe-O peaks. Annabethi chenthuram showed the presence of Fe-O peaks at 827, 664, 624, 527 and 478 cm–1. Peaks at 1179, 1149, 1085 and 1006 cm–1 showed the presence of SO4 vibrations. Over all, both raw Annabethi and Annabethi chenthuram showed the presence of Fe-O and SO4. However, intensity of Fe-O peaks was more in Annabethi chenthuram compared with raw Annabethi (Figure 2). Similarly, FTIR characterization was done for related kanta chenduram [22], Muthuchippi parpam [23] and Varatika bhasma [24].
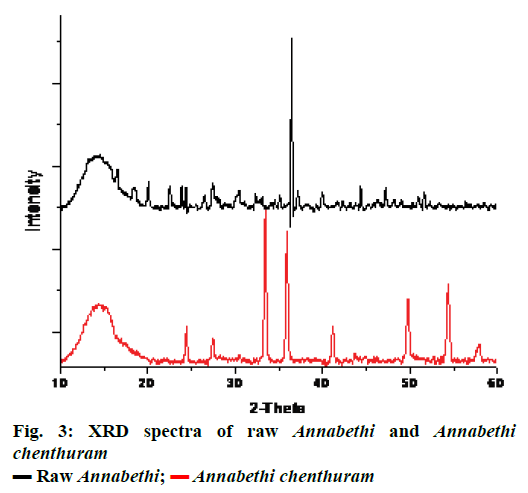
XRD spectra of raw Annabethi and Annabethi chenthuram showed the presence of peaks at 33.14, 37.17, and 47.21 Ǻ which represent the presence of FeS2 in Annabethi chenthuram. Moreover, presence of β-Fe2O3 is also evidenced by the presence of peaks at 22.56, 27.43, 30.46 and 44.36 Ǻ. Furthermore, peaks at 24.38, 35.1, 39.94, and 50.93 Ǻ represent the presence of α-Fe2O3 in the raw sample.
Though the Annabethi chenthuram showed the presence of traces of FeS2, the peaks at 35.44, 41.13, 54.34 and 57.9 Ǻ represent the presence of α-Fe2O3 and absence of β-Fe2O3. Hence it is clear that the preparation process actually increased the percentage of α-Fe2O3 and removal of β-Fe2O3 (Figure 3). Similarly, XRD characterization was done for related kanta chenduram and vanga Bhasma [22,25].
XRF analysis, it is observed that so many elements are present in Annabethi chenthuram apart from the iron and sulphate (Table 2). Some of the elements such as iron, sulphur, copper and zinc are playing a major therapeutic role in Annabethi chenthuram. Level of oxide form of iron (Fe2O3) was found to be higher (93.58 %) in Annabethi chenthuram when compared to elemental form (66.46 %). Similar trend was noted in the case of sulphur, manganese, potassium, aluminium, chromium, magnesium, sodium, copper, phosphorous, titanium and zinc. Similarly, XRF characterization was done for related vanga Bhasma [25].
| Elemental form | Oxide form | ||
|---|---|---|---|
| Fe | 66.46 | Fe2O3 | 93.58 |
| O | 30.74 | -- | -- |
| S | 0.65 | SO3 | 1.62 |
| Ca | 0.63 | -- | -- |
| Mn | 0.48 | MnO | 0.88 |
| Si | 0.44 | SiO2 | 0.44 |
| K | 0.34 | K2O | 0.42 |
| Ni | 0.29 | -- | -- |
| Al | 0.26 | Al2O3 | 0.49 |
| Cr | 0.18 | Cr2O2 | 0.26 |
| Mg | 0.16 | MgO | 0.26 |
| Na | 0.16 | Na2O | 0.21 |
| Cu | 0.087 | CuO | 0.10 |
| P | 0.03 | P2O5 | 0.14 |
| Ti | 0.02 | Ti O2 | 0.04 |
| Zn | 0.02 | Zn | 0.8 |
| -- | -- | NeO | 0.37 |
| -- | -- | Cl | 0.33 |
Table 2: XRF Data On Annabethi Chenthuram
SEM image of the raw Annabethi showed the presence of small particles placed over smooth planes (Figure 4). The size of those particles was found to be more than 100 nm. SEM analysis of Annabethi chenthuram showed the presence of particles less than 100 nm. However the particles are aggregated and approximately spherical in shape. During the preparation of Annabethi chenthuram from raw Annabethi, formation of nanoparticles with the particle size less than 100 nm was noticed (Figure 4) and our results are in agreement with earlier reports [23,25].
Particle size distribution of raw Annabethi and Annabethi chenthuram was determined by the Microtrac particle size analyser that uses three precisely placed red laser diodes. The average particle size of raw Annabethi was 85 μm whereas in the case of Annabethi chenthuram (after putam) it was found to be 71 μm. The particle size was reduced during the conversion of raw Annabethi into Annabethi chenthuram. The same analysis was already noted in previous studies in Kasisa Bhasma, Kanta chenthuram and Arka Lavana [21,22,26].
In the present study, we have prepared the Siddha formulation Annabethi chenthuram and analysed its physicochemical and structural characteristics. Physicochemical values for the raw, detoxified and final product were determined and reported for the first time, which could be useful in quality control steps.
FTIR spectra of Annabethi chenthuram revealed that the iron content was increased compare to the raw drug. XRF analytical data confirmed the presence of iron and iron oxide in large portion. XRD pattern explored the formation of crystalline compound which is again due to the impact of incineration processes. The SEM report and particle size analysis data indicated that the raw drug obtained was in nanometer size. From the present study, it is clear that Annabethi chenthuram preparation method prescribed in Siddha system involves scientific and systematic detoxification processes with enhanced therapeutic potential.
Acknowledgements
Authors thank the Honourable Vice Chancellor of SASTRA University for constant encouragement and support to carry out this research work.
Conflict of interest
The authors declare that they have no conflict of interest.
Financial support and sponsorship
Nil.
References
- Thyagarajan R. Gunapadam Thaathu Jeeva Vaguppu, 4th ed. Chennai: Department of Indian Medicine and Homeopathy; 2004.
- Ganguli MN, Singh RK, Jamadaghi SB, Mitra A, Upadhyay SN, Hazra J. Toxicological evaluation of kasisabhasma, an Ayurvedicorgana metallic preparation. Int J Res Ayurveda Pharm 2012;3:381-6.
- Nadkarani KM, Nadkarani AK. Indian Materia Medica. 3rded. Mumbai: Popular Prakash; 2005.
- Anonymous. The Siddha Formulary of India. New Delhi: Ministry of Health and Family Welfare, Department of AYUSH, Government of India; 1992.
- Kuppusamy Mudhaliyar KN. Siddha maruthuvam (Pothu). 7thed. Chennai: Department of Indian Medicine and Homeopathy; 2007.
- Murugesa Mudalier KS, Gunapadam Mooligai Vaguppu. 2nded. Chennai:Tamilnadu Siddha Medical Council; 2008.
- Anonymous. Formulary of Siddha Medicines. Chennai: Indian Medical Practitioners Cooperative Pharmacy and Stores Ltd.; 1989.
- Kuppusamy Mudaliyar KN, Uthamarayan KS. Siddha vaithiya thirattu. Chennai: Tamilnadu Siddha Medical Council; 1998.
- Narayanaswami V. Pharmacopoeia of Hospital of Indian Medicine. Chennai: TamilnaduSiddha Medical Board; 1995.
- Nishakumari PR, Dinesh Nayak J, Sathyanarayana B. Comparative antimicrobial study of shuddha Kasisaand Kasisa Bhasma. Int Ayur Med J 2016; 4: 579-83.
- Palbag S, Saha D, Gautam DNS. Toxicity studies of iron-containing Ayurvedic drug Kasisa Bhasma. BLDE Univ J Health Sci 2016; 1:39-43.
- Yogita S, Tiwari GN, Sujata S, Manoj S. Therapeutic role of Gomed-Mandur-Kasisa Bhasma compound in clinical management of sickle cell anaemia. J Pharm Sci Innov 2014; 3:459-62.
- Anonymous. Sarakku Suthi Seimuraigal. 1sted. Chennai: Department of Indian System of Medicine and Homeopathy; 2008.
- The Ayurvedic Pharmacopoeia of India. Part I, Volume IV, New Delhi: Ministry of Health and Family Welfare, Department of AYUSH; 2004.
- http://apps.who.int/iris/bitstream/10665/41986/1/9241545100.pdf.
- Joshi S, Aeri V. Practical Pharmacognosy, 1sted. New Delhi: Frank Bros and Co. Ltd; 2009. p. 290-3.
- Das PC. Text Book of Medicine. 3rded. Calcutta: Current Books International; 1991.
- Anonymous. Parameters for quality assessment of Ayurveda and Siddha drugs. Part A. New Delhi: Central Council for Research in Ayurveda and Siddha, Department of AYUSH; 2005.
- Anonymous. Pharmacopoeial Standards for Ayurvedic Formulations. New Delhi: Central Council for Research in Ayurveda and Siddha, Ministry of Health and Family Welfare; 1987.
- Akila B, Manickavasakam K, Shakila R. Chemical analysis of Gomutra Silasathu Parpam. Int J Drug Delivery 2014;6:88-93.
- Yadav S, Debnath M. Pharmaceutical and physicochemical study of Kasisa Bhasma. Indian J Ethno-Phytopharm 2016;2:43-9.
- Geetha Sudeer R, Amrutha P, Brindha P, Ramanathan V. What roles do herbs play in Kantacenturam: An iron oxide based herbo-mineral Siddha drug formulations? Indian J Tradit Know 2015;14:433-9.
- Rajalakshmi P, Devanathan R, Brindha P. Analytical studies on Muthuchippi Parpam. J Pharm Res 2010;3:2366-70.
- Devanathan R, Rajalakshmi P, Brindha P. Chemical standardisation of Varatika Bhasma. Int J Cur Pharm Res 2010:2;12-6.
- Saraswathi A, Ruckmani S, Devi AM, Ariyanathan S. Chemical analysis of Vanga Bhasma. Int J Res Ayur Pharm 2013;4:676-9.
- Devanathan R, Niraimathi KL, Karunanidhi M, Brindha P. Comparative evaluation of Arka Lavana – An Ayurvedic herbo-mineral formulation. Int J Pharm Clin Res 2013;5:37-42.

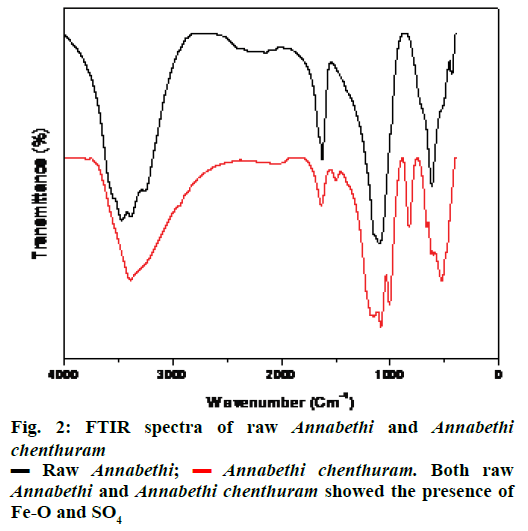
 Annabethi chenthuram. Both raw Annabethi and Annabethi chenthuram showed the presence of Fe-O and SO4
Annabethi chenthuram. Both raw Annabethi and Annabethi chenthuram showed the presence of Fe-O and SO4