- *Corresponding Author:
- B. Shekhany
Department of Medical Laboratory, Technology Shaqlawa Technical College, Erbil Polytechnic University, Erbil 44001, Iraq
E-mail: bestoon.hamed@epu.edu.iq
| Date of Received | 31 July 2022 |
| Date of Revision | 10 February 2023 |
| Date of Acceptance | 07 August 2023 |
| Indian J Pharm Sci 2023;85(4):953-961 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Chemotherapy is one of the most important treatment options in the treatment of all types of leukemia, but multi-drug resistance often precludes the success of this treatment. In this study, anticancer properties of heterocyclic salicylaldimines and their Cu(II) complexes on K562 human chronic myelogenous leukemia cell line were investigated. Doxorubicin was used as positive control. Compounds (C1-8 and doxorubicin) were placed to 96-well culture plate in triplicate order at doses of 1, 10, 100, 1000 μM and then K562 cells were seeded into the wells at the dose of 105/ml. After 72 h incubation, colorimetric 3-[4,5-dimethylthiazol-2- yl]-2,5 diphenyl tetrazolium bromide test was performed to determine half-maximal inhibitory concentration values of each compound. Antiproliferative effects of compounds were determined using carboxyfluorescein succinimidyl ester assay. Apoptosis induction potential of each compound determined by mitochondrial membrane potential analysis (Rho123), cleaved caspase-3 expression analysis by flow cytometry and immunofluorescent staining and cell morphology analysis by giemza, hematoxylin and eosin and Papanicolaou protocols. The metal complexes of the compounds had stronger cytotoxic effects and cleaved caspase-3 expression than their ligands. Mitochondrial membrane potential was found at low levels in cells treated with Schiff base compounds and doxorubicin. In the cell morphology analyzes, chromatin condensation and marginalization, changes in the cell membrane, ghost cells and apoptotic bodies were observed as evidence of apoptosis formation. Among the tested heterocyclic Schiff base compounds, the powerful cytotoxic and apoptosis-inducing potential of Bis((9-((cycloheptylimino)methyl)-1,1,7,7-tetramethyl-2,3,6,7-tetrahydro- 1H,5H-pyrido[3,2,1-ij]quinolin-8-yl)oxy)copper complex (C6) draws attention as a promising compounds withal a need for further in vitro and in vivo studies.
Keywords
Heterocyclic Schiff base, K562, cytotoxic, antiproliferative, apoptosis
Leukemia is a blood cancer that develops in the hematopoietic system and presents in the bone marrow with or without peripheral blood cell involvement[1,2]. In cancer biology, controlling leukemia remains a big challenge. Chemotherapy is one of the most important aspects of cancer treatment, although no ideal chemotherapeutic drug has yet to be developed[3]. Leukemia cells in patients are intrinsically resistant to standard chemotherapy, and resistance development in leukemia cells toward drugs results in treatment failure[4,5]. However, the applicability of such traditional chemotherapeutic approaches is primarily limited due to acquired Multi-Drug Resistance (MDR) in cells, which is acquired through the production of anti-apoptotic cascades and the overexpression of cell membrane-based drug efflux pumps, such as P-glycoprotein, for self-defense. MDR-associated protein 1 is a protein that has a big impact on the intracellular availability of MDR-prone medicines[6,7]. MDR is accountable for more than 90 % of cancer deaths who are using traditional chemotherapeutics or novel targeted therapies. Some of the MDR processes include increasing of drug efflux, increasing of deoxyribonucleic acid damage repair, decreasing of apoptosis, increasing autophagy, and/or altered drug metabolism[8-10].
Herbal or chemical compounds that kill or impede cancer cell development in vitro are being developed into new chemotherapeutic medicines. In most studies, the tested chemicals' cytotoxic or antiproliferative effects on cancer cells are investigated using similar test protocols, and the results are analyzed collectively[11]. Schiff base complexes and their related transition metal complexes have unearthed a broad field of chemistry that is drawing interest[12]. Schiff bases and its metallic complexes are instrumental in rummaging the free radicals and in this way ensure living bodies from the unfavorable impacts of these radicals[13]. Moreover, the biological activities of heterocyclic compounds derived from Schiff base exhibit a wide range of pharmacological properties, which include anticancer activities[14,15]. In our study, it was aimed to investigate the in vitro cytotoxic, antiproliferative and apoptotic properties of 8 heterozygous salicylaldimines (1,1,7,7-tetra-methyl-jululidine-N-pentylsalicylaldimine, N-hexyl, N-heptyl, N-octyl heterocyclyl salicylaldimines) copper complexes on the K562 myelomonoyctic leukemia cells.
Materials and Methods
Chemicals:
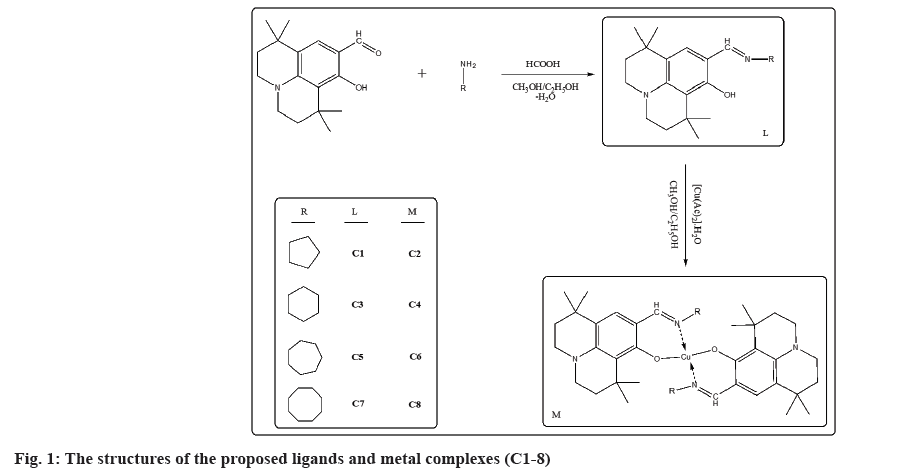
With a high yield, all ligand compounds and their metal-complexes were synthesized. As illustrated in fig. 1, the ligands (C1,3,5,7) have been prepared in moderate yield under optimal conditions by cascade reaction of 8-hydroxy-1,1,7,7-tetramethyl-2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinoline-9-carbaldehyde refluxing the 1:1 molar ratio with cyclopentanamine, cyclohexanamine, cycloheptanamine and cyclooctanamine in anhydrous ethanol and methanol (MetOH-EtOH)[16,17]. The targeted ligands (C1,3,5,7) were successfully recrystallized in good yields as solids (55-70 %), and all of the ligands displayed favorable spectroscopic results. As illustrated in fig. 1, the copper complexes (C2,4,6,8) were obtained by reactions of ligands (C1,3,5,7) with [Cu(Ac)2].H2O in good yields as solids following by recrystallization. The spectroscopic and synesthetic data of the ligands (C1,3,5,7) and their copper complexes (C2,4,6,8) were given as supportive information. The elemental analysis, Ultraviolet-visible spectra, Fourier-transform infrared spectroscopy spectra and 1H and 13C NMR spectra results supported the indicated structure of compounds, as expected. Heterocyclic Schiff base ligand derives and their Cu(II) complexes (C1-8) (fig. 1) were examined for their anticancer properties, DOX was used as positive control (Table 1). Chemicals were dissolved in ethyl alcohol in dose of 10 mM and then sterilized by syringe type filters in 20 µm pore size (Minisart®, Biotech, USA) and stored at -20°.
| Compounds | Chemical name | Structure | Molecular weight (g/m) |
|---|---|---|---|
| DOX | 7-(4-amino-5-hydroxy-6-methyloxan-2-yl)oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione | 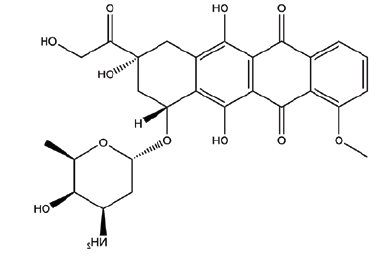 |
543.5 |
| C1 | 9-((cyclopentylimino)methyl)-1,1,7,7-tetramethyl-2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-8-ol | 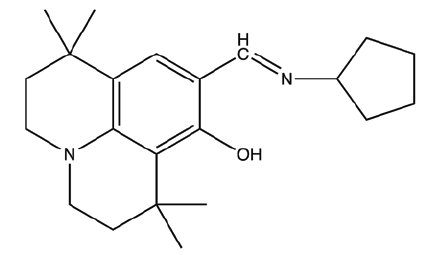 |
340.5 |
| C2 | Bis((9-((cyclopentylimino)methyl)-1,1,7,7-tetramethyl-2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-8-yl)oxy)copper | 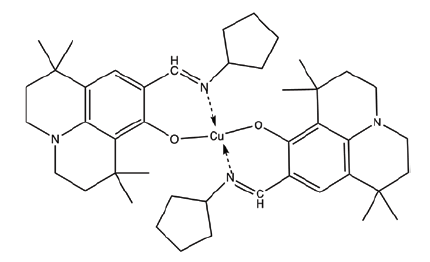 |
742.6 |
| C3 | 9-((cyclohexylimino)methyl)-1,1,7,7-tetramethyl-2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-8-ol | 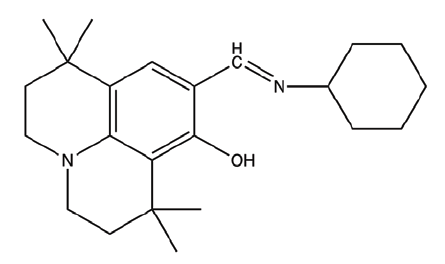 |
354.53 |
| C4 | Bis((9-((cyclohexylimino)methyl)-1,1,7,7-tetramethyl-2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-8-yl)oxy)copper | 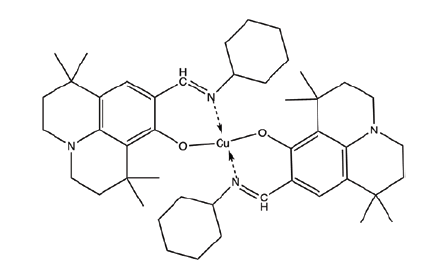 |
770.59 |
| C5 | 9-((cycloheptylimino)methyl)-1,1,7,7-tetramethyl-2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-8-ol | 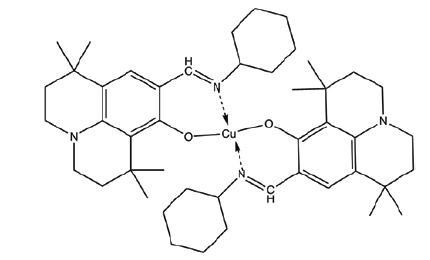 |
368.56 |
| C6 | Bis((9-((cycloheptylimino)methyl)-1,1,7,7-tetramethyl-2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-8-yl)oxy)copper | 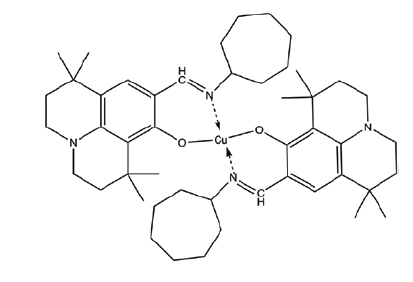 |
798.64 |
| C7 | 9-((cyclooctylimino)methyl)-1,1,7,7-tetramethyl-2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-8-ol | 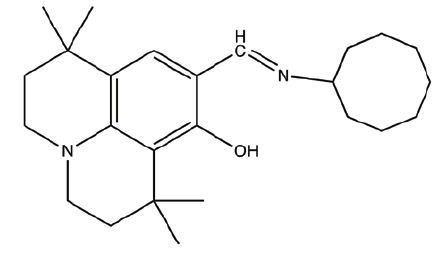 |
382.58 |
| C8 | Bis((9-((cyclooctylimino)methyl)-1,1,7,7-tetramethyl-2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-8-yl)oxy)copper | 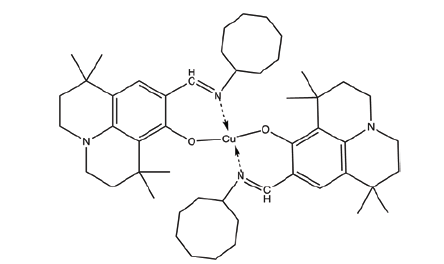 |
826.69 |
Cell line:
Chronic myelogenous leukemia cells (K562) have been suspended in Iscove's modified Dulbecco's medium (Sigma) containing fetal calf serum (10 %, Sigma) with antibiotics.
3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) analysis:
K562 cell suspension was dispensed into 96-well culture plates 105 cells/100 µl per well containing at varying doses (1, 10, 100 and 1000 µM) of DOX and (C1-8) in triplicate order. Culture flasks have been incubated in a CO2-incubator with humidifying property at 37° under 5 % CO2 condition. After 72 h of culture, 10 µl of 300 µM MTT solutions (Sigma) is added to culture plate wells and incubated for an additional 4 h. After the incubation, culture plates have been centrifuged at 3000 rpm for about 10 min. then the upper supernatant part have been decanted and replaced by adding 100 µl of Dimethyl Sulfoxide (DMSO) into each of the culture wells in order to dissolve the formazan-crystals which have been synthesized through the conversion of MTT to MTT-formazan by viable cells. The culture plates were read in an ELISA microplate reader (MD Spectramax, M5, USA) at a wavelength of 570 nm and Optical Density (OD) values for each well. OD values were expressed as mean and standard deviation. Half-Maximal Inhibitory Concentration (IC50 ) values for DOX and heterocyclic Schiff base compounds and their metal complexes were calculated by non-linear regression analyses.
Carboxyfluorescein Succinimidyl Ester (CFSE) analysis:
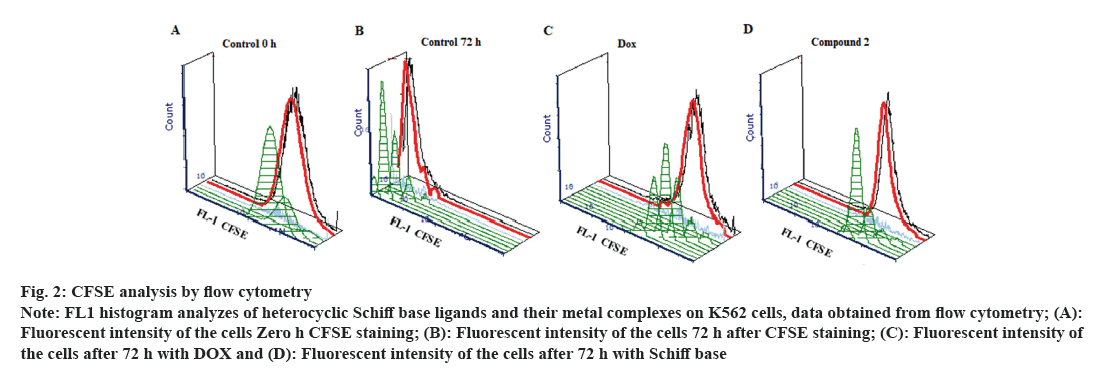
CFSE or (5(6)-CFDA N-succinimidyl ester, Sigma, USA) stain has been dissolved in DMSO solvent, and then diluted in Phosphate Buffered Saline (PBS) (adjusted to 15 μM). Cultured K562 cells have been stained with CFSE stain for about 20 min at the temperature of 37° in dark place under humidified 5 % CO2 atmosphere. Cultured cells have been washed twice, and then observed in the flow cytometry for determining maximum florescence intensity immediately after CFSE staining. CFSE stained K562 cell numbers have been adjusted to (105 cell/ml) per each well, then planted in a 96-well microplate, which previously has been treated with an IC50 dose of DOX and compounds, and incubated in CO2-incubator with humidifying property at 37° under 5 % CO2 condition for 72 h in a dark place. After incubation cells were re-analyzed by flow cytometry (Beckman coulter, USA) for determining CFSE intensities. Cells were totally gated at FS and SS histogram. Unstained cells were used for determining of auto-fluorescent, Green Fluorescence channel 1 (FL1) histogram within the range of 100-104 was used to determine CFSE level of cultured cells (fig. 2). Acquired LISTMOD data were analyzed to determine Proliferative Index (PI) values for each compounds.
Fig 2: CFSE analysis by flow cytometry
Note: FL1 histogram analyzes of heterocyclic Schiff base ligands and their metal complexes on K562 cells, data obtained from flow cytometry; (A): Fluorescent intensity of the cells Zero h CFSE staining; (B): Fluorescent intensity of the cells 72 h after CFSE staining; (C): Fluorescent intensity of the cells after 72 h with DOX and (D): Fluorescent intensity of the cells after 72 h with Schiff base
Rho123 analysis:
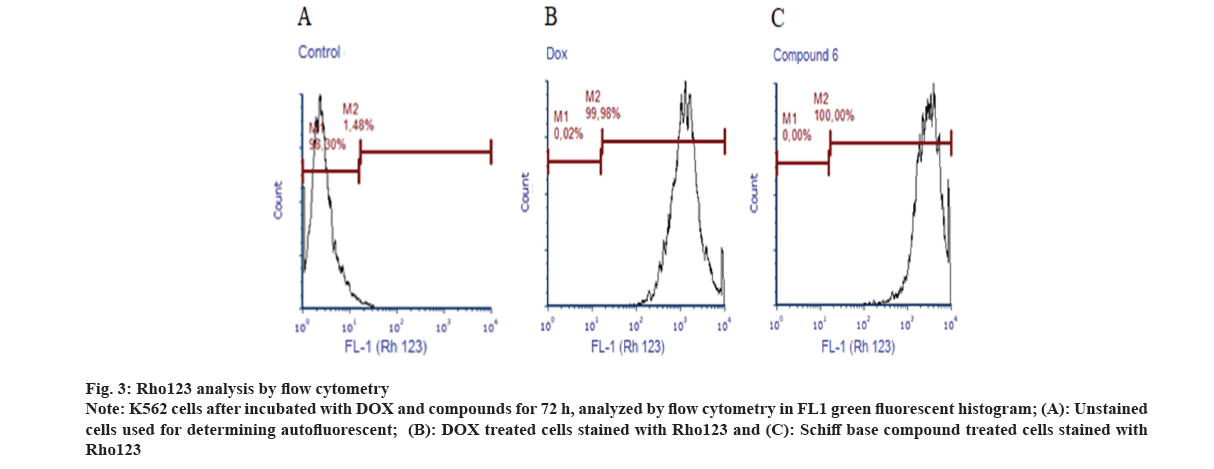
Cultured cells were incubated with 10 ml of Rho123 (1 mg/ml, Sigma) at the dose of 105/ml at 37° for 60 min in dark. After incubation, 100 ml of PBS was added to the wells and then analyzed in flow cytometry. Unstained cells were used for determining auto fluorescent, FL1 histogram used for determining Rho123 accumulation levels of the cells (fig. 3).
Fig 3: Rho123 analysis by flow cytometry
Note: K562 cells after incubated with DOX and compounds for 72 h, analyzed by flow cytometry in FL1 green fluorescent histogram; (A): Unstained cells used for determining autofluorescent; (B): DOX treated cells stained with Rho123 and (C): Schiff base compound treated cells stained with Rho123
Immunofluorescence cleaved caspase-3 analysis:
Culture wells have been rinsed within PBS and then fixed with paraformaldehyde (4 %) at 37° for 20 min. Fixed-Cells permeabilization has been done by using Triton® X-100(0, 1 %) in the PBS solution for about 15 min, then 3 % of bovine serum albumin/PBS was used in order to block them for about 30 min.
Eventually, Cells have been incubated with Alexa Fluor 488 conjugated anti-cleaved caspas-3 antibodies at room temperature for 2 h in the dark place. Samples were kept in 100 µl PBS at 4° in the dark until imaging.
Histopathological morphology analyses:
Cultured Cells were stained by Hematoxylin and Eosin (H&E), Papanicolaou (PAP) and Giemsa using standard histopathology protocols. The stained cells were examined using a light microscope (100X magnification) for morphological changes which imply apoptosis such as nuclear deterioration, chromatin condensation, chromatin marginalization and apoptotic bodies.
Results and Discussion
IC50 values of heterocyclic Schiff base ligands and their Cu(II) complexes on K562 cell are shown in Table 2. The IC50 level of the C6 (2.0±0.1 µM) were found to be more effective as equivalent to that of DOX on K562 cells (1.3±0.1 µM). The remaining tested compounds were also exerted cytotoxic effects on K562 cells.
| Compounds | IC50 (µM) | PI* | Rho123 (FL-1 mean channel**) |
|---|---|---|---|
| Control 0 h | NA | 1 | NA |
| Control 72 h | NA | 5.4 | 5547 |
| DOX | 1.3±0.1 | 1.9 | 1230 |
| C1 | 12.8±0.3 | 2.9 | 2985 |
| C2 | 9.4±0.3 | 2.8 | 2737 |
| C3 | 11.7±0.6 | 2.9 | 2729 |
| C4 | 2.4±0.1 | 3.1 | 2943 |
| C5 | 11.3±1.3 | 2.9 | 2711 |
| C6 | 2.0±0.1 | 3.0 | 2727 |
| C7 | 4.4±0.4 | 3.1 | 3195 |
| C8 | 3.4±0.1 | 3.2 | 3095 |
Note: NA: Not applicable; *PI: Proliferative index; **Fluorescent intensity measured by flow cytometry
Table 2: Cytotoxic, Antiproliferative and Apoptotic Effects of DOX and Heterocyclic Schiff Base Ligands and Their Cu (Ii) Complexes On K562 Cells
Cell proliferation inhibition by DOX and the compounds on K562 cells according to their IC50 values were represented as Proliferative Indexes (PI) which is shown in Table 2. PI of the DOX on K562 cells were found to be (PI: 1.9) while some of the compounds have greater PI values, C2 showed highest PI values and C8 have the lowest PI values on K562 cells (2.8, 3.2, respectively). Flow cytometric analyses conducted as counted 10 000 cells for each sample. Control cells showed high ratio fitted cells immediately after CFSE staining with very low noise and zero undivided cells. The cells show highest fluorescent intensity indicated that maximum staining achieved by 15 µM CFSE concentration (FL1 median channel, 3751). Cells diminished the dye by high ratio division and after 72 h incubation and fluorescent intensity measured as noise (FL1 median channel, 5.1). DOX showed powerful antiproliferative activity against K562 cells and mean channel level for FL1 histogram measured in high intensity with very low level noise showed that cell division fall behind positive control and Schiff base component applied cells (FL1 median channel, 1860). It was observed that the PI values of the compounds determined by using IC50 dose of compounds did not show any parallelism in correlation with the IC50 values of the compounds, and the PI values of each compound were found to be unique (Table 2).
Cleaved caspas-3 expression levels were determined by fluorescent intensity of the cells in FL-1 histogram. Immunefluorescent cleaved caspase-3 analysis supported the flow cytometry analysis results, and cells treated with DOX and Schiff base compounds were observed to have positive cleaved caspase-3 under fluorescent microscope analysis (fig. 4).
Rho123 analyzes were performed by flow cytometry to determine mitochondrial membrane potentials. Rho123 values fluorescent intensity of K562 cells treated with Schiff base compounds were found to be lower than the control and higher than DOX (Table 2). Rho123 accumulation represents cell activity as an indicator for mitochondrial membrane potential.
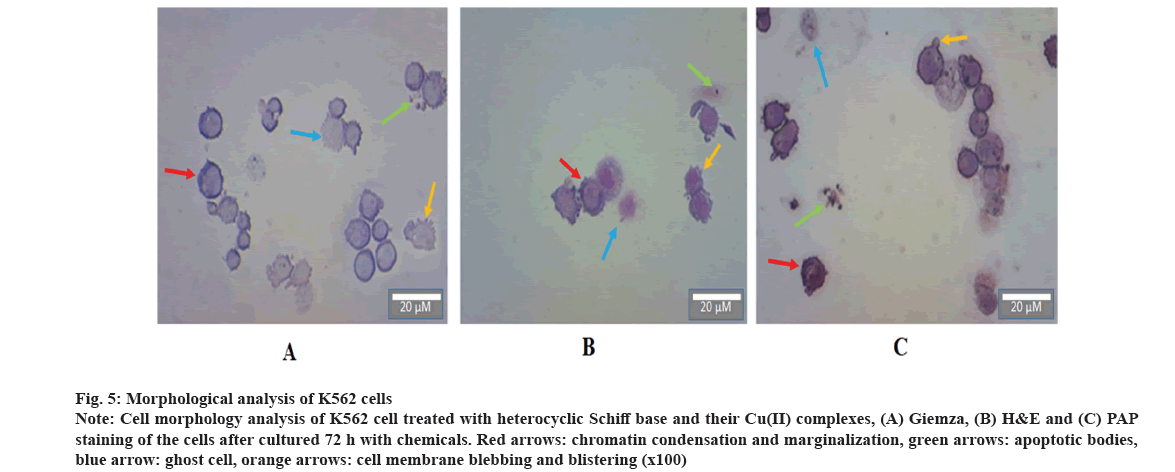
Morphological changes pointing to apoptosis were followed by giemza, H&E and PAP staining. In K562 cells treated with DOX and Heterocyclic Schiff bases, chromatin condensation and marginalization, which indicates apoptosis, were observed in cells affected by compounds and leading to apoptosis. Apoptotic bodies, which are formed by the degradation of cells after advanced destruction, were another evidence of the apoptotic effects of compounds on cells. Changes in the cell membrane, especially blebbing and blistering which is occurring in the cell membrane, were observed in some cells after the application of the compounds, and some ghost cells were formed as well (fig. 5).
Fig 5: Morphological analysis of K562 cells
Note: Cell morphology analysis of K562 cell treated with heterocyclic Schiff base and their Cu(II) complexes, (A) Giemza, (B) H&E and (C) PAP staining of the cells after cultured 72 h with chemicals. Red arrows: chromatin condensation and marginalization, green arrows: apoptotic bodies, blue arrow: ghost cell, orange arrows: cell membrane blebbing and blistering (x100)
MDR is becoming more common in cancer, necessitating the creation of novel and promising compounds with desirable properties that can combat MDR Therefore, basic science research showing that various compounds may have anticancer activity is attracting considerable attention. In particular, Schiff bases have come to the fore as the compounds that have recently been the most researched and attracted attention in promising in vitro and in vivo studies. Schiff base metal complexes have various effects such as enzyme inhibition, increasing of lipophilicity, interaction with intracellular molecules, changes in membrane functioning, and stopping cell cycle[18]. Schiff bases are often among the most important compounds considered in terms of anticancer research, and some Schiff base ligands and their complex compounds with metals have been reported to have strong anticancer effects on cell lines of many cancer types[19]. Synthesis of metal complexes of Schiff base-derived compounds with various metal ions leads to a great change in the anticancer and antioxidant properties of the coordination molecule[20]. In our study, heterocyclic Schiff base ligands and Cu(II) complexes were tested for the anticancer activity on K562 cells and metal complexes of the compounds had higher cytotoxic activity on the chronic myeloid leukemia cells.
Schiff base ligands can be used to form complexes with various metals and copper is an excellent choice. Some of the ways copper suppresses cancer cells in cancer biology are reactive oxygen species scavengers, antiangiogenic agents, apoptosis inducers, protein disulfide isomerase inhibitors and proteasome inhibitors[21,22]. To assess mitochondrial dynamics, functions and cell fate, when the K562 cell line was exposed to a non-cytotoxic copper overload, it was observed to cause elevations in mitochondrial activity and autophagic cellular activities. A smaller and denser appearance of mitochondria was also observed. An increase in cell proliferation and inhibition of cell differentiation were observed, with an increase in bioenergetic activity without a reduction in membrane potential[23]. On the other hand, in a study of leukemia cells to investigate the antiproliferative capacity of the Cu(II) complex of 1-(p-toluenesulfonyl)cytosine, a significantly higher intracellular copper concentration and higher in vitro activity were observed in K562 cells. Apoptotic morphological alterations and changes in the mitochondrial membrane potential of the cells were observed in the cells, but caspase-3 activity was not found. According to these results, it is suggested that Cu(II) in the complex compound increases its antiproliferative capacity by improving the entry of the ligand to which it is bound[24]. In our study, although we didn’t use any other metal complex than Cu(II), We discovered that metal complexes powered by Cu(II) contents were cytotoxic, similar to what other researchers discovered with Cu(II) complexes of newly synthesized Schiff base derivatives. In our study, contrary to previous studies, it was observed that metal complexes cause a significant increase in antiproliferative capacity compared to the ligands of the compounds.
A salicylaldehyde-ophenylenediamine Schiff base compound displayed a strong capability to inhibit the proliferation of leukemia cell lines K562. Moreover the compound resulted in a strong apoptotic activity against leukemia cells, and affected the cell cycle distribution[25]. In a study which is on leukemia with Schiff base ligands and their metal complexes, the cytotoxic effects of ortho and para fluorophenyl-3,5-ditert-butylsalicyildimines and their Pd(II) and Cu(II) complexes on K562 cells were investigated. It has been reported that the Cu(II) complex in the para position exhibits strong cytotoxic effects[26]. The synthesis and structure of Schiff bases and their metal complexes is fascinating because it reveals a great wealth of structural, physico-chemical and catalytic properties. Given the simplicity and ease of access to Schiff bases and their metal complexes, investigation of such compounds is essential to pinpoint and understand structure-property relationships in order to optimize and develop their use in biological applications[27]. In the heterocyclic Schiff base ligands which we used in our study, the only molecular structural difference is the changes in the number of ring structure elements. In terms of molecular structure, no negative or positive correlation was found between the changes in the ring structure of the compounds and the cytotoxic or antiproliferative effects of the compounds, both in terms of ligands and metal complexes.
Biological studies which were carried out in the K562 leukemia cell line have shown that the groups that bind to dithiocarbazate moieties and metals show marked differences in biological properties. Zinc(II) complexes showed cytotoxic activities on the K562 leukemia cell line through induced loss of mitochondrial membrane potential[28]. Fe(III) complexes of tetradentate Schiff base ligands show anti-tumor activity against K562 and Jurkat cancer cells[29]. The Rho123 levels measured in K562 cells after incubation with the compounds were found to be higher than DOX, but the fact that the activity of the compounds was found to be significantly lower than the control, which can be shown as evidence that the compounds cause induction of apoptosis in the cells. Induction of apoptosis in cells is also confirmed by cleaved caspase-3 and histopathological staining, but no significant difference was observed between the ligands of the compounds and Cu(II) complexes.
The anti-cancer activity of a water-soluble copper(II) thiosemicarbazone complex (Cu-TSC) against K562 was evaluated in a study under the assumption that thiosemicarbazones exhibit significant and selective cytotoxic potential against a wide range of cancers. It is reported that time- and dose-dependent growth inhibition of Cu-TSC treatment occurs in K562 by inducing cell accumulation in the G1 subfraction, as well as reversible arrest in G0/G1 and G2/M phases in K562. It has been reported that cell death occurs due to apoptosis, phosphatidylserine externalization and caspase-3 activation, apoptosis is shaped by the upregulation of caspase-8, -9 and the change of B- cell lymphoma protein 2 associated X protein/B-cell lymphoma protein 2 ratio, and an increase in the formation of reactive oxygen species is also observed[30]. Flow cytometric and immunofluorescent analyzes were applied to show the expression of cleaved caspase-3 in cells with the effect of the compounds in our study which show that apoptosis is effective in cell death. Although cleaved caspase-3 expression in cells was observed at close levels of heterocyclic Schiff base compounds with Cu(II) complexes in DOX-treated cells, ligands were observed to trigger expression at a lower level.
Anticancer metal complexes have been demonstrated to react with the cellular redox homeostasis system, causing it to malfunction. Activation via reductions generated by binding metal ions transported by the substances to cellular ligands is critical to know the molecular actions of anticancer medicines in metal complex structure[31]. Although certain chemical compounds are not damaging to normal healthy cells, they have considerable cytotoxic and apoptotic impacts in cancer cells from different kinds. Consequently, heterocyclic Schiff bases have become popular anticancer agents, and fresh good results are disclosed with the scientific community[32].
The cytotoxic, antiproliferative, and apoptosis-stimulating actions of heterocyclic Schiff base ligands and especially their Cu(II) complexes against chronic myeloid leukemia have shown potential biological properties. The C6 complex appears to be promising anticancer drugs candidate in leukemia treatment, however, in vivo research is needed to confirm these findings.
Acknowledgements
This study was supported by Harran University Scientific Research Project Fund (HUBAP) with project number 20161. The author would like to thank Dr. Feyza Nur TUNCER for constructive criticism, language editing and proofreading of the manuscript.
Conflict of interests:
The authors declared no conflict of interests.
References
- Poorcheraghi H, Hekmatpou D, Mehrabi F. The quality of life of patients with different leukemia types. J Client Center Nurs Care 2019;5(2):97-104.
- Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020;70(3):145-64.
[Crossref] [Google Scholar] [PubMed]
- Deveci B, Yildiz A, Yilmaz S, Ozcan B, Kargi A, Rabin SA, et al. Evaluation of the patients with hematological malignancies along with synchronous or metachronous solid tumors. Int J Hematol Oncol 2021;32(4):214-20.
- Shilpa BS, Padmamma S, Kumara AT, Walmiki RH. Mapping of scientific articles on Leukemia: A scientometric study. Libr Philos Pract 2019;1:1-4.
- Aktimur S, Gunes A, Akidan O, Karatas A, Turgut M. Efficacy of the Combination of Venetoclax and Azacitidine in elderly of frail relapsed/refractory patients with acute myeloid leukemia, first multi-institutional real world experience from Turkey. J Hematol Oncol 2020;31(4): 213.
- Kankala RK, Liu CG, Yang DY, Wang SB, Chen AZ. Ultrasmall platinum nanoparticles enable deep tumor penetration and synergistic therapeutic abilities through free radical species-assisted catalysis to combat cancer multidrug resistance. Chem Eng J 2020;383:123138.
- Gulbas Z. Advanced phase chronic myeloid leukemia treatment and transplantation. Int J Hematol Oncol 2011;33(1):18-23.
- Bukowski K, Kciuk M, Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci 2020;21(9):3233.
[Crossref] [Google Scholar] [PubMed]
- Odemis DA, Yazici H, Gurbuz O, Tuncer SB, Erdogan OS, Erciyas SK, et al. Downregulation of Forkhead Transcription Factor (FOXO3a) contributes to tumorigenesis of acute myeloid leukemia and chronic myeloid leukemia. Int J Hematol Oncol 2021;33(1):028-35.
- Ayatollahi H, Rahimi H, Jafarian AH, Allahyari A, Sadeghian MH, Keramati MR, et al. Assessment of BAALC gene expression in acute myeloid leukemia patients compared to control group in North-East of Iran. Int J Hematol Oncol 2017;33(1):237-43.
- Suzergoz F. The place and importance of CFSE method in determining cell cytotoxicity. J Harran Univ Med Faculty 2017;14(3):11.
- Fekri R, Salehi M, Asadi A, Kubicki M. Synthesis, characterization, anticancer and antibacterial evaluation of Schiff base ligands derived from hydrazone and their transition metal complexes. Inorganica Chim Acta 2019;484:245-54.
- Shah SS, Shah D, Khan I, Ahmad S, Ali U, Rahman A. Synthesis and antioxidant activities of Schiff bases and their complexes: An updated review. Bio Interface Res Appl Chem 2020;10(6):6936-63.
- Abd El?All AS, Magd?El?Din AA, Ragab FA, ElHefnawi M, Abdalla MM, Galal SA, et al. New benzimidazoles and their antitumor effects with Aurora A kinase and KSP inhibitory activities. Arch Pharm 2015;348(7):475-86.
[Crossref] [Google Scholar] [PubMed]
- Shaharyar M, Mazumder A, Ahsan MJ. Synthesis, characterization and anticancer evaluation of 2-(naphthalen-1-ylmethyl/naphthalen-2-yloxymethyl)-1-[5-(substituted phenyl)-[1, 3, 4] oxadiazol-2-ylmethyl]-1H-benzimidazole. Arab J Chem 2014;7(4):418-24.
- Aytar E, Tamar H, Kasim V. International GAP Mathematics - Engineering - Science and Health Sciences Congress, 2019.
- Tamar H. Master Thesis, Harran University, ?anl?urfa; 2018.
- Bharti SK, Singh SK. Metal based drugs: Current use and future potential. Der Pharm Lett 2009;1(2):39-51.
- Sak ZH, Süzergöz F, Kasumov VT, Gürol AO. Anticancer properties of fluorinated aminophenylhydrazines on A549 lung carcinoma cell line. Iran Public Health 2021;50(3):550-6.
[Google Scholar] [PubMed]
- Arunadevi A, Raman N. Biological response of Schiff base metal complexes incorporating amino acids–a short review. J Coordination Chem 2020;73(15):2095-116.
- Muhammad N, Guo Z. Metal-based anticancer chemotherapeutic agents. Curr Opin Chem Biol 2014;19:144-53.
[Crossref] [Google Scholar] [PubMed]
- Zehra S, Tabassum S, Arjmand F. Biochemical pathways of copper complexes: Progress over the past 5 years. Drug Discov Today 2021;26(4):1086-96.
[Crossref] [Google Scholar] [PubMed]
- Ruiz LM, Jensen EL, Rossel Y, Puas GI, Gonzalez-Ibanez AM, Bustos RI, et al. Non-cytotoxic copper overload boosts mitochondrial energy metabolism to modulate cell proliferation and differentiation in the human erythroleukemic cell line K562. Mitochondrion 2016;29:18-30.
[Crossref] [Google Scholar] [PubMed]
- Glavas-Obrovac L, Jukic M, Miskovic K, Markovic I, Saftic D, Ban Z, et al. Antiproliferative and proapoptotic activity of molecular copper (II) complex of N-1-tosylcytosine. J Trace Elements Med Biol 2019;55:216-22.
- Luo H, Xia YF, Sun BF, Huang LR, Wang XH, Lou HY, et al. Synthesis and evaluation of in vitro antibacterial and antitumor activities of novel N, N-disubstituted Schiff bases. Biochem Res Int 2017;2017:6257240.
[Crossref] [Google Scholar] [PubMed]
- Varis Y. Invest?gat?on of cytotox?c effect of fluor?ne Schiff bases on cancer cells. Harran Univ Graduate School Nat Appl Sci 2017.
- Liu X, Hamon JR. Recent developments in penta-, hexa-and heptadentate Schiff base ligands and their metal complexes. Coordination Chem Rev 2019;389(15):94-118.
- Li MX, Zhang LZ, Chen CL, Niu JY, Ji BS. Synthesis, crystal structures, and biological evaluation of Cu (II) and Zn (II) complexes of 2-benzoylpyridine Schiff bases derived from S-methyl-and S-phenyldithiocarbazates. J Inorgan Biochem 2012;106(1):117-25.
[Crossref] [Google Scholar] [PubMed]
- Shabani F, Saghatforoush LA, Ghammamy S. Synthesis, characterization and anti-tumour activity of Iron (III) Schiff base complexes with unsymmetric tetradentate ligands. Bull Chem Soc Ethiopia 2010;24(2):193-9.
- Parsa FG, Feizi MA, Safaralizadeh R, Hosseini-Yazdi SA, Mahdavi M. Molecular mechanisms of apoptosis induction in K562 and KG1a leukemia cells by a water-soluble copper (II) thiosemicarbazone complex. J Biol Inorgan Chem 2020;25:383-94.
[Crossref] [Google Scholar] [PubMed]
- Jungwirth U, Kowol CR, Keppler BK, Hartinger CG, Berger W, Heffeter P. Anticancer activity of metal complexes: Involvement of redox processes. Antioxid Redox Signal 2011;15(4):1085-127.
[Crossref] [Google Scholar] [PubMed]
- Okcanoglu TB, Susluer SY, Kayabasi C, Yelken BO, Saydam G, Gunduz C. Effect of resveratrol on apoptosis and MDM2, RUNX3, RB gene expressions in human acute myeloid leukemia cells by transfection of MATRA-Mediated miR-150. Int J Hematol Oncol 2018;33(1):147-53.